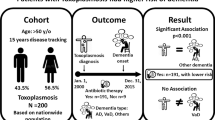
Abstract
Background
Scrub typhus is a mite-borne infectious rickettsial disease that can occur in rural and urban areas, with an especially high prevalence in older populations. This disease causes systemic vasculitis that can invade the central nervous system. Considering these characteristics, here we examined whether scrub typhus was associated with the occurrence of dementia, using large population-based cohort data.
Method
This population-based cohort study enrolled patients aged 60–89 years using data from the Health Insurance Review and Assessment database of South Korea between 2009 and 2018. We defined scrub typhus and dementia using International Classification of Diseases, Tenth Edition diagnostic codes. The control group was stratified according to age and sex at a ratio of 1:5 to the case group in the study population. The index date was set after 90 days beyond the date of the scrub typhus diagnosis, while the observation period was from the time of the index appointment to December 31, 2020. The primary outcome was newly diagnosed dementia. The secondary outcome was dementia classification, such as Alzheimer’s disease, vascular dementia, and other. All analyses were conducted by matching age, gender, and comorbidity.
Results
During the observation period, 10,460 of 71,047 (14.7%) people who had a history of scrub typhus versus 42,965 of 355,235 (12.1%) people in the control group, that is, with no history of scrub typhus, were diagnosed with dementia (adjusted hazard ratio, 1.12; 95% confidence interval, 1.10–1.15, p < 0.001). The Kaplan–Meier curves for time to cumulative incidence of dementia showed that the dementia incidence in both groups increased over time, while individuals with a past history of scrub typhus had a higher incidence of dementia than the control group. Second, the risk of Alzheimer’s disease was significantly higher among patients with a history of scrub typhus (adjusted hazard ratio, 1.15; 95% confidence interval 1.13–1.18, p < 0.001).
Conclusion
In conclusion, a history of scrub typhus infection in old age is significantly associated with an increase in dementia, especially Alzheimer’s disease. Our results suggest that prevention and appropriate treatment of scrub typhus should be emphasized as a dementia prevention measure.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Scrub typhus is an infectious disease that is transmitted by human bites from mites infected with Orienta tsutsugamushi, a gram-negative coccobacillus in the family Rickettsiaceae. This bacterium infects host vascular endothelial cells and is released from infected cells, causing systemic vasculitis such as Scrub typhus [1].
Most cases are found in Southeast Asia, China, Japan, India, Indonesia and northern Australia, especially in rural areas and both residents and visitors are susceptible to infection [2]. In 2016, there were nearly 11,000 new cases in South Korea. Since then, more than 4,000 new cases have been reported annually [3]. In terms of age distribution, the proportion of affected older individuals aged 60 years and older is high [4, 5]. Unlike other infectious diseases, scrub typhus is characterized by systemic vasculitis. In the early stages of the infection, proper antibiotic treatment results in good responses. However, delayed treatment can lead to severe systemic progression and complications ranging from pneumonia and acute renal injury to invasion of the central nervous system (CNS) resulting in meningitis or encephalitis [6]. The role of scrub typhus in the long-term development of CNS complications remains unknown.
Dementia is a degenerative disorder that affects the brain, with more than 90% of cases diagnosed after 65 years old as the age is the leading risk factor. As a result, the increase in the mean population age worldwide is associated with a steady increase in the incidence and prevalence of dementia [7]. In 2015, according to the World Alzheimer Report, there are nearly 46.8 million dementia cases worldwide, which is expected to become 131.5 million by 2050 [8]. Although the pathogenesis of dementia remains unclear, it may be associated with comorbidities affecting the blood vessels, such as hypertension and diabetes [9, 10]. Other infectious diseases such as Borrelia burgdorferi, Helicobacter pylori, Chlamydia pneumoniae and herpes simplex virus type 1 (HSV-1) may also be associated with dementia [11,12,13,14,15,16].
Considering the characteristics of scrub typhus infection (occurring frequently in old age and involving the inflammation of systemic blood vessels that can invade the CNS), scrub typhus may be associated with the occurrence of dementia.
This study aimed to investigate this hypothesis using large population-based cohort data from the Health Insurance Review and Assessment Service (HIRA) in South Korea.
Methods
Data source
HIRA is the data source responsible for claim reviews and quality assessments of the National Health Insurance (NHI) and National Medical Aid (NMA) programs, of which all medical institutions and local pharmacies are compulsory participants. All medical expenditures within the NHI and NMA programs are monitored by HIRA for redemption purposes. The HIRA claims database contains basic information on patients’ sociodemographic characteristics and prescriptions, diagnosis and diagnostic procedures [17].
This dataset contains coded information for all of the diagnoses, tests, and treatments prescribed in South Korea since January 2007.
Study population
We set the washout period for all factors that may affect the occurrence of dementia from January 2007 to December 2008.
From January 2009 to December 2018, among people aged 60 to 89, those diagnosed with scrub typhus were classified into the scrub typhus group. The index date was set after 90 days of scrub typhus diagnosis date to exclude the screening effect on dementia diagnosis. The operational definition of scrub typhus was as follows: (1) International Classification of Diseases, Tenth Edition (ICD-10) diagnostic code corresponding to scrub typhus (A75.3) during the registration period; and (2) prescription of doxycycline, the treatment of scrub typhus, for at least three days a week before or after the day when the diagnostic code was added.
The operational definition of dementia was as follows: (1) code for dementia (V-code V800, V801, V810, V811) in expending benefit coverage, reduces medical costs for patients with fatal diseases, during the follow-up period; or (2) at least one diagnostic code for dementia (F00.x, F01.x, F02.x, F03.x, G30.x), examination code (Mini-Mental State Examination [MMSE F6216] and one Global Deterioration Scale score [GDS, F6221] and Clinical Dementia Rating score [CDR, F6222]), or drug code (donepezil [1486, 6434], rivastigmine [2245], and/or galantamine [3852]) within the adjacent 4 weeks. In South Korea’s Health Insurance System, hospitals can receive medical expenses after the evaluation and approval of the Review and Assessment Service; it was considered that the possibility of falsely coding as dementia would be low.
We also classified patients by dementia subgroup into Alzheimer’s disease (AD), vascular dementia, and other, setting the operational definition of the subtype of dementia by using diagnostic codes. Among people diagnosed with dementia, those diagnosed with F00.x or G30.x code corresponding to Alzheimer’s were set as Alzheimer’s group, and those who received F01.x code corresponding to vascular dementia were set as vascular dementia group.
As a control group, among the data sets of HIRA, those aged 60–89 who were not infected with scrub typhus from January 2007 to December 2020 and applied to the exclusion criteria were randomly selected, stratified by age and sex at a ratio of 1:5.
We excluded those who were diagnosed with scrub typhus or dementia before the end of the washout period, for whom covariate data were missing, or who died during the observation period.
Institutional Review Board of Korea University Ansan Hospital (2022AS0001) and the HIRA (2022-001) approved the study. They also waived the informed consent to the study. We report the results in compliance with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guidelines [18].
Outcomes
The observation period was set from the index date to December 31, 2020. The primary outcome of this study was the event and timing of newly diagnosed dementia during the observation period. The secondary outcome was set as a subgroup of dementia that occurred during the observation period.
Covariates
All variables that were used to treat the disease at least once between January 2007 and the index date were included as covariates. Age and sex were set at the time of the index date, and age was classified as 60–69 years or 70–89 years. The other covariates were defined as those with an ICD-10 diagnostic code. The analysis was adjusted for several known risk factors, such as age, sex, central degenerative disease (G20.x and G31.x), stroke (I63.x, I64.x, and I69.x), and hypertension (I10.x–I15.x), diabetes mellitus (E11.x-E14. x), and major depressive disorder (F32.x, F33.x) [14].
Statistical analysis
We represent the Categorical variables using numbers (%) and mean ± SD for continuous variables. Fisher’s exact test, Student’s t-test and the chi-squared test were conducted for intergroup comparisons. Cox proportional hazards analysis for the incidence of dementia was performed to estimate the hazards of dementia incidence with the adjustment for age, sex, and comorbidities, and hazard ratios (HRs) were calculated with 95% confidence intervals (CI). The cumulative incidence curves of dementia were constructed and compared between groups using Kaplan–Meier curves. The follow-up period started on the index date and was censored on the date of diagnosis of dementia or the end of the study. All analyses were conducted by matching age, gender, and comorbidity. Statistical significance was considered when the two-tailed P value was < 0.05. On the other hand, R version 4.0.2 was the implemented statistical problem.
Results
At the baseline, 76,073 individuals aged 60–89 years old were confirmed to have scrub typhus between January 2009 and December 2018. After the exclusion of participants who were diagnosed with scrub typhus during the washout period (n = 233), those diagnosed with dementia before the index date (n = 3991), and those who died before the index date (n = 802), a total of 71,047 subjects were included in the scrub typhus group. A control group was randomly selected at a ratio of 1:5 with sex and age matching; thus, 355,235 subjects were included (Fig. 1). The mean follow-up period was 5.6 ± 2.9 years. The case group was followed for 5.7 ± 2.8 years versus the control group for 5.6 ± 2.9 years.
All subjects of characteristics are presented in Table 1. Since the control group was selected by matching sex and age with the case group, the ratio of males to females was 37.3–62.7%. Likewise, for age, the ratios were the same between groups: 47.1% were 60–69 years old, while 52.9% were 70–89 years old.
Regarding comorbidities, stroke (19.4% vs. 18.3%, p < 0.001), central degenerative disease (9.7% vs. 7.4%, p < 0.001), diabetes mellitus (56.3% vs. 50.5%, p < 0.001), and major depressive disorder (28.6% vs. 24.7%, p < 0.001) were significantly more prevalent in the case group. However, hypertension was more common in the control group (66.2% vs. 67.5%, p < 0.001).
Of the 426,282 participants, 53,425 (12.5%) were diagnosed with dementia during the observation period between the index date and December 31, 2020. In the scrub typhus group, 10,460 (14.7%) people were diagnosed with dementia, a significantly higher incidence comparing to the control group (n = 42,965 [12.1%]; p < 0.001) (Table 1).
Cox proportional hazards analysis revealed that dementia incidence was higher among individuals with a past history of scrub typhus than in the controls. Crude HR was 1.19 (95% CI 1.16–1.21, p < 0.001) and the adjusted HR (aHR) was 1.12 (95% CI 1.10–1.15, p < 0.001) (Table 2). When classified into subgroups, the risk of AD was higher in the scrub typhus group (aHR, 1.15; 95% CI 1.13–1.18, p < 0.001). However, there was no statistically significant differences regarding the vascular dementia risk (aHR, 0.98; 95% CI 0.83–1.17, p = 0.853) and other types of dementia (aHR, 1.05; 95% CI 1.00–1.09, p = 0.033).
The Kaplan–Meier curves for the cumulative incidence of dementia are shown in Fig. 2. It shows dementia incidence in both groups increased over time, and the incidence of dementia was higher in the scrub typhus group than in the non-history of scrub typhus group. As time goes, the gap in the incidence rate also increased.
Discussion
This nationwide retrospective population-based cohort study found that the history of scrub typhus is related to an increased risk of dementia, especially AD. The prevalence of all comorbidities except hypertension was significantly higher in the scrub typhus group than in the control group. However, the association between a high prevalence of dementia and a history of scrub typhus persisted after the adjustment for age, sex, and comorbidities. This trend became more pronounced over time, that can be attributed to the continued effects of the infection in scrub typhus over time.
Dementia, a degenerative brain disorder, is a major cause of disability. AD is the most common subtype of dementia in the world. Aging is considered an important risk factor as it increases chronic low-grade inflammation, which may be involved in the pathological progress of AD [19,20,21]. One of neuropathological features of AD is that extracellular neuronal plate containing amyloid beta (Aβ) and neural fiber tangles consisting of intracellular hyperphosphorylation tau filaments, which can be accompanied by deposition of vascular Aβ and inflammation of vessel [22,23,24]. In AD, several mechanisms contribute to the pathogenesis of neurodegeneration. Among them, neuroinflammation plays an important role in pathogenesis due to mechanisms that exacerbate Aβ and tau pathologies [25,26,27]. Increased levels of pro-inflammatory cytokines in postmortem brain and the serum of AD patients suggest that neuroinflammation plays an important role in AD pathogenesis [28].
Many studies have suggested that infectious diseases such as bacteria and viruses may be associated with AD development by this neuroinflammatory pathogenesis [29]. This eventually leads to accumulation of Aβ and hyperphosphorylation of tau by inducing excessive neuroinflammation. For example, HSV-1 is the most common cause of viral encephalitis and previous studies have shown that HSV-1 was detected in 70–100% of AD patients over the age of 65. When comparing AD patients with controls, more HSV-1 DNA was identified in AD, which was associated with Aβ plaque [30,31,32,33]. This suggests that the recent infection or reactivation of HSV-1 is associated with the pathogenesis of AD. As another example, spirochetes are the bacteria that can cause chronic inflammation in CNS, among which Borrelia burgdorperi and Treponema pallidum are widely studied [34, 35]. B. burgdorferi antigens were observed in neuron and Aβ plaques and neurofibrillary tangles in patients with both AD and neuroborreliosis [34, 36]. According to the study, spirochetal-specific antigens and T. pallidum DNA were detected on Aβ plaques, and the risk of AD increased in patients with T. pallidum infection [37, 38]. Rickettsial infections can affect CNS, and the most common neurological manifestations are meningitis, encephalitis, and acute disseminated encephalomyelitis [6]. In some studies, rickettsia lasted for a long period in the CNS of immunodeficiency mice and reappeared, causing severe neuroinflammation [39, 40]. This shows that Rickets disease is a potential risk factor for AD. The most representative involvement of CNS in rickettsia is Rocky Mountain spotted fever and epidemic typhus, followed by scrub typhus [41]. Pathological studies of brain autopsy specimens in patients with fatal scrub typhus showed the presence of mononuclear cell infiltration of the leptomeninges and typhoid nodules, microglial clusters, and hemorrhage [42]. These pathological findings are related to the significant upregulation of the pro-inflammatory cytokine gene in scrub typhus infection in murine models. Therefore, this neurotrophic O. tsutsugamushi seems to be able to induce neuroinflammation by directly inducing AD through CNS infection, or indirectly inducing AD through various systemic inflammatory effects in the brain.
Although scrub typhus is a disease that causes systemic vasculitis, the relationship between its infection and an increased incidence of vascular dementia remains unclear. Vascular dementia can only be diagnosed if there is sufficient evidence for cerebrovascular disease (CVD) on brain imaging, such as multiple infarctions, subcortical ischemic changes, or hemorrhage [43]. However, systemic vasculitis caused by scrub typhus is usually microscopic and pathologic findings and might not progress to ischemic change to brain parenchyma. Our findings are supported by a previous study that Scrub typhus showed no difference in CVD incidence rate compared to the control group, even though it showed a greater incidence rate of cardiovascular disease when analyzing the National Health Insurance Service-National Sample Cohort data in South Korea [44].
To our knowledge, this is the first to show the long-term progression of dementia-related to a history of scrub typhus infection. Its main strength is its use of medical codes that can minimize interference, such as recall bias and selection bias, and the results could be derived on a large scale. It was also designed to reduce bias through washout time and correct the risk factors for dementia. Because only computerized anonymous information was used, it may be free of ethical problems and personal information leakage.
This study has several limitations. First, since each person’s inclusion or exclusion criteria and diagnosis were made indirectly, such as via operational definitions by code, it is possible that the data may have been over-or underestimated. Second, although some potential risk factors were adjusted for, important risk factors, such as socioeconomic status and family history, could not be considered but may affect the results. Third, after January 20, 2020, there is a possibility of a potential bias due to changes in medical behavior related to the COVID-19 epidemic in Korea.
Conclusion
In conclusion, our findings suggest that a history of scrub typhus infection in old age is significantly associated with increased dementia risk, especially AD. Additionally, prevention and appropriate treatment of scrub typhus should be emphasized as a preventive measure for dementia.
Availability of data and materials
All data used in analysis of this manuscript is freely available by contacting the corresponding author.
References
Fisher J, Card G, Soong L. Neuroinflammation associated with scrub typhus and spotted fever group rickettsioses. PLoS Negl Trop Dis. 2020;14(10):e0008675.
Kelly DJ, Fuerst PA, Ching WM, Richards AL. Scrub typhus: the geographic distribution of phenotypic and genotypic variants of Orientia tsutsugamushi. Clin Infect Dis. 2009;48(Suppl 3):203–30.
Bonell A, Lubell Y, Newton PN, Crump JA, Paris DH. Estimating the burden of scrub typhus: a systematic review. PLoS Negl Trop Dis. 2017;11(9):e0005838.
Infectious diseases statistics sysmtem. https://www.index.go.kr/potal/main/EachDtlPageDetail.do?idx_cd=1442.
Chung MH, Kang JS. History of Tsutsugamushi disease in Korea. Infect Chemother. 2019;51(2):196–209.
Sekeyova Z, Danchenko M, Filipcik P, Fournier PE. Rickettsial infections of the central nervous system. PLoS Negl Trop Dis. 2019;13(8):e0007469.
Elahi FM, Miller BL. A clinicopathological approach to the diagnosis of dementia. Nat Rev Neurol. 2017;13(8):457–76.
International AsD: World Alzheimer Report 2015. In.; 2015.
Posner HB, Tang MX, Luchsinger J, Lantigua R, Stern Y, Mayeux R. The relationship of hypertension in the elderly to AD, vascular dementia, and cognitive function. Neurology. 2002;58(8):1175–81.
Schmidt R, Launer LJ, Nilsson LG, Pajak A, Sans S, Berger K, Breteler MM, de Ridder M, Dufouil C, Fuhrer R, et al. Magnetic resonance imaging of the brain in diabetes: the Cardiovascular Determinants of Dementia (CASCADE) Study. Diabetes. 2004;53(3):687–92.
Blanc F, Philippi N, Cretin B, Kleitz C, Berly L, Jung B, Kremer S, Namer IJ, Sellal F, Jaulhac B, et al. Lyme neuroborreliosis and dementia. J Alzheimers Dis. 2014;41(4):1087–93.
Jamieson GA, Maitland NJ, Wilcock GK, Yates CM, Itzhaki RF. Herpes simplex virus type 1 DNA is present in specific regions of brain from aged people with and without senile dementia of the Alzheimer type. J Pathol. 1992;167(4):365–8.
Kountouras J, Boziki M, Gavalas E, Zavos C, Deretzi G, Grigoriadis N, Tsolaki M, Chatzopoulos D, Katsinelos P, Tzilves D, et al. Increased cerebrospinal fluid Helicobacter pylori antibody in Alzheimer’s disease. Int J Neurosci. 2009;119(6):765–77.
Mawanda F, Wallace R. Can infections cause Alzheimer’s disease? Epidemiol Rev. 2013;35:161–80.
Miklossy J. Alzheimer’s disease—a spirochetosis? Neuroreport. 1993;4(7):841–8.
Paradowski B, Jaremko M, Dobosz T, Leszek J, Noga L. Evaluation of CSF-Chlamydia pneumoniae, CSF-tau, and CSF-Abeta42 in Alzheimer’s disease and vascular dementia. J Neurol. 2007;254(2):154–9.
Kim JA, Yoon S, Kim LY, Kim DS. Towards actualizing the value potential of Korea Health Insurance Review and Assessment (HIRA) data as a resource for Health Research: strengths, Limitations, applications, and strategies for optimal use of HIRA data. J Korean Med Sci. 2017;32(5):718–28.
von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP, Initiative S. The strengthening the reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370(9596):1453–7.
Chen WW, Zhang X, Huang WJ. Role of neuroinflammation in neurodegenerative diseases (review). Mol Med Rep. 2016;13(4):3391–6.
Gross AL, Walker KA, Moghekar AR, Pettigrew C, Soldan A, Albert MS, Walston JD. Plasma markers of inflammation linked to clinical progression and decline during preclinical AD. Front Aging Neurosci. 2019;11:229.
Kennedy BK, Berger SL, Brunet A, Campisi J, Cuervo AM, Epel ES, Franceschi C, Lithgow GJ, Morimoto RI, Pessin JE, et al. Geroscience: linking aging to chronic disease. Cell. 2014;159(4):709–13.
DeTure MA, Dickson DW. The neuropathological diagnosis of Alzheimer’s disease. Mol Neurodegener. 2019;14(1):32.
Goedert M, Spillantini MG. A century of Alzheimer’s disease. Science. 2006;314(5800):777–81.
Masters CL, Bateman R, Blennow K, Rowe CC, Sperling RA, Cummings JL. Alzheimer’s disease. Nat Rev Dis Primers. 2015;1:15056.
Heppner FL, Ransohoff RM, Becher B. Immune attack: the role of inflammation in Alzheimer disease. Nat Rev Neurosci. 2015;16(6):358–72.
Ising C, Venegas C, Zhang S, Scheiblich H, Schmidt SV, Vieira-Saecker A, Schwartz S, Albasset S, McManus RM, Tejera D, et al. NLRP3 inflammasome activation drives tau pathology. Nature. 2019;575(7784):669–73.
Sala Frigerio C, Wolfs L, Fattorelli N, Thrupp N, Voytyuk I, Schmidt I, Mancuso R, Chen WT, Woodbury ME, Srivastava G, et al. The major risk factors for Alzheimer’s Disease: age, sex, and genes modulate the Microglia response to Abeta plaques. Cell Rep. 2019;27(4):1293–1306.
Wang WY, Tan MS, Yu JT, Tan L. Role of pro-inflammatory cytokines released from microglia in Alzheimer’s disease. Ann Transl Med. 2015;3(10):136.
Danics K, Forrest SL, Kapas I, Erber I, Schmid S, Toro K, Majtenyi K, Kovacs GG. Neurodegenerative proteinopathies associated with neuroinfections. J Neural Transm (Vienna). 2021;128(10):1551–66.
Harris SA, Harris EA. Herpes simplex virus type 1 and other pathogens are key causative factors in sporadic Alzheimer’s Disease. J Alzheimers Dis. 2015;48(2):319–53.
Jamieson GA, Maitland NJ, Wilcock GK, Craske J, Itzhaki RF. Latent herpes simplex virus type 1 in normal and Alzheimer’s disease brains. J Med Virol. 1991;33(4):224–7.
Laval K, Enquist LW. The potential role of herpes simplex virus type 1 and neuroinflammation in the pathogenesis of Alzheimer’s Disease. Front Neurol. 2021;12:658695.
Looker KJ, Magaret AS, May MT, Turner KM, Vickerman P, Gottlieb SL, Newman LM. Global and regional estimates of prevalent and incident herpes simplex virus type 1 infections in 2012. PLoS ONE. 2015;10(10):e0140765.
Miklossy J. Alzheimer’s disease—a neurospirochetosis. Analysis of the evidence following Koch’s and Hill’s criteria. J Neuroinflamm. 2011;8:90.
Ramesh G, Alvarez AL, Roberts ED, Dennis VA, Lasater BL, Alvarez X, Philipp MT. Pathogenesis of Lyme neuroborreliosis: Borrelia burgdorferi lipoproteins induce both proliferation and apoptosis in rhesus monkey astrocytes. Eur J Immunol. 2003;33(9):2539–50.
MacDonald AB. Alzheimer’s neuroborreliosis with trans-synaptic spread of infection and neurofibrillary tangles derived from intraneuronal spirochetes. Med Hypotheses. 2007;68(4):822–5.
Maheshwari P, Eslick GD. Bacterial infection and Alzheimer’s disease: a meta-analysis. J Alzheimers Dis. 2015;43(3):957–66.
Miklossy J. Historic evidence to support a causal relationship between spirochetal infections and Alzheimer’s disease. Front Aging Neurosci. 2015;7:46.
Bohacsova M, Filipcik P, Opattova A, Valarikova J, Quevedo Diaz M, Skultety L, Novak M, Sekeyova Z. Survival of rat cerebrocortical neurons after rickettsial infection. Microbes Infect. 2015;17(11–12):845–9.
Osterloh A, Papp S, Moderzynski K, Kuehl S, Richardt U, Fleischer B. Persisting Rickettsia typhi causes fatal central nervous system inflammation. Infect Immun. 2016;84(5):1615–32.
Mahajan SK, Rolain JM, Kanga A, Raoult D. Scrub typhus involving central nervous system, India, 2004–2006. Emerg Infect Dis. 2010;16(10):1641–3.
Allen AC, Spitz S. A comparative study of the Pathology of Scrub Typhus (Tsutsugamushi Disease) and other Rickettsial Diseases. Am J Pathol. 1945;21(4):603–81.
O’Brien JT, Thomas A. Vascular dementia. Lancet. 2015;386(10004):1698–706.
Jung LY, Lee J, Jeon M, Hwang JH, Hong JY, Lee CS. Risk of cerebro- and cardiovascular disease in patients with scrub typhus. Eur J Clin Microbiol Infect Dis. 2020;39(3):451–4.
Acknowledgements
Not applicable.
Funding
This research was supported by a grant from the Korea Health Technology R&D project through the Korea Health Industry Development Institute (KHIDI) funded by the Ministry of Health & Welfare, Republic of Korea (Grant Number: HI20C0262).
Author information
Authors and Affiliations
Contributions
Conception: DWP; Design of the study: DWP, JYK, GHS; Data acquisition: HRS, JHJ, WSC; Data analysis & interpretation: DWP, JYK, GHS; Draft & revision of manuscript: DWP/JYK. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The study protocol was approved by the institutional review board of Korea University Ansan Hospital (no. 2022AS0001). This study is a retrospective analysis of clinical data obtained in the treatment process, and consent was exempted because there were no risk factors for the subject and prospective information was not collected.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Kim, J., Seok, H., Jeon, J.H. et al. Association of scrub typhus with incidence of dementia: a nationwide population-based cohort study in Korea. BMC Infect Dis 23, 127 (2023). https://doi.org/10.1186/s12879-023-08107-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-023-08107-0