Abstract
Background
COVID-19 vaccines are effective against infections and outcomes; however, breakthrough infections (VBT) are increasingly reported, possibly due to waning of vaccine-induced immunity or emerging variants. Most studies have focused on determining VBT rate based on antibody levels. This study aims at describing clinical features, risks, time trends, and outcomes of COVID-19 VBT among hospitalized patients in Egypt.
Methods
Data of SARS-CoV-2 confirmed patients hospitalized in 16 hospitals was obtained from the severe acute respiratory infections surveillance database, September 2021-April 2022. Data includes patients’ demographics, clinical picture, and outcomes. Descriptive analysis was performed and patients with VBT were compared to not fully vaccinated (UPV). Bivariate and multivariate analyses were performed using Epi Info7 with a significance level < 0.05 to identify VBT risk factors.
Results
Overall, 1,297 patients enrolled, their mean age 56.7 ± 17.0 years, 41.5% were males, 64.7% received inactivated, 25.% viral vector, and 7.7% mRNA vaccine. VBT was identified in 156(12.0%) patients with an increasing trend over time. VBT significantly was higher in (16–35 years) age, males, in those who received inactivated vaccine compared to corresponding groups of UPV (14.1 vs. 9.0%, p < 0.05 and 57.1 vs. 39.4%, p < 0.001 and 64.7 vs. 45.1, p < 0.01 respectively). Whereas receiving mRNA vaccine was significantly protective against VBT (7.7 vs. 21.6%, p < 001). VBT patients tend to have shorter hospital stays and lower case fatality (mean hospital days = 6.6 ± 5.5 vs. 7.9 ± 5.9, p < 0.01 and CFR = 28.2 vs. 33.1, p < 0.01 respectively). MVA identified younger ages, male gender, and inactivated vaccines as risks for VBT.
Conclusion
The study indicated that COVID-19 vaccines significantly reduce hospital days and fatality. VBT trend is on the rise and males, young ages, and inactivated vaccine receivers are at higher risk. Caution regarding relaxation of personal preventive measures in areas with higher or increasing incidences of COVID-19, particularly for the at-risk group even if they are vaccinated. The vaccination strategy should be revised to reduce VBT rate and increase vaccine effectiveness.
Similar content being viewed by others
Introduction
The ongoing COVID-19 pandemic evolved in 2019 ranks among the world’s deadliest epidemics. Officially between January 2020 and December 2021 SARS-CoV-2 has claimed 5.94 million lives, yet credible estimates place the pandemic’s true death toll closer at 18.2 million [1]. Amid the coronavirus crisis, safe and effective vaccines offer the most promising way out and are a game-changing tool for ending the COVID-19 pandemic. It is hugely encouraging to see so many vaccines proving and going into development.
While people who are fully vaccinated against COVID-19 have a significantly reduced risk of severe illness and fatality, some hospitalizations and deaths have been reported among fully vaccinated people. Aware of the magnitude of the current public health emergency and of the importance of facilitating the availability of vaccines to prevent COVID-19, and that the public will trust, FDA has granted Emergency Use Authorization (EUA) for different COVID-19 Vaccines. COVID-19 vaccines were evaluated through many clinical trials conducted according to the rigorous FDA standards in tens of thousands of study participants to determine its safety and effectiveness [2]. Multiphase randomized controlled trials (RCT) have shown high vaccine efficacy against symptomatic COVID-19 infection. However, RCT is usually performed under optimal conditions where vaccine storage and delivery are monitored, and participants usually in good health or selected for a specific health status [3].
Recently, SARS-CoV-2 vaccine breakthrough (VBT) infections have increasingly been reported in fully vaccinated patients. However, the fully vaccinated individuals remained less likely to become hospitalized and the number of fully vaccinated cases with breakthrough SARS-CoV-2 infection hospitalization was significantly lower when compared to the number of unvaccinated cases [4]. Observational studies are needed to understand the impact of waning immunity, viral variants, and other determinants of changing vaccine effectiveness against COVID-19 severity in the real-world conditions [5]. In addition, data is needed to characterize the clinical characteristics of patients hospitalized with COVID-19 after vaccination and monitor the performance of vaccines in preventing disease severity and outcome [6]. Monitoring vaccine effectiveness is necessary to determine how long restrictions will be required and decide on the need for vaccine booster doses or changes to the vaccine formulations or policy.
Globally, mass vaccination campaigns are underway to combat the COVID-19 pandemic with approximately 60% of world population being fully vaccinated [7]. In Egypt, vaccination was initiated in May 2021, with vaccination coverage rate reached 44.7% as of May 2022. Two inactivated vaccines (two doses: Sinovac and Sinopharm) and three viral vector vaccines (AstraZeneca, Sputnik V (two doses) and Johnson& Johnson (one dose)) and two mRNA vaccines (Pfizer and Moderna (Two doses)) are used. The most widely available and used vaccines in Egypt are the inactivated vaccines, followed by viral vector vaccines and mRNA vaccines (Ministry of Health and Population personal communication).
This study aims to evaluate the effectiveness of different COVID-19 vaccines in preventing severe outcomes and identify factors contributing to breakthrough infection after full vaccination among hospitalized patients with SARS-CoV-2 using the severe acute respiratory infections (SARI) sentinel surveillance data.
Methods
Study sites and subjects
SARI was established in Egypt in 2007 to identify viral causes of acute respiratory diseases. Currently, the system is operating in a wide network of 16 governmental hospitals and regional laboratories covering ten governorates within all Egyptian regions. As a result of the emergence of the COVID-19 pandemic in 2019, the system was adapted to include SARS-CoV-2 in the laboratory testing panel to address the emerging challenges.
WHO case definition is used to identify and enroll SARI patients after consenting to participation. During the pandemic, surveillance teams were instructed to enroll every fifth admitted patient with SARI symptoms to adapt to the increased number of patients with acute respiratory infections (ARI) symptoms.
Data collection
Enrolled patients are interviewed using a standard data collection tool that includes patients’ demographics, clinical picture, ARI risk factors, disease severity, and outcome. The tool was updated to include information on vaccination status and vaccine types following the introduction of COVID-19 vaccines in Egypt. Vaccination data are collected either from vaccination cards or as stated by patients if no cards are available at the time of the interview. An online application was developed for COVID-19 data entry and reporting, data is merged into a common ARI database at the central level.
Data of all hospitalized patients confirmed for SARS-CoV-2 by RT-PCR from September 2021-April 2022 was extracted from the surveillance database.
Laboratory procedures
Nasopharyngeal/oropharyngeal swabs are collected and shipped weekly to Central Public Health Laboratories. RT-PCR are used to confirm SARS-CoV-2, influenza types and subtypes, and Respiratory syncytial Virus (RSV). To avoid the delay in identifying and treating COVID-19 cases, two NP/OP swabs are collected from each patient, one immediately tested for COVID-19, and the other kept in nitrogen tank and transferred to the Central Public Health Laboratories (CPHL) on a weekly basis for routine influenza and RSV testing.
Case definitions
Patients who received at least one dose of the COVID-19 vaccine were classified as “vaccinated”, and those who did not complete the vaccination schedule were classified as “partially vaccinated”. Patients were categorized as “fully vaccinated” if they have received the required dose(s) of a COVID-19 vaccine at least 14 days from the single-dose vaccine or the second dose in the two-doses vaccines. The partially or unvaccinated patients were categorized as “not fully vaccinated” group. Patients who acquire SARS-CoV-2 infection after being fully vaccinated are termed “patients with VBT”.
Data analysis
Descriptive data analysis with frequencies and rates was performed for all COVID-19 lab-confirmed hospital-admitted patients at the sentinel sites. In order to identify risk factors for breakthrough infection, bivariate analysis was used to compare demographics, comorbidity, and vaccine type between patients with VBT and those without VBT. Binary logistic regression with a significance level < 0.05 was performed in order to exclude the confounders. The “fully vaccinated” patients were compared to the “not fully vaccinated” regarding disease severity and outcome in terms of ICU admission, the need for ventilation, and death at the hospital to assess different vaccines’ effectiveness in preventing severe disease and mortality. Pearson`s chi-square test and t-student test with a significance level < 0.05 were used to test the association. Analysis was conducted using epi info-7 software.
Results
Between September 2021 and April 2022, a total of 3,414 patients with SARI symptoms were enrolled including 1,447 (42.4%) positive for SARS-CoV-2 by RT-PCR. Of the SARS-CoV-2 confirmed patients, 1,297 (89.6%) had completed vaccination data (Table 1).
Their mean age was 56.7 ± 17.0 years, with 68.9% > 50 years, 41.5% were males, and 47.3% are having comorbidity (Table 2).
Of the 1,297 confirmed COVID-19 hospital admitted patients, 207 (16.0%) had received at least one dose of COVID-19 vaccine. Fully vaccinated represent 12.3% (159/1,297) of all hospitalized patients with COVID-19 and fatality among them represent 8.0% (25/312) of the in-hospital deaths due to COVID-19.
Of 159 patients with COVID-19 breakthrough infection, 101 (63.5%) received inactivated vaccines, 42 (26.5%) viral vector, 12 (7.5%) mRNA vaccine type, while 4 (2.5%) were unable to mention or document the type of vaccine they received. More than half of patients with breakthrough infection had been infected within four months of receiving the full vaccine doses (Table 1).
Bivariate analyses showed that vaccine breakthrough infections were more common among younger patients (16–35 years old), males, those with diabetes, cardiovascular disease compared to corresponding groups of the “not fully vaccinated”. (14.5% vs. 9.0%, p < 0.05 and 57.2% vs. 39.3%, p < 0.001 and 44.0% vs. 32.2%, p < 0.01 and 34.0% vs. 23.3%, p < 0.01 and 65.2% vs. 48.9%, p < 0.05 respectively). Breakthrough infection rates were higher among those who received inactivated vaccines (65.2% vs. 48.9%, p < 0.05) and lower among those who received mRNA vaccines(7.7% vs. 23.4%, p < 01), while no significant difference was observed among those who received viral vector vaccines (Table 2). By multivariate analysis, only inactivated vaccine (OR, 95% CI = 96.4, 54.7-169.8), age group 15–35 years (OR, 95% CI = 3.4, 1.8–6.8), male gender (OR, 95% CI = 2.3,1.4–3.7), cardiovascular disease (OR, 95% CI = 2.2, 1.2–3.9) remained significant (Table 3).
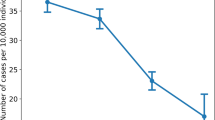
The number of vaccine breakthrough infections increases over time, peaking with the increase of COVID-19 infections (Fig. 1).
When evaluating the effectiveness of COVID-19 vaccine for the prevention of severe disease and death during hospitalization, it was found that fully vaccinated patients had significantly lower mean hospital days and case fatality rate than the “not fully vaccinated” (6.6 ± 5.5 vs. 7.9 ± 5.9, p < 0.01 and 15.7% vs. 25.2%, p < 0.01 respectively). Whereas no significant difference found between the two groups regarding rate of pneumonia infection, bilateral pneumonia, ICU admission, ICU days, mechanical ventilation, and ventilator days (Table 4).
Discussion
Despite the very high level of COVID-19 vaccines efficacy and effectiveness, still, some hospitalizations and deaths due to COVID-19 have been reported among fully vaccinated people [8]. A growing risk of breakthrough infection has been documented in long-term follow-up studies of vaccine trial participants, along with a steady decline in antibody levels among vaccinated persons [9, 10]. Unlike most studies that measured antibody levels to determine vaccine breakthrough rates in vaccinated individuals, this study investigates the characteristics of hospitalized patients with COVID-19 breakthrough infections in real-life settings [3].
Although unvaccinated people continue to represent the vast majority of COVID-19 hospitalizations, yet the rate of breakthrough infections is increasingly reported [8]. Similarly, this study identified a rising number of breakthrough infections over time. Possible reasons could include increased numbers of vaccinated persons, waning of vaccine-induced antibody levels, emerging variants with diminished susceptibility to vaccine-induced antibodies, and reducing mitigation recommendations for the community [11].
The study found that the rate of breakthrough infections peaks with the increase in COVID-19 infections, similar to other studies which reported the highest breakthrough incidence rates coincident with high positive positivity rate in the general population [12]. This might be explained by the reduced vaccine-induced immunity during the emergence of new variants where fully vaccinated people remain susceptible to infection. Studies comparing the incidence of breakthrough infections between different variant dominance periods found higher incidence in the delta period than in the alpha period [13, 14]. This should raise concern about COVID-19 vaccines effectiveness as new SARS-CoV-2 variants emerge.
Compared to other studies that reported breakthrough rates ranging from 9 to 35%, this study reported a relatively low proportion of fully vaccinated patients among hospitalized patients with COVID-19 [12, 15, 16]. However, comparing rates of breakthrough remains challenging because of study-specific differences including characteristics of study population and methods, Country-specific vaccination campaigns, vaccines used, seasonal effects, protective measures, and vaccination coverage [12]. Monitoring the change in rates of breakthrough infections in one location using surveillance data may provide early insight into vaccine effectiveness that can be confirmed by controlled studies.
Our findings show that people hospitalized with COVID-19 who were fully vaccinated are more likely to be younger individuals. The age at which individuals are more likely to develop a breakthrough infection is controversial, many studies reported higher rates of breakthrough among elderly hospitalized patients [8, 17, 18]. However, in accordance with our results, others concluded that young people are more liable to breakthrough infections [12, 19]. It was suggested that the controversy between studies could be related to the type of vaccine used. Yi S et al. found higher breakthrough rates in younger recipients of AstraZeneca, Janssen and Moderna, while this rate was higher in elderly Pfizer-BioNTech recipients. Wearing masks indoors regardless of vaccination status and age is recommended for individuals living in communities where SARS-CoV-2 transmission rates are substantial or high. [20].
Studies reported no difference in incidence of breakthrough infection by gender [12], while others suggested male predominance in accordance with our study. The gender difference was explained by physiological, genetic factors or behavioral factors. Female protection against breakthrough infection was connected to the higher expression of ACE-2 receptors found in females as compared to males. These receptors get downregulated following the entry of virus particles into the cells, prevent further viruses from entering into the host cells and rendering females less vulnerable to severities of COVID-19 [17].
In accordance with other studies, our data found that patients with comorbidities especially those with diabetes and cardiovascular disease are more liable to have a breakthrough infection [21]. It is important for people with comorbidities to stick to recommended preventive measures against COVID-19 infection despite being vaccinated.
In this study we found that the incidence of breakthrough infections among vaccine recipients varied significantly according to the type of vaccine used. The same finding was reported by Stouten et al., who found that viral vector vaccines had a higher breakthrough rate than mRNA vaccines [12]. Our study also found that inactivated vaccines had the highest rate of breakthrough infections, which was not tested in previous studies. This could be related the higher percentage of inactivated vaccines received among Egyptian population. Vaccines formulation or policy or both should be updated based on these findings.
Thomas et al., recently reported a gradual decline in vaccine efficacy of 86 to 100% across countries and in populations with diverse ages, sexes, race, or ethnic groups [9]. In this study, we found that more than half of the breakthrough infections occurred within four months from date of full vaccination. This should raise concerns regarding losing efficacy of vaccines over time and suggest considering modify vaccine formulation to enhance vaccine efficacy and effectiveness.
The decline in vaccine efficacy over time and the continuous gradual waning in population vaccine-induced immunity could also explain the insignificant difference found between the fully and not fully vaccinated patients regarding disease severity found in this study. In addition, high viral loads found in fully vaccinated individuals when infected with SARS-CoV-2 [18]. However, earlier studies reported less severe disease course and fatality in the fully vaccinated individuals [4]. In accordance with these results, our study proved that vaccines are still effective in reducing hospital stay and fatality rates among COVID-19 patients. Recent reports from the United States indicated a growing proportion of COVID-19 deaths among vaccinated individuals, however vaccination remains the best option to lower risk of severe disease course, hospitalization, and death from COVID-19 [22]. Monitoring vaccine effectiveness in preventing COVID-19 severity and fatality among breakthrough infection is essential to update vaccination policy.
Study limitations
This study results are subject to at least four limitations. First, data of hospitalized patients are insufficient to draw conclusions about the effectiveness of COVID-19 vaccines. Second, vaccinated persons are likely to represent a larger proportion of hospitalized COVID-19 cases as the rate of vaccination increases in population. Third, the demographics of cases likely reflect those of hospitalized patients rather than community, and fourth, a recall bias may occur since vaccination data were collected from the patient’s statement when vaccination cards were not available.
Conclusion
We report an increase in breakthrough infections among hospitalized patients with COVID-19 in Egypt, which usually occur within four months of completing full vaccination. Breakthrough infection rates peak with the increase in number of COVID-19 infections, possibly due to the increased number of vaccinees and the emergence of new SARS-CoV-2 variants. Factors in favor of breakthrough infections included younger ages, male gender, comorbidities, and inactivated vaccines. Study results might support caution regarding relaxation of personal preventive measures in areas with higher or increasing incidence of COVID-19, particularly for the at-risk group even if they are vaccinated.
The study showed that vaccination against COVID-19 still reduces hospital stay and mortality rates, though it does not reduce disease severity much. In order to improve COVID-19 vaccine effectiveness and reduce breakthrough infections, monitoring vaccine effectiveness and breakthrough rate is crucial. Further research is needed to better describe the at-risk group for breakthrough infection.
Data Availability
The datasets generated and/or analyzed during the current study are not publicly available to protect study subjects’ privacy but are available from the corresponding author (OD) upon reasonable request.
Abbreviations
- VBT:
-
Vaccination breakthrough infection
- RCT:
-
Randomized Controlled Trial
- SARI:
-
Severe Acute Respiratory Illness
- RT-PCR:
-
Real-Time polymerase chain reaction
- ACE:
-
Angiotensin-Converting Enzyme
- ICU:
-
Intensive care unit
References
Wang H, Paulson KR, Pease SA, Watson S, Comfort H, Zheng P, Murray CJ. Estimating excess mortality due to the COVID-19 pandemic: a systematic analysis of COVID-19-related mortality. The Lancet. 2022;399(10334):1513–36. https://doi.org/10.1016/S0140-6736(21)02796-3.
The Food and Drug Administration. : Emergency Use Authorization for Vaccines Explained https://www.fda.gov/vaccines-blood-biologics/vaccines/emergency-usauthorization-vaccines-explained.
Nasreen S, Chung H, He S. Effectiveness of COVID-19 vaccines against symptomatic SARS-CoV-2 infection and severe outcomes with variants of concern in Ontario. Nat Microbiol. 2022;7:379–85. https://doi.org/10.1038/s41564-021-01053-0.
Bahl A, Johnson S, Maine G, Garcia MH, Nimmagadda S, Qu L, et al. Vaccination reduces need for emergency care in breakthrough COVID-19 infections: a multicenter cohort study. Lancet Reg Health Am. 2021;4100065. https://doi.org/10.1016/j.lana.2021.100065.
Lipsitch M, Krammer F, Regev Yochay G. SARS-CoV-2 breakthrough infections in vaccinated individuals: measurement, causes and impact. Nat Rev Immunol. 2021 Dec;22, 57–65 (2022). https://doi.org/10.1038/s41577-021-00662-4.
Brosh-Nissimov T, Orenbuch-Harroch E, Chowers M, Elbaz M, Nesher L, Stein M, Maor Y, Cohen R, Hussein K, Weinberger M, Zimhony O, Chazan B, Najjar R, Zayyad H, Rahav G, Wiener-Well Y. (2021). BNT162b2 vaccine breakthrough: clinical characteristics of 152 fully vaccinated hospitalized COVID-19 patients in Israel. Clinical microbiology and infection: the official publication of the European Society of Clinical Microbiology and Infectious Diseases. 2021; 27(11), 1652–1657. https://doi.org/10.1016/j.cmi.2021.06.036
Our world in data. Coronavirus (COVID-19) vaccinations. https://ourworldindata.org/covid-vaccinations.
Bergwerk M, Gonen T, Lustig Y. Covid-19 breakthrough infections in vaccinated health care workers. N Engl J Med. 2021;385(16):1474–84. https://doi.org/10.1056/NEJMoa2109072.
Thomas SJ, Moreira ED Jr, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Pérez Marc G, Polack FP, Zerbini C, Bailey R, Swanson KA, Xu X, Roychoudhury S, Koury K, Bouguermouh S, Kalina WV, Cooper D, Frenck RW Jr, Hammitt LL, Türeci Ö, Nell H, Schaefer A, Ünal S, Yang Q, Liberator P, Tresnan DB, Mather S, Dormitzer PR, Şahin U, Gruber WC, Jansen KU. C4591001 clinical Trial Group. Safety and Efficacy of the BNT162b2 mRNA Covid-19 vaccine through 6 months. N Engl J Med. 2021;385(19):1761–73. https://doi.org/10.1056/NEJMoa2110345. Epub 2021 Sep 15. PMID: 34525277; PMCID: PMC8461570.
Naaber P, Tserel L, Kangro K, Sepp E, Jürjenson V, Adamson A, Haljasmägi L, Rumm AP, Maruste R, Kärner J, Gerhold JM. Anu Planken, Mart Ustav, Kai Kisand, Pärt Peterson. Dynamics of antibody response to BNT162b2 vaccine after six months: a longitudinal prospective study,The Lancet Regional Health – Europe.2021;Volume 10, 100208, ISSN 2666–7762, https://doi.org/10.1016/j.lanepe.2021.100208
Bates TA, McBride SK, Winders B et al. Antibody Response and Variant Cross-Neutralization After SARS-CoV-2 Breakthrough Infection. JAMA.2021;327(2):179–181. doi:https://doi.org/10.1001/jama.2021.22898
Stouten V, Hubin P, Haarhuis F, van Loenhout JAF, Billuart M, Brondeel R, Braeye T, Van Oyen H, Thomas W, Catteau C. Incidence and risk factors of COVID-19 vaccine breakthrough infections: a prospective cohort study in Belgium. Viruses. 2022;14(4):802. https://doi.org/10.3390/v14040802.
McEwen AE, Cohen S, Bryson C. Variants of concern are overrepresented among post-vaccination breakthrough infections of SARS-CoV-2 in Washington State. Clin Infect Dis. 2021;74(6):1089–92. https://doi.org/10.1093/cid/ciab581.
Christensen PA, Olsen RJ, Long SW, Snehal R, Davis JJ, Ojeda Saavedra M, Reppond K, Shyer MN, Cambric J, Gadd R, Thakur RM, Batajoo A, Mangham R, Pena S, Trinh T, Kinskey JC, Williams G, Olson R, Gollihar J, Musser JM. Signals of significantly increased vaccine breakthrough, decreased hospitalization rates, and less severe disease in patients with Coronavirus Disease 2019 caused by the Omicron variant of severe Acute Respiratory Syndrome Coronavirus 2 in Houston, Texas. Am J Pathol. 2022;192(4):642–52. Epub 2022 Feb 3. PMID: 35123975; PMCID: PMC8812084.
Moreno-Perez O, Ribes I, Boix V, Martinez-García M, Otero-Rodriguez S, Reus S, Sánchez-Martínez R, Ramos JM, Chico-Sánchez P, Merino E. On behalf the COVID-19 ALC research group. Hospitalized patients with breakthrough COVID-19: clinical features and poor outcome predictors. Int J Infect Dis. 2022;118:89–94. Epub 2022 Feb 13. PMID: 35172182; PMCID: PMC8841006.
Massachusetts Department of Public Health. COVID-19 Interactive Data Dashboard. Accessed October 24., 2021. https://www.mass.gov/info-details/covid-19-response-reporting#covid-19-interactive-data-dashboard
Arora G, Taneja J, Bhardwaj P, Goyal S, Naidu K, Yadav SK, Saluja D, Jetly S. Adverse events and breakthrough infections associated with COVID-19 vaccination in the Indian population. J Med Virol. 2022 July;94(7):3147–3154. doi: https://doi.org/10.1002/jmv.27708. Epub 2022 Mar 21. PMID: 35261064; PMCID: PMC9088477.
Klompas M. Understanding breakthrough infections following mRNA SARS-CoV-2 vaccination. JAMA. 2021;326(20):2018–20. https://doi.org/10.1001/jama.2021.19063.
Yi S, Choe YJ, Kim J. SARS-CoV-2 breakthrough infections after introduction of 4 COVID-19 vaccines, South Korea, 2021. Emerg Infect Dis. 2022;28(3):753–6. https://doi.org/10.3201/eid2803.212210.
Centers for Disease Control and Prevention. Use masks to slow the spread of COVID-19. Updated August 12, 2021. Accessed 24., 2021. https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/diy-cloth-face-coverings.html.
Liu C, Lee J, Ta C, Soroush A, Rogers J, Kim JH, Natarajan K, Zucker J, Perl Y, Weng C. A Retrospective Analysis of Risk Factors Associated with the SARS-CoV-2 Breakthrough in Fully mRNA Vaccinated Individuals. JMIR Public Health Surveill. 2022; 8(5):e35311. doi: https://doi.org/10.2196/35311. Epub ahead of print. PMID: 35486806.
Centers for Disease Control and Prevention. COVID data tracker weekly review. https://www.cdc.gov/coronavirus/2019-ncov/covid-data/covidview/index.html
Acknowledgements
We express our sincere appreciation and thanks to the many physicians, nurses, and clinical laboratory staff of the infectious disease hospitals involved in this study. We also thankfully acknowledge support from the staff of the Preventive Sector and the Central Public Health Laboratory of the Ministry of Health and Population who assisted with training and monitoring the activities within the infectious disease hospitals.
Funding
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
Conceptualization: AK, MAF, and AM; Data acquisition: OD, WA Writing original draft: SA; Final review and editing: MF, AM. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval statement
Ethics approval was waived by “the Ministry of Health and Population Institutional Review Board” (IRB) as a non-research study using data from public health surveillance that only involves routine data collection and procedures carried out pursuant to the Ministry of Health and Population at the Ministry hospitals.
Patient consent statement
The study used data collected during patients’ routine management procedures. All patients verbally consented to participate after they were fully informed about the aims of surveillance and the risks associated with participation. The verbal consent was approved by The Ministry of Health and Population (MoH) in Egypt. Patients’ private information is coded so investigators cannot readily determine the identities of those to whom it pertains.
Consent of publication
NA.
Conflict of Interest
The authors have no conflicts of interest to declare. All co-authors have seen and agree with the contents of the manuscript and there is no financial interest to report. We certify that the submission is original work and is not under review at any other publication.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Kandeel, A., Fahim, M., Deghedy, O. et al. Clinical features and severe outcome predictors of COVID-19 vaccine breakthrough infection among hospitalized patients: results from Egypt severe acute respiratory infections sentinel surveillance, 2021–2022. BMC Infect Dis 23, 130 (2023). https://doi.org/10.1186/s12879-023-08097-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-023-08097-z