Abstract
Background
The etiopathogenesis of idiopathic non-cirrhotic portal hypertension (INCPH) is so far poorly understood. Altered immunity, blood diseases, infections, congenital defects and drug exposure have been documented in a part of patients with INCPH owing to increased recognition of the disorder in patients with HIV, or various haematological disorders or autoimmune diseases. We aim to discuss the possible etiopathogenesis of INCPH.
Case presentation
We reported that a patient with intestinal infection of T. Marneffei and hyper-IgE syndrome, a group of rare primary immunodeficiency disorders, was finally diagnosed with INCPH for gastroesophageal variceal bleeding. The diagnosis was mainly based on histopathological features. Transjugular intrahepatic portosystemic shunt was performed and there was no recurrence of melena during the six-month follow-up.
Conclusion
In the context of immunodeficiency, INCPH may associated with intestinal infections. Thus, screening for enterogenic infection and immunological disorders in patients with unexplained portal hypertension is necessary.
Similar content being viewed by others
Background
Idiopathic non-cirrhotic portal hypertension (INCPH) is a rare vascular liver disease characterized by presinusoidal portal hypertension (PHT) without cirrhosis [1]. It has been diagnosed in up to 84% of patients with common variable immune deficiency, hyper-IgM syndrome, and et al., although mechanisms linking immunological abnormalities to INCPH remain unknown [2]. Abdominal infections have also been implicated as one of the triggers for the development of INCPH through repeated obstruction of small to medium portal branches. However the evidence underlying this association is mainly from epidemiological studies [2]. The etiopathogenesis is so far poorly understood. Herein, we reported that a patient with INCPH was previously diagnosed with intestinal infection of T. Marneffei and hyper-IgE syndrome (HIES), a group of rare primary immunodeficiency disorders [3], which had never reported in INCPH before.
Case presentation
A 26-year-old man was hospitalized due to a 6-month history of fever, cough, expectoration and melena. In the primary hospital, labs reported decreased hemoglobin and increased serum IgE (Table 1). Anti-HIV antibody was not detected but immunodeficiency was found in decreased lymphocyte subsets counts, especially the natural killer T (NKT). Chest computed tomography (CT) showed a pneumatocele in the left lung and bilateral infectious lesions (Fig. 1A). The endoscopy found an ulcer at the ileocecal valve (Fig. 2A) and mild esophageal varices (Fig. 2C). The results of a culture of the sputum yielded T. Marneffei. Periodic acid-Schiff and Hexamine silver staining of the ileocecal biopsy tissue revealed T. Marneffei infection (Fig. 3A). Conclusively, intestinal infection of T. Marneffei and hyper-IgE syndrome (HIES) were included in the diagnosis, with the score = 28 according to NIH scoring system [4] (Table 2). The whole-exon gene sequencing identified compound heterozygous mutation in dedicator of cytokinesis-8 (DOCK8), the causing of HIES. He received intravenous amphoterincin B therapy (from small dose to 0.75 mg / kg/d) for 25 days and was discharged on an oral regimen of voriconazole (400 mg / d) for 15 months until the symptoms disappeared. Recheck of colonoscopy showed the ileocecal lesion was cured (Fig. 2B).
The first colonoscopy showed an irregularly shaped ulcer at the ileocecal valve (A), which was found to be cured after the 15-month antifungal therapy (B). The esophageal varices was mild (C) when he had first esophagogastroduodenoscopy and was proved to aggravate to the severe gastroesophageal varices with the presence of red sign 29 months later (D)
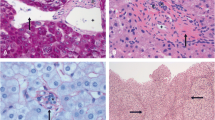
(A) Histopathologic examination for the ileocecal ulcer showed granulomatous inflammation with much round fungal structures (blue arrows; hexamine silver). (B) A fibrotic portal tract (black arrow) with obliteration of portal vein branches (H&E, magnifcation × 400). (C) Multiple thin walled vascular spaces in the portal tract (black arrow) with a periportal abnormal vessel (star). Compensatory enlargement of hepatic sinusoid (blue arrow) can be detected (H&E, magnifcation × 400)
However, the patient was readmitted to our hospital 14 months later because of recurrent melena. Laboratory tests showed decreased hemoglobin, decreased leukocyte, and normal liver function (Table 1). Endoscopy reveled the gastroesophageal varices significantly aggravated (Fig. 2D). Contrast-enhanced abdominal CT showed splenomegaly and gastroesophageal varices without obstruction of main portal vein or inferior vena cava (Fig. 4A). The liver biopsy was performed due to no evidence of chronic liver disease based on labs and previous history. The histopathological changes showed features of portal vein stenosis (Fig. 3B) and hyper-vascμlarized portal tracts (Fig. 3C) with out pseudolobule formation. Finally, the patient was diagnosed with INCPH.
A Contrast-enhanced abdominal CT angiography with three-dimensional reconstruction showed a smooth liver surface, splenomegaly and gastroesophageal varices (white arrow). B Hepatic vein-to-vein communication was detected by hepatic venography. C Portography showed irregularity and tortuosity of the peripheral portal branches with much abrupt interruptions (black arrow) and avascular area beneath the liver surface. Portovenous collaterals (white arrows) derived from gastric coronary vein were also displayed. D Post-TIPS portography revealed embolization of collaterals and clear stent flow from portal vein to systemic circulation
Transjugular intrahepatic portosystemic shunt (TIPS) was performed to prevent variceal rebleeding. During the procedure, hepatic vein-to-vein communication (HVVC) was displayed via hepatic venography (Fig. 4B). Portography showed tortuosity of portal vein branches with much interruptions (Fig. 4C). The hepatic venous pressure gradient (HVPG) was detected as only 4 mmHg while the portal pressure gradient before and after stent placement were 18 mmHg and 6 mmHg, respectively. The collaterals was disappeared after TIPS (Fig. 4D) and there was no hepatic encephalopathy and recurrence of melena during the six-mounth follow-up.
Discussion and conclusion
The diagnosis of INCPH was based on radiological examination had excluded prehepatic and posthepatic PHT (such as extrahepatic portal venous obstruction and Budd Chiari syndrome), and liver biopsy displayed portal vein branches stenosis and hyper-vascularized portal tracts, rather than severe hepatic fibrosis or pseudolobule formation [5]. HVVC and normal HVPG detected by hepatic vein catheterisation as well as the portography features also conformed with the diagnosis [6]. The etiopathogenesis of INCPH is so far poorly understood. Immunological abnormalities, including primary antibody-deficiency and HIV infection as well as autoimmune disorders seem to be associated with INCPH [1]. However, autosomal recessive HIES caused by DOCK8 deficiency is a primary combined immunodeficiency which had never reported in INCPH before. Mutations in the DOCK8 gene encoding a guanine nucleotide exchange factor highly expressed in lymphocytes that regulates the actin cytoskeleton were identified as the most common cause for autosomal recessive HIES [3]. DOCK8 deficiency impairs immune cell migration, function and survival which characterized by allergic inflammation as well as susceptibility towards infections, autoimmunity and malignancy [7]. Previous studies have suggested the abnormalities of genes and specific adhesion molecules involved in lymphocyte activation in patients with INCPH [8, 9]. These suggest lymphocyte-endothelial cell cross-talk could play a role in the pathogenesis.
Other possible mechanisms of the portal vein branches damage in patients with immunological disorders includes abdominal infections, immunosuppressants, or anti-infection drugs. In our case, the anti-fungal drugs can not explain the disease because the signs of PHT appeared much earlier. It is noteworthy that the patient had an intestinal infection with Talaromyces marneffei. The fungus is gradually emerging in southeast Asia and southern China which HIV infection is regarded as the most underlying disease [10]. Recently T. Marneffei infections in non-HIV individuals are increasing whose clinical characteristics are atypical [11]. T. Marneffei is a primary lung pathogen that disseminates to other internal organs by lymphatic or hematogenous mechanisms [12]. In our case, there were definite evidences of the presence of T. Marneffei in the lung and intestine. Coupled with a close anatomical connection between the liver and the gut it can be concluded that the patient already had a disseminated disease of T. Marneffei. In other words, T. Marneffei may enter the liver through the superior mesenteric vein and cause endothelial and vascular damage. Additionally, T. Marneffei infection and INCPH exist in sequence, whereas the primary immunodeficiency was already present. Therefore, we hypothesized that the infection of T. marneffei played a major role in the pathogenesis of INCPH. As the DOCK8 deficiency affects the long-term retention of NKT-cell in the liver [13] and may therefore increase the susceptibility to infections, the intestinal involved of T. Marneffei as well as previous occult intestinal infections combined with immune disorders may be the cause of INCPH in this patient [14]. The association between T. Marneffei infections and INCPH has not been reported in previous studies. INCPH is more common in developing countries where bacterial infection is rampant [1, 15]. Also means that as long as it produces septic emboli which cause endothelial damage, microthrombosis, sclerosis, and obstruction of the small and medium-sized portal branches any intestinal infection leads to INCPH. This hypothesis is supported by an animal study that recurrent injection of heat killed Escherichia coli into portal vein can develop the rabbit model of INCPH [16].
As for treatment, the main purpose is to prevent PHT related complications for the lack of etiotropic therapy. A retrospective study including 41 patients with INCPH have demonstrated good effectiveness and safety of TIPS for PHT related complications [17]. In the current case, there is no episode of hepatic encephalopathy or abnormal liver function tests occurred after TIPS during the six-mounth follow-up. Despite the scarcity of evidence, TIPS may precede other therapies for INCPH due to preserved liver function and less post-TIPS complications.
This case demonstrated that, like other kinds of immunodeficiency, primary combined immunodeficiency can occur in INCPH. Intestinal infections may lead to further portal vein damage in such conditions. Thus, screening for enterogenic infection and immunological disorders in patients with unexplained PHT is necessary. Futher studies on mechanisms connecting intestinal infections and INCPH are needed.
Availability of data and materials
The data and materials that support the findings of this study are available from West China Hospital, Sichuan University, but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of West China Hospital, Sichuan University.
Abbreviations
- INCPH:
-
Idiopathic non-cirrhotic portal hypertension
- HIV:
-
Human immunodeficiency virus
- PHT:
-
Portal hypertension
- T. Marneffei :
-
Talaromyces marneffei
- HIES:
-
Hyper-IgE syndrome
- NKT:
-
Natural killer T
- CT:
-
Computed tomography
- NIH:
-
National Institutes of Health
- TIPS:
-
Transjugular intrahepatic portosystemic shunt
- HVPG:
-
Hepatic venous pressure gradient
- HVVC:
-
Hepatic vein-to-vein communication
References
Hernandez-Gea V, Baiges A, Turon F, Garcia-Pagan JC. Idiopathic portal hypertension. Hepatology. 2018;68(6):2413–23.
De Gottardi A, Rautou PE, Schouten J, Rubbia-Brandt L, Leebeek F, Trebicka J, Murad SD, Vilgrain V, Hernandez-Gea V, Nery F. Porto-sinusoidal vascular disease: proposal and description of a novel entity. Lancet Gastroenterol Hepatol. 2019;4(5):399–411.
Al-Shaikhly T, Ochs HD. Hyper IgE syndromes: clinical and molecular characteristics. Immunol Cell Biol. 2019;97(4):368–79.
Grimbacher B, Schaffer AA, Holland SM, Davis J, Gallin JI, Malech HL, Atkinson TP, Belohradsky BH, Buckley RH, Cossu F, et al. Genetic linkage of hyper-IgE syndrome to chromosome 4. Am J Hum Genet. 1999;65(3):735–44.
Guido M, Alves VAF, Balabaud C, Bathal PS, Bioulac-Sage P, Colombari R, Crawford JM, Dhillon AP, Ferrell LD, Gill RM, et al. Histology of portal vascular changes associated with idiopathic non-cirrhotic portal hypertension: nomenclature and definition. Histopathology. 2019;74(2):219–26.
Seijo S, Reverter E, Miquel R, Berzigotti A, Abraldes JG, Bosch J, Garcia-Pagan JC. Role of hepatic vein catheterisation and transient elastography in the diagnosis of idiopathic portal hypertension. Dig Liver Dis. 2012;44(10):855–60.
Biggs CM, Keles S, Chatila TA. DOCK8 Deficiency: insights into pathophysiology, clinical features and management. Clin Immunol. 2017;181:75–82.
Kotani K, Kawabe J, Morikawa H, Akahoshi T, Hashizume M, Shiomi S. Comprehensive screening of gene function and networks by DNA microarray analysis in japanese patients with idiopathic portal hypertension. Mediators Inflamm. 2015;2015:349215
Yamaguchi N, Tokushige K, Haruta I, Yamauchi K, Hayashi N. Analysis of adhesion molecules in patients with idiopathic portal hypertension. J Gastroenterol Hepatol. 1999;14(4):364–9.
He L, Mei X, Lu S, Ma J, Hu Y, Mo D, Chen X, Fan R, Xi L, Xie T. Talaromyces marneffei infection in non-HIV-infected patients in mainland China. Mycoses. 2021;64(10):1170–6.
Chan J, Lau S, Yuen K-Y, Woo P. Talaromyces (Penicillium) marneffei infection in non-HIV-infected patients. Emerg Microbes Infect. 2016;5(3):1–9.
Narayanasamy S, Dougherty J, van Doorn HR, Le T. Pulmonary talaromycosis: a window into the immunopathogenesis of an endemic mycosis. Mycopathologia. 2021;186(5):707–15.
Crawford G, Enders A, Gileadi U, Stankovic S, Zhang Q, Lambe T, Crockford TL, Lockstone HE, Freeman A, Arkwright PD, et al. DOCK8 is critical for the survival and function of NKT cells. Blood. 2013;122(12):2052–61.
Eapen CE, Nightingale P, Hubscher SG, Lane PJ, Plant T, Velissaris D, Elias E. Non-cirrhotic intrahepatic portal hypertension: associated gut diseases and prognostic factors. Dig Dis Sci. 2011;56(1):227–35.
Sarin SK, Kumar A, Chawla YK, Baijal SS, Dhiman RK, Jafri W, Lesmana LA, Mazumder DG, Omata M, Qureshi H, et al. Noncirrhotic portal fibrosis / idiopathic portal hypertension: APASL recommendations for diagnosis and treatment. Hepatol Int. 2007;1:398–413.
Omanwar S, Rizvi MR, Kathayat R, Sharma BK, Pandey GK, Alam MA, Pandey GK, Malhotra V, Sarin SK. A rabbit model of non-cirrhotic portal hypertension by repeated injections of E.coli through indwelling cannulation of the gastrosplenic vein. Hepatobiliary Pancreat Dis Int. 2004;3(3):417–22.
Bissonnette J, Garcia-Pagan JC, Albillos A, Turon F, Ferreira C, Tellez L, Nault JC, Carbonell N, Cervoni JP, Abdel Rehim M, et al. Role of the transjugular intrahepatic portosystemic shunt in the management of severe complications of portal hypertension in idiopathic noncirrhotic portal hypertension. Hepatology. 2016;64(1):224–31.
Acknowledgements
We are grateful to the Department of Pathology and Radiology for the images. And we really thank the patient for his consent to participate in this study.
Funding
This study was supported by the Sichuan Science and Technology Program (Grant No. 22ZDYF0621), 1·3·5 project for disciplines of excellence – Clinical Research Incubation Project, West China Hospital, Sichuan University (2019HXFH024), Technological innovation R&D project of Chengdu Science and Technology Bureau (2021-YF05-01285-SN), and Popularization and application project of Sichuan Health Commission (Grant No. 21PJ027). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
YX and QX made substantial contributions to the conception of the work, drafted the work and substantively revised it. GX and WZ were responsible for acquisition and interpretation the date and figures. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Written informed consent to participate in the study was obtained from the patient. As a case report, our paper did not require any referral to our institutional clinical ethics committee.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor of this journal.
Competing interests
The authors have no conflicts of interest to disclose.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Ye, X., Quan, X., Guo, X. et al. Idiopathic non-cirrhotic portal hypertension in a patient with Talaromyces marneffei infection: a case report. BMC Infect Dis 23, 125 (2023). https://doi.org/10.1186/s12879-023-08090-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-023-08090-6