Abstract
Objectives
Identifying prognostic factors helps optimize the treatment regimen and promote favorable outcomes. We conducted a prospective cohort study on patients with pulmonary tuberculosis to construct a clinical indicator-based model and estimate its performance.
Methods
We performed a two-stage study by recruiting 346 pulmonary tuberculosis patients diagnosed between 2016 and 2018 in Dafeng city as the training cohort and 132 patients diagnosed between 2018 and 2019 in Nanjing city as the external validation population. We generated a risk score based on blood and biochemistry examination indicators by the least absolute shrinkage and selection operator (LASSO) Cox regression. Univariate and multivariate Cox regression models were used to assess the risk score, and the strength of association was expressed as the hazard ratio (HR) and 95% confidence interval (CI). We plotted the receiver operating characteristic (ROC) curve and calculated the area under the curve (AUC). Internal validation was conducted by 10-fold cross-validation.
Results
Ten significant indicators (PLT, PCV, LYMPH, MONO%, NEUT, NEUT%, TBTL, ALT, UA, and Cys-C) were selected to generate the risk score. Clinical indicator-based score (HR: 10.018, 95% CI: 4.904–20.468, P < 0.001), symptom-based score (HR: 1.356, 95% CI: 1.079–1.704, P = 0.009), pulmonary cavity (HR: 0.242, 95% CI: 0.087–0.674, P = 0.007), treatment history (HR: 2.810, 95% CI: 1.137–6.948, P = 0.025), and tobacco smoking (HR: 2.499, 95% CI: 1.097–5.691, P = 0.029) were significantly related to the treatment outcomes. The AUC was 0.766 (95% CI: 0.649–0.863) in the training cohort and 0.796 (95% CI: 0.630–0.928) in the validation dataset.
Conclusion
In addition to the traditional predictive factors, the clinical indicator-based risk score determined in this study has a good prediction effect on the prognosis of tuberculosis.
Similar content being viewed by others
Introduction
Tuberculosis (TB) continues to be a global concern and poses enormous threats to human health. In 2020, it caused 5.8 million new cases and 1.3 million deaths worldwide [1]. The current recommended antituberculosis treatment (ATT) regimen for drug-sensitive TB is a six-month regimen of four first-line drugs[2, 3], with a success rate of over 86%. Relapse rates varied across regions, ranging from about 3–10% in human immunodeficiency virus (HIV)-negative patients [4,5,6,7].
HIV infection, diabetes mellitus (DM), alcohol abuse, tobacco smoking, mental health have been recognized as risk factors for poor treatment outcomes[1, 8,9,10,11]. Substantial evidence has shown that age, body mass index (BMI), family income, and disease classification significantly affected the prognosis [9, 12,13,14]. Some studies have applied medical history and clinical symptom-based scores to estimate the prognosis of patients with TB, such as the Karnofsky Performance Status Scale [15] and the Charlson Comorbidity Index (CCI) [16]. Other studies have established demographic characteristics and laboratory test results, like race, acid-fast bacilli smear examination, albumin, white blood cell counts, hemoglobin, and C-reaction protein (CRP), to construct the predictive models [16,17,18].
Both baseline characteristics and dynamic clinical changes are related to the treatment outcomes. Identifying prognostic factors can help adjust for therapeutic regimens and intervention measures. In this study, we carried out a prospective cohort study on a group of patients with pulmonary TB to construct a clinical indicator-based risk score and estimate its ability to predict outcomes.
Methods
Study population
We performed a two-stage study by recruiting 346 pulmonary tuberculosis patients diagnosed between 2016 and 2018 in Dafeng city as the training cohort and 132 patients diagnosed between 2018 and 2019 in Nanjing city as the external validation population. Dafeng is located in the coastal area, and Nanjing is located in the southeast region of Jiangsu Province, China, respectively. The inclusion criteria were pulmonary TB patients who were clinically diagnosed according to the Diagnosis Criteria for Pulmonary Tuberculosis of China (WS 288–2017) and completed the baseline questionnaire investigation. Patients were excluded if they were (a) treated for < 1 month; (b) lost to follow-up; (c) HIV positive; or (d) diagnosed with other pulmonary diseases. The primary study end-point was the treatment failure or the relapse of TB, regarded as unfavorable treatment outcomes. Treatment failure was defined as bacteriologic or clinical failure, treatment interruption due to adverse drug reactions, transferring to multidrug-resistant therapy, or TB-related death. TB relapse was observed by following up until two years after the completion of ATT. This study defined favorable treatment outcomes for patients who were curative or completed the treatment without relapse within two years. The ethics committee of Nanjing Medical University approved this study. Written informed consent was obtained from study participants.
Data collection and variable coding
We collected demographic and epidemiological information of study subjects using a structured questionnaire. Patients who were not treated previously or received ATT less than one month were defined as new cases; otherwise, they were retreatment patients with an ATT history. Clinical information was obtained through the hospital information system and laboratory tests, including therapeutic regimen, sputum smear tests, sputum culture results, the onset of symptoms, date of diagnosis, chest X-ray examination, blood tests, and treatment outcomes. If TB patients took ATT drugs regularly without interruption, it was defined as good treatment compliance; otherwise, it was described as poor treatment compliance. The delayed time of ATT included the patient’s and the doctor’s delays. The patient’s delay was calculated by the interval between the symptom occurrence and the date of seeking health care. The doctor’s delay was calculated by the gap between seeking health care and initiating treatment. We figured the chest X-ray (CXR) score based on the proportion of lung lesions and the occurrence of cavitation judged by at least two experienced clinicians. According to the literature reference [19], the CXR score= proportion of total lung affected×100 + 40 (if the cavity is present). Typical symptoms of TB included cough, expectoration, fever, weight loss, dyspnea, night sweats, hemoptysis, fatigue, and chest pain, and the score represented the number of symptoms. Blood and biochemical indicators included red blood cell (RBC), white blood cell (WBC), platelet (PLT), hemoglobin (HB), packed cell volume (PCV), red blood cell distribution width (RDW), absolute value of lymphocytes (LYMPH), percentage of lymphocytes (LYMPH%), monocyte absolute value (MONO), monocyte percentage (MONO%), absolute value of neutrophil (NEUT), neutrophilic percentage (NEUT%), absolute value of eosinophils (EOS), eosinophil percentage (EOS%), absolute value of basophils (BASO), basophil percentage (BASO%), total bilirubin (TBTL), alanine aminotransferase (ALT), aspartate aminotransferase (AST), urea (UREA), creatinine (Cr), uric acid (UA), cystatin C (Cys-C), and β2-microglobulin (β2-m). They were collected at the baseline routine examination before initiating ATT.
Statistical analysis
Continuous variables were described as mean ± standard deviation (SD) or median together with interquartile range (IQR). Categorical variables were expressed as frequency and percentage. Continuous variables were compared by t-test if they were normally distributed; otherwise, they were compared by the Mann-Whitney test. Categorical variables were compared by chi-square test. Missing data of clinical data were imputed by multiple imputations shown in Supplementary materials [20, 21]. We applied the least absolute shrinkage and selection operator (LASSO) Cox regression analysis to screen clinical indices, extracted significant ones and their coefficients, and established a risk score system by predict function. The levels of clinical indicators were defined as an independent variable. The survival time and treatment outcomes of TB patients were considered as the response variables. The coefficients of each remained signature were derived from the LASSO regression analysis, and the risk score was generated using the formula of \(risk score={\sum }_{i=1}^{n}\left({Coef}_{i}\text{*}{x}_{i}\right)\). Then we utilized the univariate Cox regression model to assess the risk score and built a predictive model. Furthermore, using the bidirectional stepwise method, we conducted a multivariable Cox regression model by considering the risk score, previously reported variables, or clinically relative variables [22].
The strength of association was expressed as the hazard ratio (HR) and 95% confidence interval (CI). We plotted a nomogram based on variables in the multivariate model and showed different weighted scores for each factor. The total points were the sum of scores, which could be transferred to the predictive probability of the individual outcome event according to function transformation between total points and prognostic situation. To evaluate the prediction effects of established models, we plotted receiver operating characteristic (ROC) curves and calculated the corresponding area under the curve (AUC) both in the training and validation cohorts. Harrell’s concordance index (C-index) was also used to assess the discrimination of models. Internal validation was constructed by the 10-fold cross-validation method. The Hosmer-Lemeshow test was applied to evaluate the model’s goodness of fit.
Data analyses were performed in R version 4.1.2, using the “mice”, “randomForest”, “rms”, “survival”, “survminer”, “My.stepwise”, “VIM”, “Hmisc”, “grid”, “lattice”, “Formula”, “pROC”, “caret” and “ResourceSelection” packages. P < 0.05 was regarded as statistically significant.
Patient and public involvement
We collect anonymous information about TB patients. No individual patients or the public were involved in this study.
Results
Characteristics of study subjects
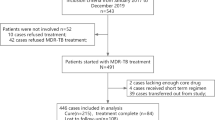
There were 402 pulmonary TB patients consecutively recruited into this study. Finally, 346 patients were kept in the training cohort dataset after excluding people who refused to participate (n = 10), diagnostic changes (n = 26), and loss to follow-up (n = 20) (Fig. 1A). There were 250 (72.3%) males and 96 (27.7%) females. Among them, 124 (35.8%) were over 60 years, 57 (16.5%) were underweight, 102 (29.5%) were ever-smokers, and 89 (25.7%) had an alcohol-drinking history. Nineteen (5.5%) subjects had an ATT history, and 34 (9.8%) were diagnosed with diabetes mellitus. The majority were farmers, and approximately half were low-income groups. Most received an ATT regimen of 2HRZE/4HR, while only 12 (3.5%) patients were treated with other regimens. Thirteen patients have an interruption of ATT (Table 1). Less than half were smear-positive. The median CXR score was 33.33, with an IQR of 40, of which 138 (39.9%) patients had pulmonary cavities. The mean symptom score was 3.39. The median time of the delayed treatment was 47 days, with an IQR of 71 days (Table 2). And the average time of follow-up was 190 days, ranging from 34 to 821 days. As a result, 35 subjects were observed with unfavorable outcomes during the follow-up, consisting of 11 adverse drug reactions, 4 TB-related deaths, 5 transferring to multidrug-resistant therapy, and 15 relapses after treatment.
We enrolled 132 TB patients in the second stage as the validation population. Detailed inclusion and exclusion criteria are shown in the diagram in Fig. 1B. Among them, 78 (59.1%) were males, 18 (13.6%) were over 60 years, 24 (18.2%) were underweight, 40 (30.3%) were ever-smokers, and 29 (22.0%) had an alcohol drinking history. There were 16 (12.1%) subjects who had an ATT history, and 12 (9.1%) were diagnosed with diabetes mellitus. There were 25 (18.9%) farmers, 60 (45.5%) patients working as laborers/sales/housekeeping, and 66 (50.0%) were low-income. Most of them received 6-month treatment with four first-line drugs, and only 2 patients had poor drug compliance (Table 1). Laboratory results of the study subjects are described in Table 2. The average follow-up time was 185 days, and we finally observed 15 patients with unfavorable outcomes, incorporating 2 treatment failures, 7 adverse drug reactions, 3 transferring to multidrug-resistant therapy, and 3 relapses.
We also compared demographics and laboratory values between two cohort populations (Tables 1 and 2). Results showed significant differences in sex, age, education levels, marriage status, occupational status, ATT history, and treatment regimens between the training cohort and validation population. Besides, there were meaningful differences in the pulmonary cavity, CXR score, symptom score, delayed treatment, and 18 clinical indicators. Overall, there existed heterogeneities in the two study populations.
Clinical indicator-based prognostic model
We performed a LASSO Cox regression analysis on 24 clinical indicators in the treatment outcomes of patients with TB. Eventually, 10 indicators (PLT, PCV, LYMPH, MONO%, NEUT, NEUT%, TBTL, ALT, UA, and Cys-C) remained in the final model. The coefficients of these indicators were utilized to calculate the risk score as follows: risk score = PLT×0.8236- PCV×0.6823- LYMPH×0.4442- MONO%×0.2720 + NEUT×0.0222 + NEUT%×0.0027- TBTL×0.5511- ALT×0.6839 + UA×0.6198 + Cys-C×0.6040. The univariate Cox regression analysis showed that the HR of the risk score was 4.980 (95% CI: 3.030–8.185, P < 0.001).
We further analyzed the effects of patient characteristics on the treatment outcomes (Table 3). Results showed that patients over 60 years, illiterate or semi-illiterate, married, with previous treatment history, treated with other regimens except for 2HRZE/4HR, with treatment interruptions, and with higher symptom scores were associated with an adverse outcome. Then, we performed a multivariate Cox regression analysis on the risk score by considering sex, age, BMI, tobacco smoking, alcohol drinking, education, marriage status, economic level, occupational status, previous medical history, ATT, treatment regimen, treatment compliance, sputum smear test at the time of diagnosis, CXR score, pulmonary cavity, symptom score, and delayed treatment (Table 4). Finally, the risk score (HR: 10.018, 95% CI: 4.904–20.468, P < 0.001), symptom score (HR: 1.356, 95% CI: 1.079–1.704, P = 0.009), pulmonary cavity (HR: 0.242, 95% CI: 0.087–0.674, P = 0.007), ATT (HR: 2.810, 95% CI: 1.137–6.948, P = 0.025), and tobacco smoking (HR: 2.499, 95% CI: 1.097–5.691, P = 0.029) constructed the optimal model. We graphed a nomogram based on this model to predict the 2-month, 6-month, and one-year favorable prognosis probability (Fig. 2).
A risk score nomogram predicts the favorable prognosis of patients with tuberculosis
First, we locate the risk score on the risk score axis and draw a vertical line up to the points axis to identify how many points the risk score contributes to a favorable prognosis (FP). Then, we use the same method for ATT history, pulmonary cavity, symptom score, and smoking. The total points are the sum of each factor. Finally, we locate the patient’s total points on the total points axis and draw a vertical line down to the probability of a 2-month, 6-month, and 12-month favorable prognosis
ATT: antituberculosis treatment; FP: favorable prognosis
Models evaluation
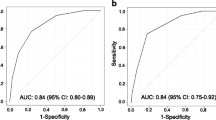
The C-index was 0.709 (95% CI: 0.548–0.870) for the univariate risk score model and 0.783 (95% CI: 0.675–0.891) for the multivariate prognostic model. The Akaike information criterion (AIC) was 271.230 for the univariate model and 265.653 for the multivariate model. Hosmer-Lemeshow tests indicated a good fit for these two models (P > 0.05). We plotted ROC curves to evaluate the predictive ability and calculated the corresponding AUC values (Fig. 3). The AUC was 0.766 (95% CI: 0.649–0.863) for the multivariate model in the training cohort and 0.796 (95% CI: (0.630–0.928) for the multivariate model in the external validation population. Internal validation showed an AUC of 0.763 (95% CI: 0.758–0.768) for the multivariate model.
Sensitivity analysis and subgroup analysis
Considering that the pulmonary cavity may affect the results, we performed a sensitivity analysis to test the consistency of this association by adding delayed time into the final prognosis model. Results showed that the pulmonary cavity remained a protective factor for the prognosis of TB (Supplementary Table 2).
Furthermore, we divided the delayed time into four groups by quartiles (25, 50, 75) and investigated its modification. In the univariate and multivariate models, no significant association was observed between the cavity and TB prognosis in each subgroup (Fig. 4).
Forest plots of the effect of the pulmonary cavity on the prognosis of patients with tuberculosis
Forest plots depicting the association between the pulmonary cavity and TB prognosis in the subgroup of treatment delay using univariate regression model (A) and multivariate regression model (B)
HR: hazard ratio; CI: confidence interval
Discussion
In the present study, we developed and validated a prognosis prediction model for patients with TB. Results showed that the clinical indicators-based risk score was significantly associated with the treatment outcomes. Findings from this study provide more references for the prognosis prediction of TB.
Previous studies have utilized different scoring systems to predict the prognosis. For example, Holden et al. constructed the CCIs-based model by considering cardiovascular disease, liver and kidney disease, mental disease, metabolic disease, and tumor [16]. Colangeli et al. calculated the Karnofsky scores to classify functional impairment to assess patients’ prognosis [15]. Nonetheless, as additional predictive tools, the aforementioned score systems relied on the patient’s recall and doctor’s judgment, prone to information bias. Blood tests and biochemistry examinations are routinely applied in clinical settings, providing accessible indicators. Therefore, we constructed a clinical indicator-based risk score to predict the treatment outcomes of TB by using PLT, PCV, LYMPH, MONO%, NEUT, NEUT%, TBTL, ALT, UA, and Cys-C.
A newly published study suggested that neutrophil, neutrophilic percentage, and neutrophil to lymphocyte ratio (NLR) were significantly related to different lung involvements among COVID-19 patients. The combination of NLR, lactate dehydrogenase, glucose, and ALT worked best to ascertain the clinical stage of COVID-19 [23]. Luo et al. established clinical indicators in routine blood tests to distinguish between active TB and latent tuberculosis infection (LTBI) [24]. Stefanescu et al. supposed that inflammatory biomarkers, including CRP, WBC, neutrophils, interferon-gamma inducible protein 10, CRP to albumin ratio (CAR), neutrophil to albumin ratio (NAR) and serum LL37, had a good prediction ability for 2-months treatment outcomes of pulmonary TB patients [25]. Previous studies also suggested that the power of a single index to predict prognosis was limited, and the combination of clinical indicators can effectively improve the prediction effectiveness.
The onset of TB always presented several clinical symptoms, including cough, expectoration, fever, weight loss, dyspnea, night sweats, hemoptysis, fatigue, and chest pain, which were closely linked to the severity of the disease. Our results uncovered the number of symptoms was positively related to the poor prognosis of TB patients, indicating that clinical symptoms and signs are associated with bacterial burden, infection site, and host immune response and thus affect treatment outcomes [26].
We found that patients with pulmonary cavities were inclined to have favorable outcomes, which seemed to be wired. This may be mediated by the fact that typical radiography findings are beneficial for accurate TB diagnosis. The discovery was in line with other studies. A cohort study has proposed atypical imaging features and sputum smear-negative at diagnosis were strongly related to delayed isolation and treatment that may cause an unfavorable prognosis [27]. Another study for hospitalized TB patients showed that noncavitary imaging manifestation might lead to misdiagnosis or delayed diagnosis, resulting in increased mortality [28]. However, it was important to note that similar radiological findings could observe in other diseases such as lung abscess, lung cancer, and pneumonia. Other characteristics like tree-in-bud appearance may be further considered. Besides, it should be noted that when patients received ATT during a relatively short period, the existence of a cavity may also be considered a risk factor for the favorable prognosis, which was shown in the subgroup analyses; this was supported by the results of Koo et al. [29]. It needs to be verified by further research through the expanded sample size.
Some studies have demonstrated that recurrent TB was a risk factor for the development and prognosis of TB [9, 14]. A nested case-control study in Vietnam elucidated that ATT history played a crucial role in the recurrence of TB, mainly due to increased drug resistance [30]. Poor treatment adherence and inadequate antibiotic therapy may also cause incomplete eradication of the causative Mycobacterium tuberculosis (M.tb) [31, 32].
Previous studies have reported that smokers were more prone to adverse treatment outcomes than non-smokers, which was consistent with our findings [33,34,35]. Harmful materials in cigarettes, like nicotine, could directly impair the human immune system and weaken the ability to kill M.tb in vivo [36]. Moreover, exposure to tobacco smoke would damage the respiratory tract, which constitutes early host defense against bacteria, thereby negatively affecting immunity [37].
However, there are several limitations to this study. First, this study was performed with a limited sample size in Jiangsu, China. Thus, caution should be exercised in extrapolating research results to other regions. Second, in this study, we excluded HIV-positive TB patients during the recruitment. Considering the critical role of HIV infections in pulmonary TB treatment effectiveness, we should bring this risk factor into further study. Third, we did not collect information about past medication history and daily ability to function, which could influence the prognosis of TB. It will be improved in future studies. Last, we only collected the baseline clinical data before treatment to calculate the risk score. It may neglect the importance of continuous follow-up and management. Future studies should consider the impact of dynamic changes in leading clinical indicators during follow-up on the prognosis, which will give more scientific justification.
In summary, we systematically generated a risk score-based model by integrating routine clinical information and demographic characteristics to predict ATT outcomes with feasibility and rationality in the clinic. Though there were population heterogeneities between the two cohorts, external validation remained a good performance of the model showing a relatively reasonable extrapolation.
Conclusion
In addition to the traditional predictive factors, the clinical indicator-based risk score determined in this study has an excellent predictive effect on the prognosis of TB.
Data Availability
All data generated or analyzed during this study are included in this published article.
Abbreviations
- AIC:
-
Akaike information criterion
- ALT:
-
Alanine aminotransferase
- AST:
-
Aspartate aminotransferase
- ATT:
-
Antituberculosis treatment
- AUC:
-
Area under the curve
- BASO:
-
Basophils
- BMI:
-
Body mass index
- CCI:
-
Charlson comorbidity index
- CI:
-
Confidence interval
- Cr:
-
Creatinine
- CXR:
-
Chest X-ray
- Cys-C:
-
Cystatin C
- DM:
-
Diabetes mellitus
- EOS:
-
Eosinophils
- HB:
-
Hemoglobin
- HIV:
-
human immunodeficiency virus
- HR:
-
Hazard ratio
- IQR:
-
Interquartile range
- LASSO:
-
Least absolute shrinkage and selection operator
- LTBI:
-
Latent tuberculosis infection
- LYMPH:
-
Lymphocytes
- MONO:
-
Monocyte
- M.tb :
-
Mycobacterium tuberculosis
- NEUT:
-
Neutrophil
- NLR:
-
Neutrophil to lymphocyte ratio
- PCV:
-
Packed cell volume
- PLT:
-
Platelet
- RBC:
-
Red blood cell
- RDW:
-
Red blood cell distribution width
- ROC:
-
Receiver operating characteristic
- SD:
-
Standard deviation
- TB:
-
Tuberculosis
- TBTL:
-
Total bilirubin
- UA:
-
Uric acid
- UREA:
-
Urea
- WBC:
-
White blood cell
- β2-m:
-
β2-microglobulin
References
WHO. Global tuberculosis report 2021. Geneva: World Health Organization; 2021.
Borisov AS, Bamrah Morris S, Njie GJ, Winston CA, Burton D, Goldberg S, Yelk Woodruff R, Allen L, LoBue P, Vernon A. Update of recommendations for use of once-weekly isoniazid-rifapentine regimen to treat latent Mycobacterium tuberculosis infection. MMWR Morbidity and mortality weekly report. 2018;67(25):723–6.
Targeted tuberculin testing and treatment of latent tuberculosis infection. American Thoracic Society. MMWR Recommendations and reports: Morbidity and mortality weekly report Recommendations and reports. 2000, 49(RR-6):1–51.
Sonnenberg P, Murray J, Glynn JR, Shearer S, Kambashi B, Godfrey-Faussett P. HIV-1 and recurrence, relapse, and reinfection of tuberculosis after cure: a cohort study in south african mineworkers. Lancet. 2001;358(9294):1687–93.
Panjabi R, Comstock GW, Golub JE. Recurrent tuberculosis and its risk factors: adequately treated patients are still at high risk. Int J tuberculosis lung disease: official J Int Union against Tuberculosis Lung Disease. 2007;11(8):828–37.
Fitzpatrick LK, Okwera A, Mugerwa R, Ridzon R, Ehiner J, Onorato I. An investigation of suspected exogenous reinfection in tuberculosis patients in Kampala, Uganda. Int J tuberculosis lung disease: official J Int Union against Tuberculosis Lung Disease. 2002;6(6):550–2.
Lambert ML, Hasker E, Van Deun A, Roberfroid D, Boelaert M. Van der Stuyft P: recurrence in tuberculosis: relapse or reinfection?. Lancet Infect Dis. 2003;3(5):282–7.
Peetluk LS, Rebeiro PF, Ridolfi FM, Andrade BB, Cordeiro-Santos M, Kritski A, Durovni B, Calvacante S, Figueiredo MC, Haas DW, et al. A clinical prediction model for unsuccessful pulmonary tuberculosis treatment outcomes. Clin Infect diseases: official publication Infect Dis Soc Am. 2022;74(6):973–82.
Pefura-Yone EW, Balkissou AD, Poka-Mayap V, Fatime-Abaicho HK, Enono-Edende PT, Kengne AP. Development and validation of a prognostic score during tuberculosis treatment. BMC Infect Dis. 2017;17(1):251.
Qiu B, Wu Z, Tao B, Li Z, Song H, Tian D, Wu J, Zhan M, Wang J. Risk factors for types of recurrent tuberculosis (reactivation versus reinfection): a global systematic review and meta-analysis. Int J Infect diseases: IJID : official publication Int Soc Infect Dis. 2022;116:14–20.
Agbeko CK, Mallah MA, He B, Liu Q, Song H, Wang J. Mental Health Status and its impact on TB Treatment and its outcomes: a scoping literature review. Front Public Health. 2022;10:855515.
Kornfeld H, Sahukar SB, Procter-Gray E, Kumar NP, West K, Kane K, Natarajan M, Li W, Babu S, Viswanathan V. Impact of diabetes and low body Mass Index on Tuberculosis Treatment Outcomes. Clin Infect diseases: official publication Infect Dis Soc Am. 2020;71(9):e392–8.
Nagai K, Horita N, Sato T, Yamamoto M, Nagakura H, Kaneko T. Age, dehydration, respiratory failure, Orientation Disturbance, and blood pressure score predicts In-hospital mortality in HIV-negative non-multidrug-resistant smear-positive pulmonary tuberculosis in Japan. Sci Rep. 2016;6:21610.
Velen K, Nhung NV, Anh NT, Cuong PD, Hoa NB, Cuong NK, Dung NH, Sy DN, Britton WJ, Marks GB, et al. Risk factors for tuberculosis (TB) among Household Contacts of patients with smear-positive TB in 8 provinces of Vietnam: a nested case-control study. Clin Infect diseases: official publication Infect Dis Soc Am. 2021;73(9):e3358–64.
Colangeli R, Jedrey H, Kim S, Connell R, Ma S, Chippada Venkata UD, Chakravorty S, Gupta A, Sizemore EE, Diem L, et al. Bacterial factors that predict relapse after tuberculosis therapy. N Engl J Med. 2018;379(9):823–33.
Holden IK, Lillebaek T, Andersen PH, Wejse C, Johansen IS. Characteristics and predictors for tuberculosis related mortality in Denmark from 2009 through 2014: a retrospective cohort study. PLoS ONE. 2020;15(6):e0231821.
Horita N, Miyazawa N, Yoshiyama T, Sato T, Yamamoto M, Tomaru K, Masuda M, Tashiro K, Sasaki M, Morita S, et al. Development and validation of a tuberculosis prognostic score for smear-positive in-patients in Japan. Int J tuberculosis lung disease: official J Int Union against Tuberculosis Lung Disease. 2013;17(1):54–60.
Nguyen DT, Graviss EA. Development and validation of a risk score to predict mortality during TB treatment in patients with TB-diabetes comorbidity. BMC Infect Dis. 2019;19(1):10.
Ralph AP, Ardian M, Wiguna A, Maguire GP, Becker NG, Drogumuller G, Wilks MJ, Waramori G, Tjitra E, Sandjaja, et al. A simple, valid, numerical score for grading chest x-ray severity in adult smear-positive pulmonary tuberculosis. Thorax. 2010;65(10):863–9.
Blazek K, van Zwieten A, Saglimbene V, Teixeira-Pinto A. A practical guide to multiple imputation of missing data in nephrology. Kidney Int. 2021;99(1):68–74.
Austin PC, White IR, Lee DS, van Buuren S. Missing Data in Clinical Research: a tutorial on multiple imputation. Can J Cardiol. 2021;37(9):1322–31.
Collett D. Modelling Binary Data. Second Edition: Taylor & Francis; 2002.
Liang YL, Ma YT, Huang KK, Luo HD, Wang AM, Xiong Z, Luo Y, Yi B. [The clinical application of blood routine and liver and kidney function test in COVID-19 disease prediction]. Chin J Prev Med. 2021;55(1):89–95.
Luo Y, Tang G, Yuan X, Lin Q, Mao L, Song H, Xue Y, Wu S, Ouyang R, Hou H, et al. Combination of blood routine examination and T-SPOT.TB assay for distinguishing between active tuberculosis and latent tuberculosis infection. Front Cell Infect Microbiol. 2021;11:575650.
Stefanescu S, Cocos R, Turcu-Stiolica A, Shelby ES, Matei M, Subtirelu MS, Meca AD, Stanciulescu EC, Popescu SO, Biciusca V, et al. Prediction of treatment outcome with inflammatory biomarkers after 2 months of Therapy in Pulmonary Tuberculosis Patients: preliminary results. Pathogens (Basel Switzerland). 2021;10(7):789.
Cadena AM, Fortune SM, Flynn JL. Heterogeneity in tuberculosis. Nat Rev Immunol. 2017;17(11):691–702.
Kim CJ, Kim Y, Bae JY, Kim A, Kim J, Son HJ, Choi HJ. Risk factors of delayed isolation of patients with pulmonary tuberculosis. Clin Microbiol infection: official publication Eur Soc Clin Microbiol Infect Dis. 2020;26(8):1058–62.
Lui G, Wong RY, Li F, Lee MK, Lai RW, Li TC, Kam JK, Lee N. High mortality in adults hospitalized for active tuberculosis in a low HIV prevalence setting. PLoS ONE. 2014;9(3):e92077.
Koo HK, Min J, Kim HW, Lee J, Kim JS, Park JS, Lee SS. Prediction of treatment failure and compliance in patients with tuberculosis. BMC Infect Dis. 2020;20(1):622.
Bestrashniy J, Nguyen VN, Nguyen TL, Pham TL, Nguyen TA, Pham DC, Nghiem LPH, Le TNA, Nguyen BH, Nguyen KC, et al. Recurrence of tuberculosis among patients following treatment completion in eight provinces of Vietnam: a nested case-control study. Int J Infect diseases: IJID : official publication Int Soc Infect Dis. 2018;74:31–7.
Lan NT, Lien HT, Tung le B, Borgdorff MW, Kremer K, van Soolingen D. Mycobacterium tuberculosis Beijing genotype and risk for treatment failure and relapse, Vietnam. Emerg Infect Dis. 2003;9(12):1633–5.
Gegia M, Winters N, Benedetti A, van Soolingen D, Menzies D. Treatment of isoniazid-resistant tuberculosis with first-line drugs: a systematic review and meta-analysis. Lancet Infect Dis. 2017;17(2):223–34.
Qiu F, Liang CL, Liu H, Zeng YQ, Hou S, Huang S, Lai X, Dai Z. Impacts of cigarette smoking on immune responsiveness: Up and down or upside down? Oncotarget 2017, 8(1):268–284.
Burusie A, Enquesilassie F, Addissie A, Dessalegn B, Lamaro T. Effect of smoking on tuberculosis treatment outcomes: a systematic review and meta-analysis. PLoS ONE. 2020;15(9):e0239333.
Lin HH, Ezzati M, Murray M. Tobacco smoke, indoor air pollution and tuberculosis: a systematic review and meta-analysis. PLoS Med. 2007;4(1):e20.
Wang H, Yu M, Ochani M, Amella CA, Tanovic M, Susarla S, Li JH, Wang H, Yang H, Ulloa L, et al. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature. 2003;421(6921):384–8.
Sopori M. Effects of cigarette smoke on the immune system. Nat Rev Immunol. 2002;2(5):372–7.
Acknowledgements
Not applicable.
Funding
This study was funded by the National Natural Science Foundation of China (81973103), Medical Research Project of Jiangsu Health Commission (ZDB2020013), Nanjing Major Science and Technology Project (2021–11005), and Yancheng Medical Science and Technology Development Program (YK2016021). The funding agencies had no role in the study design, data collection, analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
Mengyao Zhan, Hao Xue, and Yuting Wang: Conceptualization, Methodology, Data Collection, Data Collation, Software, Formal analysis, Writing - Original Draft, Visualization. Zhuchao Wu, Qin Wen, and Xinling Shi: Data Collation, Data Collation, Resources, Writing - Review & Editing, Supervision. Jianming Wang: Conceptualization, Resources, Writing - Review & Editing, Visualization, Supervision, Project administration, Funding acquisition.
Corresponding author
Ethics declarations
Ethical approval
This study was approved by the Institutional Review Board of Nanjing Medical University (ID: 2020-65). Written informed consent was obtained from study participants. The study was conducted in accordance with the Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhan, M., Xue, H., Wang, Y. et al. A clinical indicator-based prognostic model predicting treatment outcomes of pulmonary tuberculosis: a prospective cohort study. BMC Infect Dis 23, 101 (2023). https://doi.org/10.1186/s12879-023-08053-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-023-08053-x