Abstract
Background
China has a double burden of diabetes mellitus and tuberculosis. Diabetes mellitus and tuberculosis are both important risk factors for Aspergillus infection. Aspergillus lentulus is an emerging fungal pathogen in China and invasive aspergillosis due to A. lentulus is associated with high mortality.
Case presentation
A 79-year-old man was admitted to our hospital, complaining of a 7-day history of fever. Five days before admission, he was diagnosed with pulmonary infection at a local hospital, but his symptoms did not relieve after antibiotic therapy. The patient was diagnosed with diabetes mellitus two months ago. About 20 days ago, he began to present chest tightness and shortness of breath after physical activity. After admission, he developed continuous fever and rapid respiratory deterioration, and finally died after his family abandoned treatment. Pulmonary coinfection with M. tuberculosis and A. lentulus was identified by metagenome next-generation sequencing (mNGS) from bronchoalveolar lavage fluid.
Conclusions
Clinicians and laboratories should be alert to the emerging A. lentulus infection in China due to its drug-resistance and high mortality. In comparison with conventional methods, mNGS has a great advantage for the diagnosis of mixed pulmonary infection.
Similar content being viewed by others
Background
There is a double burden of diabetes mellitus (DM) and tuberculosis (TB) in China: the TB cases account for 7.4% of the global total in 2021; the prevalence of DM has increased from less than 1% in 1980 to 11.2% in 2017 [1, 2]. Strong evidence has proved the association between the two diseases. In patients with active TB, DM can lead to a poor TB treatment outcome, a higher chance of relapse, and an increased risk of death [3].
TB and DM are both important risk factors for Aspergillus infection. Coinfection with M. tuberculosis and Aspergillus is common in Asia and Africa countries. The combined prevalence of Aspergillus coinfection among patients with pulmonary tuberculosis was 15.4% [4]. DM is widely recognized as a risk factor for invasive pulmonary aspergillosis. Diabetic patients have an immune system with a lower ability to respond to and deal with aspergillosis, and prolonged hyperglycemia results in unfavorable outcomes in infected patients [5]. Aspergillus lentulus is an emerging fungal pathogen in China and invasive aspergillosis due to A. lentulus is associated with high mortality [6]. Herein, we report a case of pulmonary coinfection with M. tuberculosis and A. lentulus in a diabetic patient diagnosed by metagenome next-generation sequencing.
Case presentation
A 79-year-old man was admitted to the cardiology department of our hospital, complaining of a 7-day history of fever, with a temperature up to 39.5 ℃. He denied cough, phlegm, nasal obstruction, pharyngalgia, chest pain, dizziness, or headache. Five days before admission, he was diagnosed with pulmonary infection by chest X-ray and given antibiotic therapy at a local hospital, but his symptoms did not relieve. The patient was diagnosed with diabetes mellitus two months ago and drank 2000–3000 ml of water per day. About 20 days ago, he began to present chest tightness and shortness of breath after physical activity. In addition, the patient had a history of high blood pressure for more than 20 years.
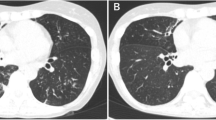
On admission (day 0), physical examination showed conscious state, temperature of 36.5 ℃, heart rate of 78/min, respiratory rate of 20/min, blood pressure of 120/62 mmHg, and crackles on pulmonary auscultation. The patient reported a weight loss of 10 kg in the last six months. Laboratory investigations showed white blood cell of 7.77 × 109/L (with 90.1% neutrophil, 2.5% monocytes, and 7.2% lymphocytes), procalcitonin of 3.24 ng/ml, erythrocyte sedimentation rate of 52 mm/h, hemoglobin A1c of 9.5%, and fasting blood sugar of 8.26 mmol/L. A chest computed tomography (CT) scan showed multiple nodules and patchy infiltration in both lungs (Fig. 1A, B), which did not exclude the possibility of tuberculosis. The preliminary diagnosis was pulmonary infection, and empiric antibiotic treatment with intravenous piperacillin/tazobactam (4.5 g, q12h) and moxifloxacin (2 g, q12h) was given. Intravenous insulin (4 U, qd) and oral miglitol (50 mg, q12h) were used to control blood glucose level.
After admission, the patient developed continuous fever and daily temperature peak exceeded 38.5 ℃. On day 1, he developed respiratory failure and received oxygen treatment. Arterial blood gas analysis showed PH 7.50, PaO2 of 57.7 mmHg, PaCO2 of 31.9 mmHg, and SaO2 of 90.2%. Serological tests for influenza A IgM, influenza B IgM, parainfluenza virus IgM, adenovirus IgM, respiratory syncytial virus IgM, Mycoplasma pneumoniae IgM, and Chlamydia pneumoniae IgM proved negative. Tuberculosis immune spot test was positive. On day 2, the patient presented polyuria and polydipsia, and his symptom of tachypnea was not relieved during oxygen treatment. Then he was transferred to the intensive care unit (ICU) and given mechanical ventilation. On day 3, the patient had a PASS score of − 1, and received analgesia and sedation with propofol and alfentanil. Bronchoscopy found bronchial mucosal inflammation and production of purulent sputum, and bronchoalveolar lavage fluid (BALF) was obtained for further detection.
On day 5, the patient underwent a decreased oxygenation index (155) compared with before. No abnormalities were found in his blood culture. Serum galactomannan (1.74 ng/ml) and 1,3-β-d-glucan (424.50 ng/L) were positive. Acid-fast staining and culture of BALF were negative. M. tuberculosis DNA detection from BALF was positive. The BALF sample was also sent for metagenomic next-generation sequencing (Genskey, Beijing, China). A total of 86,398,947 raw reads were generated and 60,161,047 high quality reads were obtained after removing low quality reads. Among the high quality reads, 47,232,566 were aligned with the human reference genome (HG38) and 12,928,481 were used for downstream analysis. The mNGS of BALF reported M. tuberculosis complex (7,453,374 reads), A. lentulus (37,868 reads), and several oral colonizing microorganisms. Metagenome data is now available at NCBI under the Sequence Read Archive (SRA) database with accession no. PRJNA917778. Based on these findings, the patient was diagnosed as coinfection of M. tuberculosis and A. lentulus. Initial antibiotic treatment was changed to oral isoniazid (0.3 g, qd), rifapentine (0.45 g, qd) for anti-tuberculosis, and intravenous caspofungin (50 mg, qd) for anti-fungal.
On day 6, the patient's condition aggravated again. He developed multiple organ dysfunction and fell into a light coma state. His family decided to abandon treatment because of financial problems and grave prognosis. Artificial respiration was stopped subsequently and the patient expired 1.5 h later.
Discussion and conclusion
TB and DM are both significant public health problems in China, and they have mutual risk factors. A higher prevalence of TB among DM patients and vice versa were found in many previous studies worldwide [7]. TB and DM patients are usually immunocompromised and susceptible to fungal infections. Among patients with M. tuberculosis and Aspergillus coinfection, the most frequency of Aspergillus spp. was A. fumigatus with a prevalence of 57.6% [4]. Here, we report a rare case of pulmonary infection caused by M. tuberculosis and A. lentulus in a diabetic patient, and believe that A. lentulus infection was secondary to TB and DM.
Pulmonary TB and aspergillosis have similar clinical symptoms including fever, chest tightness, shortness of breath, cough, chest pain, sputum with blood streaks, weight loss, and night sweats [8]. Therefore, they might be misdiagnosed and mistreated in clinical practice, and a definitive diagnosis relies on etiological detection results. However, traditional methods often have limitations for the detection of rare pathogens or mixed infections.
In recent years, the application of mNGS shed light on detecting pathogens much faster and more efficiently. Miao et al. demonstrated that the mNGS method had higher sensitivity and specificity than microbial culture, especially for the detection of M. tuberculosis, fungi, anaerobic bacteria, and viruses [9]. As an unbiased and rapid diagnostic method, mNGS can sequence thousands to billions of DNA fragments and provide information on a broad spectrum of organisms simultaneously.
mNGS has a great advantage for the diagnosis of mixed pulmonary infection. Mixed pulmonary infection is defined when coinfection with two or more pathogens is identified. A study showed that the sensitivity of mNGS in mixed pulmonary infection diagnosis was seven times higher than that of conventional tests (97.2% vs 13.9%) [10]. Another study showed that the rate of confirmed mixed pulmonary infection detected by mNGS was four times higher than that detected by conventional methods [11]. Yan et al. reported a case on mixed pulmonary infection of Nocardia nova, M. tuberculosis, and A. fumigatus based on mNGS. Although sputum and BALF were sent several times for smear and culture, only A. fumigatus was detected by culture [8]. Qin et al. reported a case of mixed pulmonary infection with Corynebacterium striatum, Pseudomonas aeruginosa, Streptococcus pneumoniae, and Cryptococcus neoformans through mNGS, while microbial culture and immunological tests were all negative [12]. Mixed pulmonary infection is very common in clinical experience, especially in immunocompromised patients, so early accurate diagnosis and appropriate treatment may reduce the mortality.
Invasive aspergillosis remains a major invasive fungal infection with serious clinical consequences among immunocompromised patients, and A. fumigatus is the most common cause [13]. As a sibling species of A. fumigatus, A. lentulus was first described in 2004, which caused fatal infections in four hematopoietic stem cell transplant patients [14, 15]. This species is usually difficult to distinguish from A. fumigatus based on phenotypic typing, and can be misidentified as A. fumigatus [16]. A. lentulus is genetically distinct from A. fumigatus and highly drug resistant. Molecular identification and matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF MS) can be used to distinguish them [17].
A. lentulus tends to cause infections associated with high mortality (> 60%) and poor clinical outcomes [18]. In China, the first A. lentulus strain was isolated from a patient with acute pulmonary infection in 2011, who died due to aggressive lung fungal infection after active treatment [19]. In 2020, Yu et al. reported six patients with proven or probable A. lentulus infection from China. Four of the patients had poor prognosis. All the six isolates were in vitro resistant to multiple anti-fungal drugs, including amphotericin B, itraconazole, voriconazole, and posaconazole. Immunocompromise, critical illness, and prior anti-fungal were common risks for invasive A. lentulus infection [6]. Although A. lentulus infection is not commonly found in China, we still need to pay attention to its pathogenicity in clinical practice.
Collectively, we report the first case of pulmonary coinfection caused by M. tuberculosis and A. lentulus in China, to the best of our knowledge. Clinicians and laboratories should be alert to the emerging A. lentulus infection in China due to its drug-resistance and high mortality. In comparison with conventional methods, mNGS has a great advantage for the diagnosis of mixed pulmonary infection.
Availability of data and materials
All data generated or analysed during this study are included in this published article.
Abbreviations
- mNGS:
-
Metagenome next-generation sequencing
- DM:
-
Diabetes mellitus
- TB:
-
Tuberculosis
- CT:
-
Computed tomography
- ICU:
-
Intensive care unit
- BALF:
-
Bronchoalveolar lavage fluid
- MALDI-TOF MS:
-
Matrix-assisted laser desorption ionization-time of flight mass spectrometry
References
WHO. Global tuberculosis report 2022. 2022.
Li Y, Teng D, Shi X, et al. Prevalence of diabetes recorded in mainland China using 2018 diagnostic criteria from the American Diabetes Association: national cross sectional study. BMJ. 2020;369: m997.
Du Q, Wang L, Long Q, Zhao Y, Abdullah AS. Systematic review and meta-analysis: prevalence of diabetes among patients with tuberculosis in China. Trop Med Int Health. 2021;26(12):1553–9.
Hosseini M, Shakerimoghaddam A, Ghazalibina M, Khaledi A. Aspergillus coinfection among patients with pulmonary tuberculosis in Asia and Africa countries; a systematic review and meta-analysis of cross-sectional studies. Microb Pathog. 2020;141: 104018.
Rafat Z, Hashemi SJ, Ashrafi K, et al. Fungal isolates of the respiratory tract in symptomatic patients hospitalized in pulmonary units: a mycological and molecular epidemiologic study. J Multidiscip Health. 2020;13:661–7.
Yu SY, Guo LN, Xiao M, et al. Clinical and microbiological characterization of invasive pulmonary aspergillosis caused by Aspergillus lentulus in China. Front Microbiol. 2020;11:1672.
Cheng J, Zhang H, Zhao YL, Wang LX, Chen MT. Mutual impact of diabetes mellitus and tuberculosis in China. Biomed Environ Sci. 2017;30(5):384–9.
Yan H, Li Z, Xia H, Li Q, Bai H. A case report on mixed pulmonary infection of Nocardia nova, Mycobacterium tuberculosis, and Aspergillus fumigatus based on metagenomic next-generation sequencing. Front Public Health. 2022;10: 927338.
Miao Q, Ma Y, Wang Q, et al. Microbiological diagnostic performance of metagenomic next-generation sequencing when applied to clinical practice. Clin Infect Dis. 2018;67(suppl_2):S231–40.
Wang J, Han Y, Feng J. Metagenomic next-generation sequencing for mixed pulmonary infection diagnosis. BMC Pulm Med. 2019;19(1):252.
Wei P, Wu L, Li Y, et al. Metagenomic next-generation sequencing for the detection of pathogenic microorganisms in patients with pulmonary infection. Am J Transl Res. 2022;14(9):6382–8.
Qin Z, Zou Y, Huang Z, et al. Metagenomic next-generation sequencing contributes to the diagnosis of mixed pulmonary infection: a case report. Ann Clin Microbiol Antimicrob. 2022;21(1):52.
Sugui JA, Peterson SW, Figat A, et al. Genetic relatedness versus biological compatibility between Aspergillus fumigatus and related species. J Clin Microbiol. 2014;52(10):3707–21.
Balajee SA, Weaver M, Imhof A, Gribskov J, Marr KA. Aspergillus fumigatus variant with decreased susceptibility to multiple antifungals. Antimicrob Agents Chemother. 2004;48(4):1197–203.
Balajee SA, Gribskov JL, Hanley E, Nickle D, Marr KA. Aspergillus lentulus sp. nov., a new sibling species of A. fumigatus. Eukaryot Cell. 2005;4(3):625–32.
Nematollahi S, Permpalung N, Zhang SX, Morales M, Marr KA. Aspergillus lentulus: an under-recognized cause of antifungal drug-resistant aspergillosis. Open Forum Infect Dis. 2021;8(8):ofab392.
Lamoth F. Aspergillus fumigatus-related species in clinical practice. Front Microbiol. 2016;7:683.
Won EJ, Shin JH, Kim SH, et al. Antifungal susceptibilities to amphotericin B, triazoles and echinocandins of 77 clinical isolates of cryptic Aspergillus species in multicenter surveillance in Korea. Med Mycol. 2018;56(4):501–5.
Zhang LJ, Wang XD, Ji MS, Hasimu H, Abliz P. Characterisation of a clinical isolated Aspergillus lentulus strain using a Galleria mellonella infection model. J Thorac Dis. 2021;13(2):803–11.
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
HZ and GML collected clinical and laboratory data. LH, YZ, and HBT assisted with data analysis. SFZ integrated the data and wrote the manuscript. HZ and GML contributed equally to this work. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Written informed consent was obtained from the patient’s family for the publication of this case report.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhang, H., Liu, G., He, L. et al. Life-threatening pulmonary coinfection with Mycobacterium tuberculosis and Aspergillus lentulus in a diabetic patient diagnosed by metagenome next-generation sequencing. BMC Infect Dis 23, 88 (2023). https://doi.org/10.1186/s12879-023-08052-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-023-08052-y