Abstract
Background
Malaria remains a public health problem in Kenya despite sustained interventions deployed by the government. One of the major impediments to effective malaria control is a lack of accurate diagnosis and effective treatment. This study was conducted to assess clinical malaria incidence and treatment seeking profiles of febrile cases in western Kenya.
Methods
Active case detection of malaria was carried out in three eco-epidemiologically distinct zones topologically characterized as lakeshore, hillside, and highland plateau in Kisumu County, western Kenya, from March 2020 to March 2021. Community Health Volunteers (CHVs) conducted biweekly visits to residents in their households to interview and examine for febrile illness. A febrile case was defined as an individual having fever (axillary temperature ≥ 37.5 °C) during examination or complaints of fever and other nonspecific malaria related symptoms 1–2 days before examination. Prior to the biweekly malaria testing by the CHVs, the participants' treatment seeking methods were based on their behaviors in response to febrile illness. In suspected malaria cases, finger-prick blood samples were taken and tested for malaria parasites with ultra-sensitive Alere® malaria rapid diagnostic tests (RDT) and subjected to real-time polymerase chain reaction (RT-PCR) for quality control examination.
Results
Of the total 5838 residents interviewed, 2205 residents had high temperature or reported febrile illness in the previous two days before the visit. Clinical malaria incidence (cases/1000people/month) was highest in the lakeshore zone (24.3), followed by the hillside (18.7) and the highland plateau zone (10.3). Clinical malaria incidence showed significant difference across gender (χ2 = 7.57; df = 2, p = 0.0227) and age group (χ2 = 58.34; df = 4, p < 0.0001). Treatment seeking patterns of malaria febrile cases showed significant difference with doing nothing (48.7%) and purchasing antimalarials from drug shops (38.1%) being the most common health-seeking pattern among the 2205 febrile residents (χ2 = 21.875; df = 4, p < 0.0001). Caregivers of 802 school-aged children aged 5–14 years with fever primarily sought treatment from drug shops (28.9%) and public hospitals (14.0%), with significant lower proportions of children receiving treatment from traditional medication (2.9%) and private hospital (4.4%) (p < 0.0001). There was no significant difference in care givers' treatment seeking patterns for feverish children under the age of five (p = 0.086). Residents with clinical malaria cases in the lakeshore and hillside zones sought treatment primarily from public hospitals (61.9%, 60/97) traditional medication (51.1%, 23/45) respectively (p < 0.0001). However, there was no significant difference in the treatment seeking patterns of highland plateau residents with clinical malaria (p = 0.431).The main factors associated with the decision to seek treatment were the travel distance to the health facility, the severity of the disease, confidence in the treatment, and affordability.
Conclusion
Clinical malaria incidence remains highest in the Lakeshore (24.3cases/1000 people/month) despite high LLINs coverage (90%). The travel distance to the health facility, severity of disease and affordability were mainly associated with 80% of residents either self-medicating or doing nothing to alleviate their illness. The findings of this study suggest that the Ministry of Health should strengthen community case management of malaria by providing supportive supervision of community health volunteers to advocate for community awareness, early diagnosis, and treatment of malaria.
Similar content being viewed by others
Background
Malaria remains a major public health problem in Kenya, despite increased efforts by the Ministry of Health to scale up intervention strategies [1,2,3]. Approximately 70% of the country's 47 million inhabitants are at risk of the disease, with the western Kenya region having the highest burden of infection [4]. The disease accounts for about 30% out-patients attendance in both public and private health care facilities [1, 2, 5, 6]. The most common symptom of clinical malaria is fever which drives people to seek treatment when it becomes severe [7]. Access to health care, housing type, proximity of human settlements to vector breeding sites [8], socioeconomic status, and bednet use [9, 10] may all influence clinical malaria risk in the community. Children and pregnant women are the most vulnerable groups to infection [11, 12]. School-aged children serve as a reservoir for malaria parasites, and the prevalence of infection among this age group is high [13]. Variation in the ecological landscape may result in differential risk exposures to malaria contributing to variation in febrile incidences in the community [14] driving residents to seek alternative treatment routes. Understanding the health-seeking behavior of clinical malaria cases across different topographical zones may aid in addressing the year-round infection in the community.
The Kenya National Guidelines for the Diagnosis, Treatment, and Prevention of Malaria call for seeking medical attention within 24 h of becoming ill [15]. Adherence to the guidelines, on the other hand, is becoming a problem as people suffering from malaria-related symptoms either self-medicate with over-the-counter medications or do nothing and only seek treatment from health facilities when their symptoms become severe. The COVID-19 pandemic has had a negative impact on fever care, with outpatient clinic attendance significantly lower since the virus was first detected in Kenya [16]. Prior to COVID-19, the majority of caregivers of children with fever sought treatment from public health facilities [15]. While the majority of parents seek medical attention for their feverish children, many do not do so right away. Some parents wait until their child's symptoms become severe before seeking medical help. Doing nothing about febrile symptoms, resulting in a delay in seeking treatment until the illness worsens, may lead to complicated malaria [17]. Although treatment in public health facilities is free, there is vast majority of underreported malaria cases at the community [18]. The availability and affordability of the local herbs is easier as they can be obtained from the fields or traditional healers as these traditional healers have good knowledge of symptoms of malaria [19,20,21]. The reasons for informing the decision to use the various treatment seeking rotes are unknown.
Self-medication for fever relief is a common practice, particularly in the early stages of illness when symptoms are mild [19]. Self-prescription and the use of antimalarial drugs without a confirmed diagnosis may result in antimalarial misuse, which may contributes to selection pressure [22]. In the absence of a confirmed diagnosis, alternative methods of treating malaria symptoms may result in disease complications. Monitoring the impact of topography on treatment seeking profiles in rural communities, as well as adherence to MOH-recommended prompt diagnosis and treatment guidelines, is critical for effective malaria control. As a result, more efforts must be made to encourage prompt fever treatment, as well as the adoption of more sensitive and accurate diagnostic tools to aid in community case management of malaria. The current study aims at assessing clinical malaria incidences and treatment seeking patterns across topography in a rural community in Kisumu County, western Kenya.
Methods
Study site
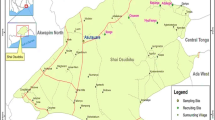
This study was carried out in Nyakach Sub-County of Kisumu in Western Kenya. Based on malaria prevalence and topographical features, the study area was divided into three eco-epidemiological zones based on a previous study [18]: Lakeshore, Hillside, and Highland plateau (Fig. 1). Landscapes in the three zones are very different from each other based on altitude and topography [18]. The altitude of the three eco-epidemiological zones varies, with the lakeshore zone located on the lakeside of the Lake Victoria region at an altitude ranging from 1100 to 1200 m above sea level and prone to flooding during the rainy season. The Highland plateau zone has an altitude range of 1500–1700 m and has more stable larval habitats, whereas the Hillside zone has an altitude range of 1300–1450 m and is located between the Lakeshore and Highland plateau zones. Permanent aquatic habitats are uncommon and larval habitats unstable in this zone. The study area is approximately 327 square kilometers in size, with a population of 168,140 people living in 35,553 households at a population density of 460 people per square km [23]. This region's economic activities are primarily fishing, subsistence farming, rock mining, and small-scale trading.
Study design and data collection
A longitudinal study was conducted in three eco-epidemiologically distinct zones: Lakeshore, Hillside, and Highland plateau zones, between March 2020 and March 2021.Community health volunteers (CHVs) were trained on how to record febrile cases in each household, as well as how to take blood sample for ultra-sensitive malaria RDT and RT-PCR analysis. The survey was conducted during the Covid-19 era, and all infection prevention and control protocols were followed in accordance with Ministry of Health guidelines [24]. A febrile suspected malaria case was defined as an individual with fever (axillary temperature ≥ 37.5 °C) at the time of examination or complaints of fever and other nonspecific symptoms within 48 h prior to examination, according to the WHO definition [25]. The survey sought to ascertain the community's health, demographic, and socioeconomic characteristics. The study questionnaire collected self-reported information on age, gender, and active fever, treatment seeking method prior to the CHVs testing, primary occupation, travel history, and ITN use. Participants were divided into three age groups (< 5 years old, 5–14 years old, and ≥ 15 years old). Active fever was defined as an individual axillary temperature ≥ 37.5 °C at the time of examination. The participants' treatment seeking methods were based on their behaviors in response to the illness prior to the biweekly malaria testing by the CHVs. There are five types of treatment seeking methods (public hospital, private hospital, drug shops, traditional medication, and do nothing) Occupation was divided into four categories (farmer, small scale business, office worker, unemployed, student, non-school child, and others. Travel history was defined as having traveled outside the study zones within the previous two weeks. ITN use was defined as sleeping under an ITN the night before the survey. The study sought to identify the socioeconomic and demographic factors associated with the decision to seek treatment. Furthermore, the study questionnaire gathered self-reported data on wall material type, health insurance, and malaria information and awareness, marital status, and distance to the health facility, severity of the disease, confidence in treatment choice, affordability, and medication availability. The wall material type was used to assess house structure categorized into the following groups: Brick/Block, Mud & Wood, and Mud & Cement. The health insurance was classified as the mode of payment at the health facility, as either the hospital payment was by health insurance or cash. Malaria information was defined as the medium through which participants learned about malaria prevention, symptoms, and control. Malaria information was divided into three categories: no information received, information received from the media, and information received from the CHV. Participants who knew the symptoms and severity of malaria were classified as "aware," while those who didn't know were classified as "not aware." The marital status was divided into four categories: under age, single, married, and widowed/divorced. The travel distance to the health facility, the severity of the disease, confidence in the choice of treatment, affordability, and medication availability were all evaluated to see if they played a role in treatment selection. These information was reviewed daily by team supervisors for quality assurance. A total of 2205 finger-prick blood samples were taken from febrile cases for parasite examination with ultra-sensitive Alere® malaria RDT (Reference number: 05FK140, Republic of Korea) and RT-PCR on dry blood spots [26]. The samples were then transported to the International Centre of Excellence for Malaria Research (ICEMR) at Tom Mboya University College in Homa Bay, Kenya, for further analysis. The CHVs administered AL to all RDT positive febrile residents. Residents who tested negative were referred to the nearest health facility for follow-up care.
DNA extraction and screening for Plasmodium falciparum infection
975 of the 2205 dried blood spots were randomly selected for DNA extraction to determine the sensitivity and specificity of the ultrasensitive malaria RDT. Chelex resin (Chelex-100) saponin method was used with slight modifications [26]. Plasmodium species-specific primers and probes targeting 18S ribosomal RNA were used [27]. PCR reaction volume was constituted as follows; 6 µL of PerfeCTa® qPCR ToughMix™, Low ROX™ Master mix (2X), 0.4 µL each of the forward and reverse species-specific primers (10 µM), 0.5 µL of the species-specific probe, 0.1 µL of double-distilled water and 2 µL of parasite DNA. Thermocycler conditions were set as follows, 50 °C for 2 min, (95 °C for 2 min, 95 °C for 3 s and 58 °C for 30 s) for 45 cycles (QuantStudio™ 3 Real-Time PCR System).
Data analysis
Data were analyzed using SPSS Version 21 software. The demographic profiles of the study participants were described using descriptive statistics. The Chi-square test, odds ratio, incidence ratio, and risk ratio were used to identify the factors associated with clinical malaria incidences and the treatment seeking patterns. Multiple regression was used to predict malaria febrile incidence across topography. Artificial neural network model was used to identify the variables importance associated with the decision to seek treatment. Frequency tables were used to describe categorical variables (counts and percentages). For all analyses, p ≤ 0.05 was considered statistically significant.
Results
Demographic information of the study participants
A total of 1,599 households were surveyed, with 5,838 residents participating in the study. The three zones' residents’ age structure and gender were all similar. Farming was the most important source of income (21.7%). Individuals aged > 15 years made up approximately 56.6% of the study population and literacy rates were high, with 54.7% completing primary school and 26.2% completing secondary school education (Table 1).
Clinical malaria incidence across topography by age and gender
In the study zone, 2205 residents reported febrile illness out of a total of 5838 residents. The Lakeshore zone had the highest clinical malaria incidence, with 24.3 cases/1000 people/month, followed by the hillside zone (18.7 cases/1000 people/month) and the highland plateau zone (10.3 cases/1000 people/ month).
A further Chi square test revealed a statistically significant difference in clinical malaria incidence by gender across topographic zones (χ2 = 7.57; df = 2, p = 0.0227). Males had a higher incidence of 26.7 in the lakeshore zone than females, who had an incidence of 22.3. In the hillside and the highland plateau, the females had the higher incidence of infection at 21.7 and 12.7, respectively (Table 2).
The chi square test revealed a significant difference in the incidence of clinical malaria by age group across topographical zones (χ2 = 58.34; df = 4, p < 0.0001). In the Lakeshore zone, hillside and the highland plateau the school going children aged between 5 and 14 years old had the highest incidence of infection at 38.8, 28.1, and 13.9 cases/1000 people/month, respectively (Table 2).
Among the females in the lakeshore zone, the risk of clinical malaria incidences was 1.72 times higher among the 5–14 years old school going children (IR:1.72, 95% CI = 1.24–2.18) and 0.51 times lower among individuals ≥ 15 years old (IR:0.51, 95% CI = 0.25–0.77) compared to children under 5 years old (Table 2). Among the males, the incidence risk of infection was 0.18 times lower among individuals ≥ 15 years old (IR: 0.18, 95% CI = 0.04–0.31) compared to children under five years old in the lake zone. In the hillside zone, the school going children had the highest incidence risk of infection compared to the children under five years old (IR: 2.66, 95% CI = 1.53–3.62) (Table 2).
Risk factors associated with clinical malaria incidences
Multivariate analysis found that residency in the lakeshore and hillside zone, being male, being between the ages < 5 years and 5–14 years, having a subjective fever, and an elevated body temperature at the time of the visit were all associated with an increased risk of clinical malaria incidences (p < 0.05) (Table 3). When compared to the highland plateau, the odds of clinical malaria cases were 2.01(95% CI = 1.63–2.49, p < 0.0001) and 1.47 times higher in the lakeshore and hillside zones, respectively. Females were less likely than males to suffer from clinical malaria (OR: 0.83, 95% CI = 0.70–0.99, p = 0.042). When compared to individuals over the age of 15, school-aged children aged 5–14 years and under 5 years were 2.00 times (95% CI = 1.66–2.43, p < 0.0001) and 1.98 (95% CI = 1.54–2.54, p < 0.0001) more likely to suffer from clinical malaria, respectively. Residents who did not have active fever at the time of testing by the CHVs were less likely to test positive for malaria than those who did (OR: 0.27 95% CI = 0.21–0.34, p < 0.0001). However, seasonality, recent travel history, and bed net use were not associated with the risk of clinical malaria incidences (p < 0.05) (Table 3).
Symptoms presented by ultrasensitive malaria RDT positive and negative residents
The Chi square test revealed a significant difference in symptoms between residents who tested positive for malaria by ultrasensitive malaria RDT and those who tested negative (χ2 = 20.273, df = 7, p = 0.005). Fever, headache, chills, and vomiting were the most common symptoms among ultrasensitive malaria RDT positive residents, while fatigue, muscle and joint pain were common among ultrasensitive malaria RDT negative residents (Fig. 2).
The Chi square test revealed no significant differences in the symptoms reported by ultrasensitive malaria RDT positive residents across age groups (χ2 = 16.537, df = 14, p = 0.282). Furthermore, the test revealed no significant difference in symptoms among those who tested negative for ultrasensitive malaria RDT across age groups (χ2 = 6.577, df = 14, p = 0.950) (Fig. 3).
Treatment seeking patterns of clinical malaria cases
The identified treatment seeking patterns of the clinical malaria cases were doing nothing, buying medicine from drug stores/chemists, and seeking treatment in public, private, and traditional medication which was mainly herbal remedies. There was significant difference in the treatment seeking profiles of the clinical malaria cases (χ2 = 21.875; df = 4, p < 0.0001). The most common health seeking behavior among the total 2205 febrile cases assessed was doing nothing (48.7%), buying medicine from drug shops/chemists (38.1%), and seeking treatment in public (12.5%), private hospitals (4.1%), and traditional medication (3.5%) (Table 4). Treatment seeking patterns for the clinical malaria cases differed significantly by the lakeshore (χ2 = 22.471, df = 4, p < 0.0001), hillside zones (χ2 = 27.813, df = 4, p < 0.0001), female sex (χ2 = 19.447, df = 4, p = 0.001), school going children (χ2 = 21.717, df = 4, p < 0.0001), residents with active fever (temperature ≥ 37.5 °C) at the time of visit (χ2 = 11.943, df = 4, p = 0.018), bednet users(χ2 = 16.355, df = 4, p = 0.003), and bednet non users (χ2 = 15.945, df = 4, p = 0.003) (Table 4). 28.9% (232/802) of caregivers of school-aged children aged 5 to 14 years old with fever sought treatment from drug shops, while 14.0% (112/802) sought treatment from public health facilities, with much lower proportions of children receiving fever treatment from traditional medication (2.9%, 23/802) and private health facility (4.4%, 35/802) (p < 0.0001). However, There was no significant difference in care givers' treatment seeking patterns for feverish children under the age of five (p = 0.086). Although the majority of children receive fever treatment at government facilities, the proportion of children seeking treatment varies by topography (p < 0.0001).
Residents with clinical malaria cases in the lakeshore zones sought treatment primarily from public hospitals (61.9%, 60/97) and purchased medication from drug shops (51.4%, 75/146) (p < 0.0001). Residents in the hillside zone with clinical malaria cases sought treatment primarily through traditional medication (51.1%, 23/45) (p < 0.0001). There was, however, no significant difference in treatment seeking patterns between Highland plateau residents with clinical malaria cases (p = 0.431) (Table 4).
Females with clinical malaria sought treatment primarily from traditional medications (50.9%, 27/53) and at public hospitals (43.8%, 64/146). Children, on the other hand, are unable to make treatment decisions on their own, and their treatment seeking pattern is determined by their parents/guardians (Table 4).
Majority of the clinical malaria cases were from school going children under traditional medication (60.9%, 14/23) and at the public hospital (51.8%, 58/112). Of the residents with active fever at the time of visits, clinical malaria cases were mostly from those who sought prior treatment from: public hospital (82.0%, 50/61) and those who did nothing (66.5%, 127/191) (Table 4).
Factors associated with decision to seek treatment
Artificial neural network model was used to identify the main factors associated with the decision to seek treatment. Independent variable importance analysis showed that distance (Importance = 0.184, normalized importance = 100%), severity of disease (Importance = 0.163, normalized importance = 88.7%), confidence in the treatment (Importance = 0.108, normalized importance = 58.5%), affordability (Importance = 0.100, normalized importance = 54.4%), availability of medication (Importance = 0.090, normalized importance = 49.1%), marital status (Importance = 0.072, normalized importance = 39.2%), health insurance (Importance = 0.057, normalized importance = 30.8%), malaria awareness (Importance = 0.054, normalized importance = 29.50%), socio-economic status (wall type: Importance = 0.054, normalized importance = 29.4% and floor type: Importance = 0.038, normalized importance = 20.70%), Knowledge of malaria (Importance = 0.034, normalized importance = 18.8%), net usage (Importance = 0.028, normalized importance = 15.0%) and gender (Importance = 0.018, normalized importance = 10.0%) were the main factors associated with decision to seek treatment. (Training: cross entropy error = 167.712, incorrect prediction = 25.1%; Testing: cross entropy error = 110.675; incorrect prediction = 35.4%) (Additional file 1: Table S1).
A subsequent analysis revealed a significant relationship between treatment seeking pattern and distance to the health facility (χ2 = 98.816, df = 4, p < 0.0001). Residents who reported distance to the health facility as a factor in their decision to seek treatment did nothing (34.4%, 65/188). Residents who reported that distance was not a factor in their decision to seek treatment, on the other hand, primarily sought treatment from private (31.6%, 67/212) and public hospitals (27.4%, 58/212) (Additional file 1: Table S2).
The severity of the diseases was significantly related to the treatment seeking preference (χ2 = 121.246, df = 4, p < 0.0001). Residents who said the severity of the malaria disease influenced their decision to seek treatment did so primarily through traditional medicine (26.2%, 67/256) and private hospitals (25.8%, 66/256). Residents who reported that the severity of the diseases was not a factor in their decision to seek treatment, on the other hand, largely did nothing (48.6%, 70/144) (Additional file 1: Table S2).
Residents' confidence in their treatment options was significantly related to their decision to seek treatment (χ2 = 33.442, df = 4, p < 0.0001). The majority of residents were confident in seeking treatment in a public hospital (25.1%, 55/219), traditional medication (24.7, 54/219), and private hospital (20.1%, 44/219). Residents who did nothing (31.5%, 57/181) and bought drugs from drug shops (20.4%, 37/181), on the other hand, reported having little confidence in their treatment option (Additional file 1: Table S2).
The cost of the medication had a significant impact on the decision to seek treatment. (χ2 = 80.640, df = 4, p < 0.0001). The majority of residents could easily afford treatment from traditional medications (27.2%, 68/250) and public hospitals (25.6%, 64/250). On the other hand, most residents could not afford medication from private hospitals (38.7%, 58/150) (Additional file 1: Table S2).
Furthermore, the availability of medication was strongly related to the decision to seek treatment (χ2 = 93.594, df = 4, p < 0.0001). The majority of residents stated that medication was easily accessible from traditional medication (29.4%, 67/228), drug shops (27.6%, 63/228), and private hospitals (20.6%, 47/228). Those who did nothing (38.4%, 66/172) and those who sought medication from public hospitals (25.0%, 43/172) both reported that medication was not readily available (Additional file 1: Table S2).
Discussion
The current study looked at clinical malaria incidences, treatment seeking profiles of febrile cases and factors associated with the decision to seek treatment in western Kenya. Clinical malaria incidence (cases/1000people/month) was highest in the lakeshore zone (24.3), followed by the hillside (18.7) and the highland plateau zone (10.3). The most common health-seeking behaviors among the 2205 febrile residents were doing nothing (48.7%) and purchasing antimalarial from local drug shops (38.1%). Malaria was present in the residents who did nothing, sought traditional medication, and purchased antimalarial from drug shops, and from public hospitals at the time of the visit. The decision to seek treatment was heavily influenced by the distance to the health facility, the severity of the disease, confidence in the treatment, affordability, and socioeconomic status. Furthermore, the current study observed that ultrasensitive malaria RDT diagnosis is highly specific (98.7%) and has good sensitivity (65.5%).
The high clinical malaria incidences and the positivity rates in the Lakeshore zone may be attributed to the area's flat plains and frequent flooding during the rainy seasons, resulting in water stagnation and the presence of permanent mosquito breeding habitats, as well as households' proximity to open water sources, which are stable larval habitats and potential mosquito breeding grounds. The findings corroborate previous research from western Kenya that found a high prevalence of malaria along the lake basin [28,29,30,31,32,33]. The primary economic activities in the current study region are subsistence farming and small-scale businesses such as fishing and rock mining. Residents' economic activities, such as night fishing and dusk small-scale businesses, may cause them to remain outside without protective measures, exposing themselves to mosquito bites. However, the current study did not investigate the relationship between economic activity and malaria burden.
In the current study, male in the lakeshore zones were more likely to contract malaria than females. This could be attributed to socioeconomic differences, with the majority of adult males engaging in nighttime outdoor activities that expose them to mosquito bites if no protective measures are taken [34, 35]. Females, on the other hand, were more likely to contract malaria in the hillside and highland plateau zones, most likely as a result of dusk activities such as selling vegetables and outdoor cooking at night, which exposes them to mosquito bites [36]. Females have pre-natal clinic appointments during pregnancy and frequently take their children to seek treatment, which may explain their high hospital seeking behavior and, as a result, their lower clinical malaria incidences when compared to males [37, 38]. Clinical malaria incidences were high among school-aged children aged 5–14 years in all study zones, according to the findings. Lower bednet usage among school-aged children exposes them to high mosquito bites at night, which may explain why clinical malaria incidences are higher in this age group [34, 39, 40]. The low infection rate among children under the age of five compared to school-age children could be attributed to the children being cared for by their parents and sleeping under mosquito nets at night [12, 41,42,43]. Children who sleep under insecticide-treated mosquito nets were less likely to contract malaria than those who did not sleep under bednets [41]. A similar study in Mozambique discovered that self-reported symptomatic malaria is extremely common among children, and that factors facilitating access to health care are associated with symptomatic malaria diagnosis [7]. Individuals in malaria-endemic areas develop adaptive immunity to the P. falciparum parasite, resulting in a decreasing rate of infection with age [44]. Similarly, a study in Burkina Faso linked increased fever cases among children to malaria infection (27). The current study, on the other hand, found that health seeking profiles did not differ by age group. Children, unlike adults, are unable to make treatment decisions on their own because their parents or guardians determine the treatment pattern [37, 38].
Individuals suspected of having malaria often start by doing nothing, then self-medicate with drugs from drug stores or traditional medications, and when the condition worsens, they seek treatment at health facilities. Doing nothing was most commonly reported among febrile residents, but when their febrile condition worsened, these residents were more likely to seek other alternative treatment options. In the current study, more than 80% of residents either self-medicate or do nothing when they have febrile illness, with only less than 20% seeking treatment in a health facility. In the current study, less than 20% of residents were reported to seek malaria treatment in health facilities, with an estimated 80% of febrile cases being underreported, with a proportion of whom could be malaria cases not being recorded in health facilities. A large proportion of the community does not seek treatment at health care facilities for a variety of reasons, including a lack of antimalarial in health facilities, the affordability of malaria diagnosis and distance to health facilities, confidence in the treatment, and socioeconomic status. As a result, approximately 31% of febrile cases self-diagnose and self-treat with drugs obtained from local drug shops located in nearly every shopping center. A Nigerian study found that approximately 88% of residents prefer to manage malaria at home, with only about 12% visiting health facilities [17]. The use of antimalarial drugs in the absence of a confirmed test is a major source of concern. Despite seeking treatment from drug stores and traditional medication, inappropriate treatment may have contributed to the observed higher clinical malaria cases in the current study.
Traditional medicine is commonly used to treat fever in African communities, especially during the early stages of illness or when the symptoms are mild [17, 21, 45]. According to the current study, the hillside zone, which is mostly hilly and has a lot of herbs and shrub plantation, explains why the majority of the residents are more likely to seek traditional medicine. Local herbs are more accessible and affordable because they can be obtained from the fields or traditional healers. According to studies in the Democratic Republic of the Congo, Guinea, and Kenya, traditional healers have a good understanding of malaria symptoms and causes, resulting in consistent knowledge of antimalarial plants [19,20,21]. It has been reported that herbal medications are involved in parasite clearance [17, 21, 45]. Furthermore, healthcare facilities in the hillside zone were scarce, which could explain the decision to seek traditional medication form of treatment. The current study, however, did correlate the availability of health facilities across topographies and treatment seeking profiles. The current study, however, did not follow up on the parasite clearance by traditional herbs. The study showed socio-economic status such as that the type of housing wall and the floor type, distance to medication access, and hospital payment method all influenced the decision to seek treatment. Residents from the lake zone, for example, were more likely to seek treatment in a public hospital and purchase antimalarial drugs from local drug stores. This was greatly influenced by the distance and ease of access. The severity of fever as a result of P. falciparum infection drives people to seek treatment [7] which is heavily influenced by accessibility, availability, and affordability of treatment services [22]. The current study residents reported taking analgesics to relieve pain before taking antimalarials, which may explain why there were fewer active fever cases in the study zone.
The rapid emergence and spread of the COVID-19 has resulted in massive global disruptions that are affecting people's lives and well-being. The devastation caused by the pandemic could be greatly exacerbated if the response jeopardizes the provision of life-saving malaria services [46]. COVID-19-related challenges have contributed to an increase in antimalarial and RDT stockout rates, resulting in a drop in test-and-treat policy adherence [16]. Reduced funding for vector interventions, combined with competing public health challenges such as the ongoing COVID-19 pandemic, may result in a rollback of malaria control gains, leading to increased morbidity and mortality from malaria [47,48,49]. Furthermore, fear and stigma were generated as a result of the COVID-19 situation. Fear of contracting COVID-19 in a health facility, for example, as well as the stigma of being tested for COVID-19 infection, influenced facility attendance. The number of people visiting health-care facilities decreased as a result of such concerns. The Kenya malaria indicator survey has also reported similar findings [50].
According to the current study, ultrasensitive malaria RDT diagnosis had a higher specificity (99%) and a good sensitivity (66%) in detecting malaria febrile cases. The study's findings confirm the high sensitivity of the ultrasensitive malaria RDT when compared to RT-PCR, as previously reported [51,52,53]. Malaria intervention strategies are dependent on whether malaria patients can easily access and afford appropriate diagnosis and treatment. To reduce the complication of malaria cases, the government should invest in supportive supervision of CHVs as well as the provision of more sensitive RDTs and antimalarial to strengthen community malaria case management.
Conclusion
Malaria case treatment-seeking habit is critical in determining malaria infection at the community level. Despite high bednet coverage, the current study found that the community has a high rate of clinical malaria incidences and positivity rates with the lakeshore zones bearing the greatest burden. The number of febrile cases is high because only about 20% of residents seek diagnosis and treatment in health care facilities, while the other 80% self-medicate or do nothing. These health seeking behavior suggests that a portion of the community's reported 80% of febrile cases may be infected with malaria but not reported in the Kisumu's monthly DHIS-2 reporting system. More research should be done to determine the true number of malaria-infected people who aren't reported in the DHIS-2. This information will help the Ministry of Health strengthen its community case management strategy for malaria.
Availability of data and materials
The dataset used in this study is available from the corresponding author upon request.
Abbreviations
- CI:
-
Confidence Interval
- CHV:
-
Community Health Volunteer
- DBS:
-
Dried blood spots
- DNA:
-
Deoxyribonucleic acid
- ICEMR:
-
International Center of Excellence for Malaria Research
- RDT:
-
Rapid diagnosis test
- RT-PCR:
-
Real-time polymerase chain reaction
References
National Malaria Control Programme Ministry of Health. The Kenya Malaria Communication Strategy 2016–2021. 2016.
Kenya Malaria Control Programme. Kenya Malaria Programme Review 2018. Nairobi, Kenya, and Rockville, Maryland, USA: NMCP, KNBS, and ICF International; 2019.
Kenya Malaria Control Programme. The Kenya Malaria Strategy 2019–2023. 2019.
National Malaria Control Programme. Kenya Malaria Indicator Survey 2015. Nairobi, Kenya, and Rockville, Maryland, USA: NMCP, KNBS, and ICF International; 2016.
Githinji S, Oyando R, Malinga J, Ejersa W, Soti D, Rono J, et al. Completeness of malaria indicator data reporting via the District Health Information Software 2 in Kenya, 2011–2015. Malar J. 2017;16:344.
Maina JK, Macharia PM, Ouma PO. Coverage of routine reporting on malaria parasitological testing in Kenya, 2015–2016. Glob Health Action. 2017;10.
Carlucci JG, Blevins Peratikos M, Cherry CB, Lopez ML, Green AF, González-Calvo L, et al. Prevalence and determinants of malaria among children in Zambézia Province, Mozambique. Malar J. 2017;16:1–13.
WHO. Guidelines for Malaria Vector Control [Internet]. World Health Organization. 2019.
Guerra M, De SB, Mabale NN, Berzosa P, Arez AP. Malaria determining risk factors at the household level in two rural villages of mainland Equatorial Guinea. Malar J. 2018;17:203.
Bannister-tyrrell M, Srun S, Sluydts V, Gryseels C, Mean V, Kim S, et al. Importance of household-level risk factors in explaining micro- epidemiology of asymptomatic malaria infections in Ratanakiri Province , Cambodia. Sci Rep. 2018;1–15.
Kwenti TE, TayongDizzleBitaKwenti AL, Njunda LA, Nkuo-Akenji T. Epidemiological and clinical profile of paediatric malaria: a cross sectional study performed on febrile children in five epidemiological strata of malaria in Cameroon. BMC Infect Dis. 2017;17:1–13.
Hajison PL, Feresu SA, Mwakikunga BW. Malaria in children under-five: a comparison of risk factors in lakeshore and highland areas, Zomba district, Malawi. PLoS ONE. 2018;13: e0207207.
Cohee L, Laufer M. Tackling malaria transmission in sub-Saharan Africa. Lancet Glob Heal. 2018;6:e598–9.
Mwakalinga VM, Sartorius BKD, Limwagu AJ, Mlacha YP, Msellemu DF, Chaki PP, et al. Topographic mapping of the interfaces between human and aquatic mosquito habitats to enable barrier targeting of interventions against malaria vectors. R Soc Open Sci. 2018;5: 161055.
Kenya Malaria Control Programme. National Guidelines for the diagnosis, treatment and prevention of malaria in Kenya. 2016.
U.S. President’s Malaria Initiative. Kenya Malaria Operational Plan FY 2022 [Internet]. 2022.
Dave-agboola IO, Raji J. Health-Seeking Behaviour of Malaria Patients in Lagos, Nigeria. Int J Heal Sci Res. 2018;8:259–64.
Otambo WO, Omondi CJ, Ochwedo KO, Onyango O, Atieli H, Ming-chiehLee CW, et al. Risk associations of submicroscopic malaria infection in lakeshore, plateau and highland areas of Kisumu County in western Kenya. PLoS ONE. 2022;17:e0268463.
Manya MH, Keymeulen F, Ngezahayo J, Bakari AS, Kalonda ME, Kahumba BJ, et al. Antimalarial herbal remedies of Bukavu and Uvira areas in DR Congo: an ethnobotanical survey. J Ethnopharmacol. 2020;249: 112422.
Traore M, Baldé M, Diallo M, Baldé E, Camara A, et al. Ethnobotanical survey on medicinal plants used by Guinean traditional healers in the treatment of malaria. J Ethnopharmacol. 2013;150:2013.
Gathwira JW, Rukunga GM, Mwitari PG, Mwikwabe MN, Kimani CW, Muthaura CN, et al. Traditional herbal antimalarial therapy in Kilifi district, Kenya. J Ethnopharmacol. 2010;134:434–42.
Karyana M, Devine A, Kenangalem E, Burdarm L, Poespoprodjo JR, Vemuri R, et al. Treatment—seeking behaviour and associated costs for malaria in Papua, Indonesia. Malar J. 2016;15:536.
Kisumu County. Kisumu County Integrated Development Plan II, 2018–2022 [Internet]. 2018.
Ministry of Health K. Interim guidelines on management of COVID-19 in Kenya. Repub Kenya. 2020;1–76.
WHO. WHO informal consultation on fever management in peripheral health care settings: a global review of evidence and practice [Internet]. Vol. 148. 2013.
Plowe CV, Djimde A, Bouare M, Doumbo O, Wellems TE. Pyrimethamine and proguanil resistance-conferring mutations in Plasmodium falciparum dihydrofolate reductase: polymerase chain reaction methods for surveillance in Africa. Am J Trop Med Hyg. 1995;52:565–8.
Veron V, Stephane S, Bernard C. Experimental parasitology multiplex real-time PCR detection of P. falciparum, P. vivax and P. malariae in human blood samples. Exp Parasitol. 2009;121:346–51.
Matsushita N, Kim Y, Ng CFS, Moriyama M, Igarashi T, Yamamoto K, et al. Differences of rainfall-Malaria associations in lowland and highland in Western Kenya. Int J Environ Res Public Health. 2019;16:19.
Amek N, Bayoh N, Hamel M, Lindblade KA, Gimnig JE, Odhiambo F, et al. Spatial and temporal dynamics of malaria transmission in rural Western Kenya. Parasit Vectors. 2012;5:86.
Matsushita N, Kim Y, Ng CFS, Moriyama M, Igarashi T, Yamamoto K, et al. Differences of rainfall–malaria associations in lowland and highland in western Kenya. Int J Environ Res Public Health. 2019;16:3693.
Dellicour S, Hill J, Bruce J, Ouma P, Marwanga D, Otieno P, et al. Effectiveness of the delivery of interventions to prevent malaria in pregnancy in Kenya. Malar J. 2016;15:1–13.
Ng’ang’a PN, Aduogo P, Mutero CM. Long lasting insecticidal mosquito nets (LLINs) ownership, use and coverage following mass distribution campaign in Lake Victoria basin, Western Kenya. BMC Public Health. 2021;21:1046.
Kapesa A, Kweka EJ, Zhou G, Atieli HE, Kamugisha E, Mazigo HD, et al. Utility of passive malaria surveillance in hospitals as a surrogate to community infection transmission dynamics in western Kenya. Arch Public Heal. 2018;76:1–11.
Ochwedo KO, Omondi CJ, Magomere EO, Olumeh JO, Debrah I, Onyango SA, et al. Hyper-prevalence of submicroscopic Plasmodium falciparum infections in a rural area of western Kenya with declining malaria cases. Malar J. 2021;20:427.
Diiro GM, Affognon HD, Muriithi BW, Wanja SK, Mbogo C, Mutero C. The role of gender on malaria preventive behaviour among rural households in Kenya. Malar J. 2016;15:14.
Jenkins R, Omollo R, Ongecha M, Sifuna P, Othieno C, Ongeri L, et al. Prevalence of malaria parasites in adults and its determinants in malaria endemic area of Kisumu County, Kenya. Malar J. 2015;14:263.
Franckel A, Lalou R. Health-seeking behaviour for childhood malaria: household dynamics in rural Senegal. J Biosoc Sci. 2009;41:1–19.
Budu E, Seidu AA, Armah-Ansah EK, Sambah F, Baatiema L, Ahinkorah BO. Women’s autonomy in healthcare decisionmaking and healthcare seeking behaviour for childhood illness in Ghana: analysis of data from the 2014 Ghana Demographic and Health Survey. PLoS ONE. 2020;15: e0241488.
Kamau A, Nyaga V, Bauni E, Tsofa B, Noor AM, Bejon P, et al. Trends in bednet ownership and usage, and the effect of bednets on malaria hospitalization in the Kilifi Health and Demographic Surveillance System (KHDSS): 2008–2015. BMC Infect Dis. 2017;17:720.
Minakawa N, Kongere JO, Sonye GO, Lutiali PA, Awuor B, Kawada H, et al. Long-lasting insecticidal nets incorporating piperonyl butoxide reduce the risk of malaria in children in Western Kenya: a cluster randomized controlled trial. Am J Trop Med Hyg. 2021;105:461–71.
Abossie A, Yohanes T, Nedu A, Tafesse W, Damitie M. Prevalence of malaria and associated risk factors among febrile children under five years: a cross-sectional study in arba minch zuria district, south Ethiopia. Infect Drug Resist. 2020;13:363–72.
Maketa V, Mavoko HM, Inocêncio R, Zanga J, Lubiba J, Kalonji A, et al. The relationship between Plasmodium infection, anaemia and nutritional status in asymptomatic children aged under five years living in stable transmission zones in Kinshasa, Democratic Republic of Congo. Malar J. 2015;14:83.
Roberts D, Matthews G. Risk factors of malaria in children under the age of five years old in Uganda. Malar J. 2016;15:246.
Agwu E, Ihongbe JC, Okogun GRA, Inyang NJ. High incidence of co-infection with malaria and typhoid in febrile HIV infected and AIDS patients in Ekpoma, Edo State, Nigeria Brazilian. J Microbiol. 2009;40:329–32.
Heng S, Durnez L, Mao S, Siv S, Tho S, Mean V, et al. Passive case detection of malaria in Ratanakiri Province (Cambodia) to detect villages at higher risk for malaria. Malar J. 2017;16:1–11.
WHO. Tailoring malaria interventions in the COVID-19 response [Internet]. 2020.
Ajayi IO, Ajumobi OO, Falade C. Malaria and COVID-19: commonalities, intersections and implications for sustaining malaria control. Pan Afr Med J. 2020;37:1–10.
Weiss DJ, Bertozzi-villa A, Rumisha SF, Amratia P, Arambepola R, Battle KE, et al. Indirect effects of the COVID-19 pandemic on malaria intervention coverage, morbidity, and mortality in Africa: a geospatial modelling analysis. Lancet Infect Dis. 2021;21:59–69.
Heuschen AK, Lu G, Razum O, Mumin AA, Sankoh O, Von SL, et al. Public health—relevant consequences of the COVID-19 pandemic on malaria in sub-Saharan Africa: a scoping review. Malar J. 2021;20:339.
Division of National Malaria Program. Kenya Malaria Indicator Survey 2020 [Internet]. Nairobi, Kenya and Rockville, Maryland, USA: DNMP and ICF; 2021.
Reichert EN, Hume JCC, Sagara I, Healy SA, Assadou MH, Guindo MA, et al. Ultra-sensitive RDT performance and antigen dynamics in a high-transmission Plasmodium falciparum setting in Mali. Malar J. 2020;19:323.
Danwang C, Kirakoya-Samadoulougou F, Samadoulougou S. Assessing field performance of ultrasensitive rapid diagnostic tests for malaria: a systematic review and meta-analysis. Malar J. 2021;20:245.
Acquah FK, Donu D, Obboh EK, Bredu D, Mawuli B, Amponsah JA, et al. Diagnostic performance of an ultrasensitive HRP2-based malaria rapid diagnostic test kit used in surveys of afebrile people living in Southern Ghana. Malar J. 2021;20:125.
Acknowledgements
We would like to thank the Nyakach Sub-County study participants for their participation in the study. We would like to express our gratitude to all the CHVs, and field and lab team members lead by Charles Omboko who worked tirelessly to complete this project.
Funding
This research is supported by grants from the National Institutes of Health (U19 AI129326 and D43 TW001505).
Author information
Authors and Affiliations
Contributions
WOO Conceptualization, designed the study, oversaw its implementation, performed laboratory assays, interpretations, analyses, drafted the original manuscript and edited and reviewed the final manuscript. KO, JO, SO, PO aided in the coordination of sample collection, conducted laboratory analysis and reviewing the manuscript. HA provided administrative support. MCL helped in designing the figure, CW, DZ and GZ contributed to study design, data analysis, editing and reviewing the manuscript, AG contributed to study design, editing and reviewing the manuscript. JG conceived the study design, reviewed and revised the manuscript. CO and PO provided input in data analysis, supervision, editing and reviewed the manuscript. GY contributed to study design, editing and review of the manuscript, and funded the project. JK contributed to study design and editing and reviewed the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Maseno University's Ethics Review Committee (MSU/DRPI/MUERC/00778/19) and the University of California, Irvine's Institutional Review Board (HS# 2017-3512) provided ethical approval for this study. The Kisumu County Director of Health (GN133VOLIX-413) and the Deputy County Commissioner (NYK/PH/13/1-200) gave their approval for the study to be conducted in the villages. The survey was open to all residents who were willing to participate in the study, regardless of their demographics. Residents who refused to participate or changed their willingness to participate at any time were excluded from participating. Before the study began, adults provided signed informed consent, and minors provided assents through their parents/guardians.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1
. Independent variable importance associated with decision to seek treatment. Table S2. Factors associated with decision to seek treatment.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Otambo, W.O., Onyango, P.O., Ochwedo, K. et al. Clinical malaria incidence and health seeking pattern in geographically heterogeneous landscape of western Kenya. BMC Infect Dis 22, 768 (2022). https://doi.org/10.1186/s12879-022-07757-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-022-07757-w