Abstract
Background
Infections caused by Klebsiella oxytoca are the second most common cause of Klebsiella infections in humans. Most studies have focused on K. oxytoca outbreaks and few have examined the broader clinical context of K. oxytoca.
Methods
Here, we collected all clinical isolates identified as K. oxytoca in a hospital microbiological diagnostic lab across a 15-month period (n = 239). Whole genome sequencing was performed on a subset of 92 isolates (all invasive, third-generation cephalosporin resistant (3GCR) and non-urinary isolates collected > 48 h after admission), including long-read sequencing on a further six isolates with extended-spectrum beta-lactamase or carbapenemase genes.
Results
The majority of isolates were sensitive to antimicrobials, however 22 isolates were 3GCR, of which five were also carbapenem resistant. Genomic analyses showed those identified as K. oxytoca by the clinical laboratory actually encompassed four distinct species (K. oxytoca, Klebsiella michiganensis, Klebsiella grimontii and Klebsiella pasteurii), referred to as the K. oxytoca species complex (KoSC). There was significant diversity within the population, with only 10/67 multi-locus sequence types (STs) represented by more than one isolate. Strain transmission was rare, with only one likely event identified. Six isolates had extended spectrum beta-lactamase (blaSHV−12 and/or blaCTX−M−9) or carbapenemase (blaIMP−4) genes. One pair of K. michiganensis and K. pasteurii genomes carried identical blaIMP−4 IncL/M plasmids, indicative of plasmid transmission.
Conclusion
Whilst antimicrobial resistance was rare, the resistance plasmids were similar to those found in other Enterobacterales, demonstrating that KoSC has access to the same plasmid reservoir and thus there is potential for multi-drug resistance. Further genomic studies are required to improve our understanding of the KoSC population and facilitate investigation into the attributes of successful nosocomial isolates.
Similar content being viewed by others
Background
The Klebsiella oxytoca species complex (KoSC) is a group of Gram-negative bacilli within the order Enterobacterales consisting of nine species [1,2,3] including Klebsiella michiganensis [4], Klebsiella grimontii [5] and Klebsiella pasteurii [6]. KoSC are the second most common Klebsiella group identified as the cause of clinical infections in humans after the Klebsiella pneumoniae species complex (KpSC) [7]. Like its sister complex KpSC, KoSC has the ability to persist in a variety of niches, including wet environments [8, 9], hostile environments such as handwashing soaps [10, 11], prosthetic material like central venous catheters [12], and within gastrointestinal flora [13], all of which contribute to its ability to cause opportunistic infections in healthcare settings.
Acquired antimicrobial resistance (AMR) is an emerging concern for KoSC. There are now several reports of multi-drug resistant (MDR) K. oxytoca outbreaks carrying either carbapenemases or extended-spectrum beta-lactamases (ESBLs), which confer third-generation cephalosporin resistance (3GCR) [1, 9, 14]. In part, this resistance is due to a large shared gene pool with KpSC [1] that can act as a potent source and reservoir of new AMR genes [15]. Additionally, 3GCR can emerge through point mutations in the promoter region of the chromosomal blaOXY gene (which normally confers only ampicillin resistance), leading to over-production and an ESBL phenotype [16, 17]. The most well-characterised virulence factors in KoSC is the kleboxymycin-biosynthetic gene cluster that encodes for the cytotoxin kleboxymycin or tilivallin, both of which are implicated in the pathological changes caused by antibiotic-associated haemorrhagic colitis [3]. Most of the remaining virulence information about KoSC has been extrapolated from established K. pneumoniae data [1], often without phenotypic evidence.
Despite its clinical relevance and the increasing concerns about AMR, little is known about the epidemiology and broader population structure of KoSC within healthcare institutions. Like KpSC, members of the KoSC remain difficult to distinguish both phenotypically and by matrix-assisted laser desorption/ionization mass spectrometry (MALDI–TOF) [11, 18], and as such are usually reported as K. oxytoca by clinical laboratories.
Here, we describe the clinical characteristics of a collection of 239 KoSC infection isolates from hospitalised patients within a single tertiary hospital network across a 15-month period. We sequenced a subset of 92 isolates, including all invasive isolates, to characterise their genetic diversity, assess for evidence of nosocomial transmission, and determine the genetic context of acquired AMR determinants.
Methods
Ethics
Approval was given by the Alfred Health Hospital Ethics Committee for prospective storage of clinical isolates for future research (HREC 533-16); and later for analysis of the K. oxytoca isolates and medical record review for the purpose of this study (HREC 169-19). A consent waiver was granted as the study utilised bacteria that were isolated during routine diagnostic procedures, and hospital records were reviewed only by those who would normally have access to them.
Specimen collection, identification and antimicrobial susceptibility testing
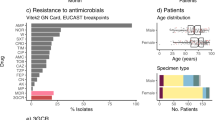
All KoSC isolated from clinical and screening specimens from 1 to 2018 until 7 April 2019 at the Alfred Hospital Microbiological Diagnostics Laboratory in Melbourne, Australia (serving four hospitals in the network), were eligible for inclusion (n = 239). Screening isolates were included to ensure we obtained the most complete picture of the KoSC population as was feasible. Bacterial identification was performed using MALDI–TOF (Vitek MS; bioMérieux, Marcy-l’Etoile, France). Antimicrobial susceptibility testing (AST) was performed in the clinical laboratory at the time of isolation using Vitek2 AST-246 (bioMérieux, Marcy-l’Etoile, France), with the exception of urinary isolates where initial disk diffusion susceptibility testing was performed. All MICs and zone of inhibitions were re-interpreted as per EUCAST 2021 guidelines for analysis in this study. Phenotypic blaOXY hyperexpression was identified via double disc synergy testing with ceftriaxone, ceftazidime and aztreonam +/- clavulanic acid, which demonstrated susceptibility to ceftazidime and resistance to ceftriaxone and aztreonam that was inhibited by clavulanic acid [19].
Clinical data collection
Clinical information was collected on all 239 isolates from the electronic medical record (EMR) (PowerChart, Cerner, Kansas City, USA). Information regarding microbiological investigations were recovered from the PathNet laboratory information system (LIS) (Cerner, Kansas City, USA). Details on all isolates can be found in Additional file 1: Table S1.
DNA extraction and sequencing
We selected 92 isolates (38% of the total collection) to sequence using the Illumina platform. This subset included (i) all invasive isolates and all isolates with acquired 3GCR (with the exception of a single isolate that could not be found in storage which was both from a blood culture and had acquired 3GCR, n = 39); (ii) all non-urinary isolates collected > 48 h after admission (n = 22); (iii) all urinary isolates collected > 48 h after admission with pyuria (WCC > 100 × 106/L, isolated in the absence of co-pathogens or flora, n = 6); (iv) all isolates collected during admission to the ICU (n = 14); and (v) all non-urinary isolates collected from outpatients or inpatients after < 48 h provided there had been an admission within the prior 12 months (n = 11). DNA was extracted from purified cultures using the Agencourt Genfind v2 kit (Beckman Coulter, Beverly, USA) with a paramagnetic based extraction protocol. Library preparation was performed using the Nextera DNA Flex kit (Illumina, San Diego, USA) and libraries were sequenced on the Illumina NextSeq. Six isolates (Kox26, Kox58, Kox71, Kox100, Kox101 and Kox205) were selected for additional long-read sequencing because they carried carbapenemase genes or were involved in suspected transmission events using Nanopore MinION (Oxford Nanopore Technologies, Oxford, UK) with a R9.4.1 flow cell as previously described [20]. Whole genome sequencing reads have been deposited under BioProject PRJNA781656 (see Additional file 1:Table S1 for individual accessions).
Genome assembly and pairwise comparisons
To generate draft genome assemblies for genotyping, Illumina reads were assembled using Unicycler v0.4.7 [21] with default parameters. Draft genome assemblies were generated for pairwise single nucleotide variant (SNV) analyses using SKESA v2.2.1 [22] with default parameters, because this comparatively conservative assembly approach removes lower depth sequence regions that are more likely to contain sequence inaccuracies. Catpac (https://github.com/rrwick/Catpac) was used to calculate pairwise SNVs for all pairs of genomes assigned the same ST. SNV counts ≤ 5 per Mbp were considered indicative of recent transmission, as previously described for the closely related organism K. pneumoniae [23].
The six genomes with ONT reads were assembled using Flye v2.8 [24] and polished using Medaka v1.4.3 (https://github.com/nanoporetech/medaka) using the r941_min_hac_g507 model, followed by Illumina polishing using Pilon v1.23 [25]. Completed plasmids were compared using BLASTn and comparisons were visualised using genoPlotR v0.8.1 [26]. All six completed genome assemblies have been deposited in GenBank, see Additional file 1:Table S1 for genome accessions.
Species confirmation, MLST, AMR, metal resistance and virulence gene screening
Kleborate v2.0.4 [27] was used to identify species, and calculate assembly quality control metrics. Multi-locus sequence types were determined using mlst v2.17 (https://github.com/tseemann/mlst) with the K. oxytoca scheme [28].
AMR and metal resistance genes were detected using AMRFinder Plus v3.10.5 [29], specifying species Klebsiella and the plus option. Putative pathogenicity and virulence loci were identified through a combination of tBLASTn search for homologues of the key K. pneumoniae pathogenicity and virulence determinants using the following loci from the SGH10 reference genome (GenBank accessions CP025080.1 and CP025081.1); SGH10_001765- SGH10_001775 (yersiniabactin, ybt, synthesis locus); SGH10_001728-SGH10_001744 (colibactin, clb, synthesis locus); SGH10_001661 (wzi, capsule synthesis locus marker); SGH10_001680-SGH10_001681 (wzm and wzt outer lipopolysaccharide synthesis locus markers); SGH10_005357-SGH10_005360 (salmochelin, iro, synthesis locus); SGH10_005387- SGH10_005391 (aerobactin, iuc, synthesis locus); SGH10_005363 (rmpA, regulator of mucoid phenotype gene). tBLASTn hits with ≥ 70% predicted amino acid identity and ≥ 95% query coverage were reported (thresholds determined by inspection of the empirical distribution). Additionally, Kaptive v0.7.3 [30] was used to screen for additional evidence of the capsule (K) and outer lipopolysaccharide (O) loci, which were recorded as present if (i) Kaptive identified a match to a locus in the K. pneumoniae database with ‘good’ or better confidence; and/or (ii) the wzi or wzm/wzt marker genes, respectively, were detected by tBLASTn. Putative yersiniabactin locus deletion variants were investigated by manual inspection of the corresponding genome assembly graph in Bandage v0.8.1 [31].
Identification of the kleboxymycin cluster was performed by screening all genome assemblies against the full locus (GenBank accession MF401554) using BLASTn. Genes with > 90% coverage and > 90% nucleotide identity were considered present. Genomes carrying all 12 genes in the locus were considered to harbour kleboxymycin.
Core genome phylogeny
All assemblies were annotated with Prokka v1.14.6 [32] and a pan-genome was generated using panaroo v1.2.4 [33] with default parameters. A total of 3,502 core genes were detected by panaroo and aligned, the resulting core genome alignment was used to create a core gene phylogeny using IQTree v2 [34] with a GTR + F + I + G4 substitution model. The phylogeny was midpoint rooted and visualised using the R package ggtree [35].
Results
KoSC contribute to a diverse range of infections
A total of 239 clinical and screening isolates of KoSC were identified by MALDI-TOF over the 15-month study period, in specimens from 206 individual patients. The patients were 60% male (n = 123) with a median age of 67 years (range 20–98) (Additional file 1:Table S1). Specimen types included urine (n = 126, 53%), respiratory (n = 45, 19%), wound (n = 26, 11%), blood (n = 19, 8%), tissues and fluids (n = 11, 4.6%), and other (n = 12, 5%). There were 219 non-sterile specimens with KoSC recovered, of these 51% (n = 112) had other microorganisms isolated, 44% of these were urine specimens. Twenty-six patients had multiple isolations of KoSC (median 2, range 2–4). Eighty percent (n = 191) of isolates were recovered from inpatients, 38% (n = 92) of these were collected > 48 h after admission. Of the 48 isolates collected from outpatients, 58% (n = 28) were from individuals with hospital admission or procedures in the prior 12 months. Amongst the 19 isolates recovered from blood, seven were from inpatients > 48 h after admission and nine were from individuals with hospitalisations or procedures in the prior 12 months. The vast majority of isolates were sensitive to antimicrobials except ampicillin, with only 15% (n = 22) of isolates displaying a 3GCR phenotype (MIC > 2 mg/L to ceftriaxone), of which five were also carbapenem resistant (MIC > 2 mg/L to meropenem).
Genomic analysis revealed species and substantial sequence type diversity
We sequenced a subset of 92 isolates from the full collection (38%), including all isolates associated with invasive, nosocomial and/or drug-resistant infections (see "Methods"). These isolates represented 31 respiratory specimens (34%), 20 urine (22%), 19 blood (21%), 12 wound/swab (13%), 7 tissue/sterile fluid (7%) and 3 other (3%). We detected four species from the KoSC complex in our collection—43 K. oxytoca (47%), 37 K. michiganensis (40%), 8 K. grimontii (9%), and 4 K. pasteurii (4%) (Fig. 1). Amongst the 21 invasive isolates (19 from blood, two from tissue/sterile fluid), we found all four species represented—K. oxytoca and K. michiganensis were the most common (n = 10 and n = 8 respectively). The remaining three isolates belonged to K. grimontii (n = 2) and K. pasteurii (n = 1).
Core genome phylogeny of the KoSC. Left, maximum-likelihood core genome phylogeny (midpoint rooted) of all 92 sequenced isolates in this study, with tips coloured by species. Scale indicates substitutions per site. STs with ≥ 2 members are labelled. Black stars indicate isolates with completed genomes. Heatmap shows presence (coloured) or absence (white) of the virulence loci kleboxymycin and yersiniabactin (as per legend), in addition to resistance to third-generation cephalosporins, key AMR mutations and genes (coloured by mutation type or resistance gene class as per legend). Barchart indicates total number of acquired AMR genes per isolate
A total of 67 distinct sequence types (STs) were identified, including 43 that were novel to this study. Fifty-seven STs had only a single representative isolate, and a further 10 STs were represented by ≥ 2 isolates each (Fig. 1, discussed further below).
Diversity of resistance, pathogenicity and virulence determinants
The vast majority of genomes (64%, n = 59/92) had no acquired AMR genes (Fig. 1). Of the remaining 33 genomes with at least 1 AMR gene, the majority (n = 24, 79%) carried only a chromosomal copy of aph(3’)-Ia (which confers aminoglycoside resistance) and no other acquired AMR genes (Fig. 1). These 24 genomes all belonged to K. michiganensis, and overall 70% (n = 26) of the K. michiganensis population in our study carried a chromosomal aph(3’)-Ia gene. Nine genomes carried > 1 AMR gene (median 9, range 2–23); six had acquired ESBL and/or carbapenemase genes, accounting for 46% of sequenced 3GCR isolates—one with blaIMP−4 only (Kox101), two with blaSHV−12 only (Kox58 and Kox100), one with blaIMP−4 and blaSHV−12 (Kox205), one with blaIMP−4 and blaCTX−M−9 (Kox26) and one with blaIMP−4, blaCTX−M−9 and blaSHV−12 (Kox71) (Fig. 1). Of these six isolates, all except Kox58 carried ≥ 9 AMR genes, conferring resistance to aminoglycosides, macrolides, sulphonamides, tetracyclines, trimethoprim and multiple beta-lactams (Fig. 1, Additional file 1:Table S1). Three of the six also carried mcr9.1, which has been associated with colistin resistance in certain circumstances but was not associated with resistance in these isolates [36]. Kox58 was an outlier, carrying only blaSHV−12 (plasmid-borne) and aph(3’)-Ia (chromosomal).
Nine isolates were recognised during phenotypic testing to over-express their chromosomal blaOXY gene, eight of these were sequenced (Additional file 1:Table S1). Over-expression leading to 3GCR has been previously associated with point mutations in the blaOXY promoter −10 motif at positions 1, 5, 8 and 12, and position 4 in the −35 motif [3, 16, 37]. We identified mutations at position 1 (G → T) and position 5 (G → A) of the −10 motif in 12 of our isolates (n = 4 G → T and n = 8 G → A) (Additional file 1:Table S1); all eight of the isolates identified as over-expressing their blaOXY gene during phenotypic testing carried one of these two promotor mutations. However, these mutations were not always associated with 3GCR. Of the eight 3GCR isolates with no acquired ESBL gene detected, six had blaOXY promoter mutations but two did not. Additionally, six isolates with blaOXY promoter mutations were not 3GCR (MIC range ≤ 1–< 2 mg/L). Of the five isolates with phenotypic carbapenem resistance, four carried the carbapenemase blaIMP−4, and one (Kox219) had no known carbapenemase detected. Overall, 86% (n = 12/14) of phenotypic 3GCR and 80% of carbapenem resistance in sequenced isolates could be explained by known resistance mechanisms.
The most well-known virulence factor in KoSC is the cytotoxin kleboxymycin. We screened for the full locus (12 genes) required for the production of this toxin (see "Methods"). The locus was present in 56% of genomes (n = 52); all K. pasteurii genomes (n = 4) carried the locus, as well as 87.5% (n = 7/8) K. grimontii genomes and 79% (n = 34/43) of K. oxytoca genomes (Fig. 1). Kleboxymycin was rarer in K. michiganensis, present in only 19% (n = 7/37) of genomes (Fig. 1). We also investigated the presence of factors with homology to key virulence determinants recognised for KpSC. We found a K locus (which encodes for the polysaccharide capsule) and an O locus (encoding the outer lipopolysaccharide O antigen) in all isolates, with 35% (n = 32/92) of K locus hits and 96% (n = 88/92) of O locus hits demonstrating good or very high similarity to previously described KpSC loci (Additional file 1: Table S1). Among the key KpSC acquired virulence loci, only yersiniabactin was detected; the full locus was present in 80% (n = 74/92) of genomes, and partially present in 3% (n = 3/92) (Fig. 1). All K. oxytoca and K. pasteurii genomes carried yersiniabactin, however it was variably present in K. michiganensis (70%) and K. grimontii (50%) genomes.
Limited evidence of strain and plasmid transmission
Ten STs (see Fig. 1) were represented by more than one isolate from either the same or different patients. We conducted a pairwise SNV analysis within each ST to identify putative transmissions between patients (defined as ≤ 5 SNVs per Mbp, see "Methods", n = 60 pairwise comparisons). The vast majority of pairs (88%, n = 53) differed by greater than > 100 SNVs (range 5–7066). Five pairs differed by 38–104 SNVs (Table 1); whilst this does not meet the cut-off for patient-to-patient transmission, it does indicate that these isolates may have been acquired from a common source. Two pairs of isolates differed by a total of ≤ 30 SNVs (equivalent to ≤ 5 SNVs per Mbp). One pair comprised two strains isolated from the same patient 87 days apart which differed by 6 SNVs (Kox170 and Kox211, ST266, Table 1). The other pair (Kox26 and Kox71, ST178, Table 1) differed by a total of 5 SNVs (0.8 SNVs per Mbp) and were isolated from two different patients 67 days apart, consistent with transmission. These patients were admitted to different wards, and were notable for both carrying a blaIMP−4 IncHI2A plasmid (see below).
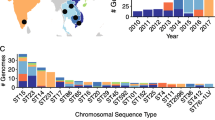
In order to determine if there was any evidence of ESBL/carbapenemase plasmid transmission within our hospital we generated completed plasmid sequences for all six isolates that harboured either type of enzyme. We found two distinct plasmids harbouring blaIMP−4 amongst four genomes (Fig. 2). Kox101 (K. michiganensis ST231) and Kox205 (K. pasteurii ST300) carried ~ 87 kbp blaIMP−4 IncL/M plasmids, which we named pKox101_2 and pKox205_2, respectively (accessions CP089409.1 and CP089405.1). The plasmid sequences were identical in both isolates, suggestive of plasmid transmission between these two species, which were isolated 28 weeks apart from different wards (Fig. 2a). The blaIMP-4 IncL/M plasmid carried eight additional AMR genes (aac(6’)-Ib4, aac(3)-IId, catB3, two copies of sul1, qnrB2, mph(A) and blaTEM-1), conferring resistance to six different antimicrobial drug classes (aminoglycosides, chloramphenicol, sulphonamides, quinolones, macrolides and penicillins). This blaIMP-4 IncL/M plasmid is identical to one previously sequenced from an Enterobacter genome in Sydney in 2012 (100% nucleotide identity, accession JX101693) [38].
Comparison of carbapenemase and ESBL plasmids. a Comparison of blaIMP−4 carrying IncL/M plasmids in ST231 and ST300. Blocks indicate genes, top row are genes on forward strand and bottom row are genes on reverse strand. Genes are coloured by type as per legend. Red block indicates > 99% nucleotide identity between plasmids. b Comparison of blaIMP−4 and blaSHV−12 IncHI2A plasmids in ST178 and ST278. Blocks indicate genes as in a, with genes coloured by type as per legend. Red and blue blocks indicate segments with > 99% nucleotide identity between pairs of plasmids, blue indicates the segment is in reverse orientation
The remaining two blaIMP-4 positive genomes (both K. oxytoca) carried blaIMP-4 on an ~ 300 kbp IncHI2A plasmid, along with a large number of other AMR genes (n = 31 in Kox71, n = 28 in Kox26), including the ESBLs blaSHV-12 and/or blaCTX-M-9 (Fig. 2b). Overall, the IncHI2A plasmids conferred resistance to nine antimicrobial drug classes, in addition to heavy metals including mercury. Both plasmids had zero SNVs between them (consistent with strain transmission described above, see Table 1), however there has been at least one inversion event and the loss of blaSHV-12 in Kox26 (Fig. 2b). A version of this IncHI2A plasmid lacking blaIMP-4 was detected in Kox100 (ST278), however it was significantly shorter (269 kbp vs. ~ 300 kbp in ST178), had 212 SNVs in comparison to the ST178 version, and had lost both blaIMP-4 and blaCTX-M-9 (though still harboured the ESBL blaSHV-12, see Fig. 2b). The Kox100 variant carried 12 additional AMR genes, conferring resistance to eight drug classes. Finally, Kox58 (K. michiganensis ST309) carried the ESBL blaSHV-12 on a 136 kpb IncFII plasmid that harboured silver and copper resistance operons, but zero additional AMR genes.
Discussion
To our knowledge, this is the first description of the population structure of a non-outbreak collection of isolates identified as K. oxytoca from a single hospital network. Isolates from urine were most common, followed by isolates from respiratory and wound specimens, similar to what has been observed for a collection of KpSC infections from the same hospital across a similar time period [39].
We preferentially sequenced hospital onset isolates, invasive isolates and isolates with acquired drug resistance, and found that isolates identified as K. oxytoca by the clinical laboratory instead represented four distinct species, with significant diversity within each. Amongst invasive isolates, we found that K. oxytoca and K. michiganensis were most common, however these species were also the most common across the whole collection. As we did not sequence a representative sample of isolates from all specimen types, we are unable to draw conclusions regarding differences in the rates of urinary tract infections and gastrointestinal carriage for the different species within the KoSC. Improvement in laboratory species identification in the KoSC will be key for bettering our understanding of the clinical syndromes and the likelihood of acquired resistance associated with each species. Models for correctly identifying KoSC species using MALDI-TOF now exist [40, 41], however these models aren’t yet implemented in the MALDI-TOF instrument databases. Whilst spectra can be exported from the instruments and analysed independently, this requires significant additional expertise.
Consistent with earlier studies, we found the virulence factor kleboxymycin gene cluster in all four species in our data, though not conserved in any one species [42]. We did not identify any K. pneumoniae virulence factors apart from yersiniabactin in our isolate collection, in contrast to some previous studies [1, 43]. Given our decision to focus on nosocomial isolates we may have been less likely to identify hypervirulent isolates associated with community-acquired infections, although we did include all invasive isolates with community onset. These findings suggest that these key K. pneumoniae virulence factors, apart from yersiniabactin, are uncommon in KoSC. Significantly more work is required to characterise novel virulence factors in KoSC and this will require both phenotypic and genotypic studies.
We observed a marked amount of genomic diversity in our sampled population, collected over a single year from one hospital. The amount of diversity found (where most STs were represented by a single genome) was similar to that found in an earlier study that examined a 10-year collection of K. oxytoca genomes from multiple clinical labs in the UK and Ireland [1]. Our collection did not include any of the globally distributed STs (ST2 or ST9) [44], or ST315 which was responsible for a K. michiganensis outbreak in a neonatal care unit in Australia [11]. There was limited evidence of strain transmission in our collection, with only one likely transmission event, of an isolate with an acquired MDR plasmid including blaIMP−4. However, this event involved patients that were admitted two months apart, and to separate wards, with no overlap in admission times. This may suggest an environmental reservoir or an unrecognised intermediate patient or healthcare worker with carriage or unsampled infection. This low frequency of identified transmission is consistent with previous work in K. pneumoniae demonstrating most infections are from a patient’s own flora [23]. Previous studies of KoSC have shown close relatedness between environmental and clinical specimens suggesting a possible environmental source for infection [1, 11]. We found a number of isolate pairs in our collection with > 30 but < 105 SNVs, consistent with transmission from an environmental source. Further work sequencing environmental and screening isolates from within the community and hospital will provide context to describe the relative contribution of environmental isolates to carriage and clinical infection and additionally allow for some assessment of candidate virulence factors.
Within our collection AMR was quite rare—only six isolates had acquired multi-drug resistance that included ESBLs. Notably four of these isolates had also acquired the carbapenemase blaIMP-4. Of these four carbapenemase isolates, two carried an identical IncL/M plasmid that has previously been described in Enterobacter cloacae from Sydney [38]. The remaining two isolates carried an IncHI2A plasmid that has been found to be circulating in multiple species within our hospital [36], and is highly similar to a plasmid previously described in Brisbane [45], consistent with the previously described widespread dissemination of blaIMP-4 within the Enterobacteriaceae along Australia’s east coast [46,47,48,49]. Internationally, IncHI2A plasmids carrying mcr-9 with blaIMP-4 or blaNDM-1 have also been described in Klebsiella species and other Enterobacteriaceae, suggesting that these plasmids are widespread [50,51,52]. Given that these plasmids are distributed across multiple species in the KoSC and STs, this suggests regular horizontal transfer of plasmids amongst species within this family.
This study provides insight into the clinical epidemiology and population structure of KoSC within a single hospital network, and has identified significant population diversity within what was identified by the clinical laboratory as simply K. oxytoca. K. oxytoca was not an uncommon pathogen in our hospital, and whilst AMR was rare, the MDR plasmids identified in our isolates indicate that KoSC has access to the same plasmid reservoir as KpSC, providing the potential for any isolate to become highly drug-resistant with the acquisition of a single plasmid. Future genomic studies will increase our understanding of both community and environmental population structures, which will allow us to further investigate virulence factors and other attributes of successful nosocomial isolates.
Availability of data and materials
All genome sequencing data generated for this study is available in the National Centre for Biotechnology Information (NCBI) database under BioProject PRJNA781656. Individual accessions can be found in Additional file 1: Table S1.
References
Moradigaravand D, Martin V, Peacock SJ, Parkhill J. Population structure of multidrug resistant Klebsiella oxytoca within hospitals across the UK and Ireland identifies sharing of virulence and resistance genes with K. pneumoniae. Genome Biol Evol. 2017;9:evx019.
Fevre C, Jbel M, Passet V, Weill F-X, Grimont PAD, Brisse S. Six groups of the OXY β-lactamase evolved over millions of years in Klebsiella oxytoca. Antimicrob Agents Ch. 2005;49:3453–62.
Yang J, Long H, Hu Y, Feng Y, McNally A, Zong Z. Klebsiella oxytoca complex: update on taxonomy, antimicrobial resistance, and virulence. Clin Microbiol Rev. 2021;35:e00006-21.
Saha R, Farrance CE, Verghese B, Hong S, Donofrio RS. Klebsiella michiganensis sp. nov., a new bacterium isolated from a tooth brush holder. Curr Microbiol. 2013;66:72–8.
Passet V, Brisse S. Description of Klebsiella grimontii sp. nov. Int J Syst Evol Micr. 2017;68:377–81.
Merla C, Rodrigues C, Passet V, Corbella M, Thorpe HA, Kallonen TVS, Zong Z, Marone P, Bandi C, Sassera D, Corander J, Feil EJ, Brisse S. Description of Klebsiella spallanzanii sp. nov. and of Klebsiella pasteurii sp. nov. Front Microbiol. 2019;10:2360.
Hansen DS, Gottschau A, Kolmos HJ. Epidemiology of Klebsiella bacteraemia: a case control study using Escherichia coli bacteraemia as control. J Hosp Infect. 1998;38:119–32.
Vergara-López S, Domínguez MC, Conejo MC, Pascual Á, Rodríguez‐Baño J. Wastewater drainage system as an occult reservoir in a protracted clonal outbreak due to metallo‐β‐lactamase‐producing Klebsiella oxytoca. Clin Microbiol Infect. 2013;19:E490–8.
Lowe C, Willey B, O’Shaughnessy A, Lee W, Lum M, Pike K, Larocque C, Dedier H, Dales L, Moore C, McGeer A, Team MSHIC. Outbreak of extended-spectrum β-Lactamase-producing Klebsiella oxytoca infections associated with contaminated handwashing sinks. Emerg Infect Dis. 2012;18:1242–7.
Dieckmann R, Hammerl JA, Hahmann H, Wicke A, Kleta S, Dabrowski PW, Nitsche A, Stämmler M, Dahouk SA, Lasch P. Rapid characterisation of Klebsiella oxytoca isolates from contaminated liquid hand soap using mass spectrometry, FTIR and Raman spectroscopy. Faraday Discuss. 2015;187:353–75.
Chapman P, Forde BM, Roberts LW, Bergh H, Vesey D, Jennison AV, Moss S, Paterson DL, Beatson SA, Harris PNA. Genomic investigation reveals contaminated detergent as the source of an ESBL-producing Klebsiella michiganensis outbreak in a neonatal unit. J Clin Microbiol. 2020;58:e01980-19.
Watson JT, Jones RC, Siston AM, Fernandez JR, Martin K, Beck E, Sokalski S, Jensen BJ, Arduino MJ, Srinivasan A, Gerber SI. Outbreak of catheter-associated Klebsiella oxytoca and Enterobacter cloacae bloodstream infections in an oncology chemotherapy center. Arch Intern Med. 2005;165:2639–43.
Herruzo R, Ruiz G, Gallego S, Diez J, Sarria A, Omeñaca F. VIM-Klebsiella oxytoca outbreak in a Neonatal Intensive Care Unit. This time it wasn’t the drain. J Prev Med Hyg. 2017;58:E302–7.
Decré D, Burghoffer B, Gautier V, Petit J-C, Arlet G. Outbreak of multi-resistant Klebsiella oxytoca involving strains with extended-spectrum β-lactamases and strains with extended-spectrum activity of the chromosomal β-lactamase. J Antimicrob Chemoth. 2004;54:881–8.
Wyres KL, Holt KE. Klebsiella pneumoniae as a key trafficker of drug resistance genes from environmental to clinically important bacteria. Curr Opin Microbiol. 2018;45:131–9.
Fournier B, Lu CY, Lagrange PH, Krishnamoorthy R, Philippon A. Point mutation in the pribnow box, the molecular basis of beta-lactamase overproduction in Klebsiella oxytoca. Antimicrob Agents Ch. 1995;39:1365–8.
Sato T, Hara T, Horiyama T, Kanazawa S, Yamaguchi T, Maki H. Mechanism of resistance and antibacterial susceptibility in extended-spectrum β-lactamase phenotype Klebsiella pneumoniae and Klebsiella oxytoca isolated between 2000 and 2010 in Japan. J Med Microbiol. 2015;64:538–43.
Stojowska-Swędrzyńska K, Krawczyk B. A new assay for the simultaneous identification and differentiation of Klebsiella oxytoca strains. Appl Microbiol Biot. 2016;100:10115–23.
Livermore DM. beta-Lactamases in laboratory and clinical resistance. Clin Microbiol Rev. 1995;8:557–84.
Wick RR, Judd LM, Gorrie CL, Holt KE. Completing bacterial genome assemblies with multiplex MinION sequencing. Microb Genom. 2017;3:e000132–e000132.
Wick RR, Judd LM, Gorrie CL, Holt KE. Unicycler: resolving bacterial genome assemblies from short and long sequencing reads. Plos Comput Biol. 2017;13:e1005595–e1005595.
Souvorov A, Agarwala R, Lipman DJ. SKESA: strategic k-mer extension for scrupulous assemblies. Genome Biol. 2018;19:153.
Gorrie CL, Mirceta M, Wick RR, Edwards DJ, Strugnell RA, Pratt N, Garlick J, Watson K, Pilcher D, McGloughlin S, Spelman DW, Jenney AWJ, Holt KE. Gastrointestinal carriage is a major reservoir of K. pneumoniae infection in intensive care patients. Clin Inf Dis. 2017;2:208–15.
Kolmogorov M, Yuan J, Lin Y, Pevzner PA. Assembly of long, error-prone reads using repeat graphs. Nat Biotechnol. 2019;37:540–6.
Walker BJ, Abeel T, Shea T, Priest M, Abouelliel A, Sakthikumar S, Cuomo CA, Zeng Q, Wortman J, Young SK, Earl AM. Pilon: an integrated tool for comprehensive microbial variant detection and genome assembly improvement. PLoS ONE. 2014;9:e112963.
Guy L, Kultima JR, Andersson SGE. genoPlotR: comparative gene and genome visualization in R. Bioinformatics. 2010;26:2334–5.
Lam MMC, Wick RR, Watts SC, Cerdeira LT, Wyres KL, Holt KE. A genomic surveillance framework and genotyping tool for Klebsiella pneumoniae and its related species complex. Nat Commun. 2021;12:4188.
Herzog KAT, Schneditz G, Leitner E, Feierl G, Hoffmann KM, Zollner-Schwetz I, Krause R, Gorkiewicz G, Zechner EL, Högenauer C. Genotypes of Klebsiella oxytoca isolates from patients with nosocomial pneumonia are distinct from those of isolates from patients with antibiotic-associated hemorrhagic colitis. J Clin Microbiol. 2014;52:1607–16.
Feldgarden M, Brover V, Haft DH, Prasad AB, Slotta DJ, Tolstoy I, Tyson GH, Zhao S, Hsu C-H, McDermott PF, Tadesse DA, Morales C, Simmons M, Tillman G, Wasilenko J, Folster JP, Klimke W. Validating the AMRFinder Tool and resistance gene database by using antimicrobial resistance genotype-phenotype correlations in a collection of isolates. Antimicrob Agents Ch. 2019;63:e00483-19.
Wyres KL, Wick RR, Gorrie C, Jenney A, Follador R, Thomson NR, Holt KE. Identification of Klebsiella capsule synthesis loci from whole genome data. Microb Genom. 2016;2:e000102–e000102.
Wick RR, Schultz MB, Zobel J, Holt KE. Bandage: interactive visualization of de novo genome assemblies. Bioinformatics. 2015;31:3350–2.
Seemann T. Prokka: rapid prokaryotic genome annotation. Bioinformatics. 2014;30:2068–9.
Tonkin-Hill G, MacAlasdair N, Ruis C, Weimann A, Horesh G, Lees JA, Gladstone RA, Lo S, Beaudoin C, Floto RA, Frost SDW, Corander J, Bentley SD, Parkhill J. Producing polished prokaryotic pangenomes with the Panaroo pipeline. Genome Biol. 2020;21:180.
Minh BQ, Schmidt HA, Chernomor O, Schrempf D, Woodhams MD, von Haeseler A, Lanfear R. IQ-TREE 2: new models and efficient methods for phylogenetic inference in the genomic era. Mol Biol Evol. 2020;37:1530–4.
Yu G, Smith DK, Zhu H, Guan Y, Lam TT. ggtree: an R package for visualization and annotation of phylogenetic trees with their covariates and other associated data. Methods Ecol Evol. 2017;8:28–36.
Macesic N, Blakeway LV, Stewart JD, Hawkey J, Wyres KL, Judd LM, Wick RR, Jenney AW, Holt KE, Peleg AY. Silent spread of mobile colistin resistance gene mcr-9.1 on IncHI2 ‘superplasmids’ in clinical carbapenem-resistant Enterobacterales. Clin Microbiol Infec. 2021;27:1856.e7-e13.
Fournier B, Gravel A, Hooper DC, Roy PH. Strength and regulation of the different promoters for chromosomal β-Lactamases of Klebsiella oxytoca Antimicrob Agents Ch. 1999;43:850–5.
Partridge SR, Ginn AN, Paulsen IT, Iredell JR. pEl1573 carrying blaIMP-4, from Sydney, Australia, is closely related to other IncL/M plasmids. Antimicrob Agents Ch. 2012;56:6029–32.
Gorrie CL, Mirčeta M, Wick RR, Judd LM, Lam MMC, Gomi R, Abbott IJ, Thomson NR, Strugnell RA, Pratt NF, Garlick JS, Watson KM, Hunter PC, Pilcher DV, McGloughlin SA, Spelman DW, Wyres KL, Jenney AWJ, Holt KE. Genomic dissection of Klebsiella pneumoniae infections in hospital patients reveals insights into an opportunistic pathogen. Nat Commun. 2021;13:3017.
Cuénod A, Wüthrich D, Seth-Smith HMB, Ott C, Gehringer C, Foucault F, Mouchet R, Kassim A, Revathi G, Vogt DR, von Felten S, Tschudin-Sutter Bassetti S, Hettich S, Schlotterbeck T, Homberger G, Casanova C, Moran-Gilad C, Sagi J, Rodríguez-Sánchez O, Müller B, Aerni F, Gaia M, Dessel V, van Kampinga H, Müller GA, Daubenberger C, Pflüger C, Egli V. Whole-genome sequence-informed MALDI-TOF MS diagnostics reveal importance of Klebsiella oxytoca group in invasive infections: a retrospective clinical study. Genome Med. 2021;13:150.
Bridel S, Watts SC, Judd LM, Harshegyi T, Passet V, Rodrigues C, Holt KE, Brisse S. Klebsiella MALDI TypeR: a web-based tool for Klebsiella identification based on MALDI-TOF mass spectrometry. Res Microbiol. 2021;172:103835.
Shibu P, McCuaig F, McCartney AL, Kujawska M, Hall LJ, Hoyles L. Improved molecular characterization of the Klebsiella oxytoca complex reveals the prevalence of the kleboxymycin biosynthetic gene cluster. Microb Genom. 2021;7:000592.
Jiang J, Tun HM, Mauroo NF, Ma APY, Chan SY, Leung FC. Complete genome sequence and comparative genome analysis of Klebsiella oxytoca HKOPL1 isolated from giant panda feces. BMC Res Notes. 2014;7:827.
Izdebski R, Fiett J, Urbanowicz P, Baraniak A, Derde LPG, Bonten MJM, Carmeli Y, Goossens H, Hryniewicz W, Brun-Buisson C, Brisse S, Gniadkowski M, Herda M, Dautzenberg MJ, Adler A, Kazma M, Navon-Venezia S, Malhotra-Kumar S, Lammens C, Legrand P, Chalfine A, Giamarellou H, Petrikkos GL, Balode A, Dumpis U, Stammet P, Aragăo I, Esteves F, Martí AT, Lawrence C, Salomon J, Paul M, Lerman Y, Rossini A, Salvia A, Samso JV, Fierro J. Phylogenetic lineages, clones and β-lactamases in an international collection of Klebsiella oxytoca isolates non-susceptible to expanded-spectrum cephalosporins. J Antimicrob Chemoth. 2015;70:3230–7.
Roberts LW, Catchpoole E, Jennison AV, Bergh H, Hume A, Heney C, George N, Paterson DL, Schembri MA, Beatson SA, Harris PNA. 2020. Genomic analysis of carbapenemase-producing Enterobacteriaceae in Queensland reveals widespread transmission of blaIMP-4 on an IncHI2 plasmid. Microb Genom 6.
Espedido BA, Partridge SR, Iredell JR. blaIMP-4 in different genetic contexts in Enterobacteriaceae isolates from Australia. Antimicrob Agents Ch. 2008;52:2984–7.
Leung GH, Gray TJ, Cheong EY, Haertsch P, Gottlieb T. Persistence of related blaIMP-4 metallo-beta-lactamase producing Enterobacteriaceae from clinical and environmental specimens within a burns unit in Australia—a six-year retrospective study. Antimicrob Resist Infect. 2013;2:35–35.
Peleg AY, Franklin C, Bell JM, Spelman DW. Dissemination of the metallo-beta-lactamase gene blaIMP-4 among gram-negative pathogens in a clinical setting in Australia. Clin Infect Dis. 2005;41:1549–56.
Sidjabat HE, Townell N, Nimmo GR, George NM, Robson J, Vohra R, Davis L, Heney C, Paterson DL. Dominance of IMP-4-Producing Enterobacter cloacae among carbapenemase-producing Enterobacteriaceae in Australia. Antimicrob Agents Ch. 2015;59:4059–66.
Liu Z, Hang X, Xiao X, Chu W, Li X, Liu Y, Li X, Zhou Q, Li J. Co-occurrence of blaNDM-1 and mcr-9 in a conjugative IncHI2/HI2A plasmid from a bloodstream infection-causing carbapenem-resistant Klebsiella pneumoniae. Front Microbiol. 2021;12:756201.
Faccone D, Martino F, Albornoz E, Gomez S, Corso A, Petroni A. Plasmid carrying mcr-9 from an extensively drug-resistant NDM-1-producing Klebsiella quasipneumoniae subsp. quasipneumoniae clinical isolate. Infect Genetics Evol. 2020;81:104273.
Sun L, Zhao X, Wang L, Guo X, Shi X, Hu L. Coexistence of mcr-9 and blaNDM-1 in a multidrug-resistant Enterobacter hormaechei strain recovered from a bloodstream infection in China. J Glob Antimicrob Re. 2021;24:440–2.
Acknowledgements
Not applicable.
Funding
This work was supported by a National Health and Medical Research Council of Australia Investigator Grant (APP1176192, awarded to KLW). It was also supported in part by the Bill & Melinda Gates Foundation [OPP1175797]. Under the grant conditions of the Foundation, a Creative Commons Attribution 4.0 Generic License has already been assigned to the Author Accepted Manuscript version that might arise from this submission. Funding for the genome sequencing was provided by an Alfred Hospital Research Trust Small Project Grant (T11938, awarded to JS).
Author information
Authors and Affiliations
Contributions
Conceptualization: JS, AJ; Formal analysis: JS, JH, KLW; Funding acquisition: JS, AJ, KEH; Investigation: JS, LMJ, JH, KLW; Resources: JS, AJ, LMJ; Supervision: AJ, JH, KLW; Visualization: JH, KLW; Writing (original draft): JS; Writing (review and editing): all authors. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Approval was given by the Alfred Health Hospital Ethics Committee for prospective storage of clinical isolates for future research (HREC 533 − 16); and later for analysis of the K. oxytoca isolates and medical record review for the purpose of this study (HREC 169 − 19). An informed consent waiver was granted by the Alfred Health Hospital Ethics Committee because of the retrospective nature of the study, and bacteria were isolated for routine diagnostic purposes. All methods were carried out in accordance with relevant guidelines and regulations.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1
. Details of all isolates included in this study. NA’s indicate that no sequence data was generated for these isolates, therefore genome information is not available.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Stewart, J., Judd, L.M., Jenney, A. et al. Epidemiology and genomic analysis of Klebsiella oxytoca from a single hospital network in Australia. BMC Infect Dis 22, 704 (2022). https://doi.org/10.1186/s12879-022-07687-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-022-07687-7