Abstract
Background
Healthcare workers (HCW) are at increased risk of infection with SARS-CoV-2. Vulnerable patient populations in particular must be protected, and clinics should not become transmission hotspots to avoid delaying medical treatments independent of COVID. Because asymptomatic transmission has been described, routine screening of asymptomatic HCW would potentially be able to interrupt chains of infection through early detection.
Methods
A systematic search was conducted in the Cochrane COVID-19 Study Register, Web of Science and WHO COVID‐19 Global literature on coronavirus with regard to non-incident related testing of healthcare workers using polymerase chain reaction on May 4th 2021. Studies since January 2020 were included. An assessment of risk of bias and representativeness was performed.
Results
The search identified 39 studies with heterogeneous designs. Data collection of the included studies took place from January to August 2020. The studies were conducted worldwide and the sample size of the included HCW ranged from 70 to 9449 participants. In total, 1000 of 51,700 (1.9%) asymptomatic HCW were tested positive for SARS-CoV-2 using PCR testing. The proportion of positive test results ranged between 0 and 14.3%. No study reported on HCW-screening related reductions in infected person-days.
Discussion and conclusions
The heterogeneous proportions might be explained by different regional incidences, lock-downs, and pre-analytical pitfalls that reduce the sensitivity of the nasopharyngeal swab. The very high prevalence in some studies indicates that screening HCW for SARS-CoV-2 may be important particularly in geographical regions and pandemic periods with a high-incidence. With low numbers and an increasing rate of vaccinated HCW, a strict cost–benefit consideration must be made, especially in times of low incidences. Since we found no studies that reported on HCW-screening related reductions in infected person-days, re-evaluation should be done when these are available.
Similar content being viewed by others
Introduction
To control the global SARS-CoV-2 pandemic, measures such as personal protective equipment (PPE), disinfection, virucidal gargling and nasal spray [1], window ventilation or mechanical ventilation systems, public restrictions such as business closures, contact and visitor restrictions, vaccination etc. are being used. The long-term effects of these measures, especially on social life and the economic situation are difficult to assess. HCW have an increased risk of infection due to their exposure and occupational intensity of contacts [2]. The possibility of asymptomatic infection in HCW increases the risk of nosocomial transmission to "non-COVID" patients and to other HCW [3]. Nosocomial infection or even unprotected exposure of HCW necessitates interruptions in their availability and aggravates any pre-existing shortage of HCW in specialised inpatient services. In addition, HCW might suffer from associated fears of infection, isolation, and transmission to their own families [4]. Ultimately, material shortages of PPE in the past meant that staff safety could not be guaranteed at all times. Nosocomial infections, which account for approximately 20% of patient and 89% of HCW infections with SARS-CoV-2 in the United Kingdom [5, 6], have been described as sometimes even having a more severe and complex course [7]. Therefore, many hospitals screen patients on admission, regardless of contacts or symptoms, while HCW are tested only when symptomatic. But the disease may present with minimal or no symptoms [8] and asymptomatic transmission has been described in up to 50% of cases [9]. Nosocomial infections account for 12–29% of these [10]. Similar numbers and durations of viral infection were observed as in symptomatic individuals [11, 12]. Considering these risks, regular routine screening of HCW would be a conceivable tool to control the pandemic as it may protect the hospital staff themselves and, in particular, the vulnerable patient populations from transmission by HCW [7].
Additionally HCW morale and mental health have been boosted by screening programs in past pandemics [13]. Hospitals have special roles in pandemics, as patients with serious comorbidities or new-onset diseases sometimes delay seeking medical treatment in fear of infection with SARS-CoV-2, which may worsen their prognosis [14]. Limitations to extend screening programs by also considering asymptomatic HCW include financial as well as capacity and logistical problems, and the risk of massive workforce losses if a considerable number of HCW are tested positive, sometimes also false-positive [15]. Thus, appropriate screening programs must be well considered and planned. We conducted a systematic review to summarise the existing literature on routine SARS-CoV-2 screening of HCW in acute care hospitals using PCR to demonstrate the usefulness of screening for HCW.
Methods
Systematic literature search
This systematic review is reported according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) 2020 guideline [16].
For the identification of studies systematic literature searches were performed by an information specialist and peer reviewed by a second information specialist.
On May 4th 2021 we searched for studies that screened for SARS-CoV-2 with PCR in HCW. The following sources were searched: the Cochrane COVID-19 Study Register (comprising MEDLINE, Embase, CENTRAL ClinicalTrials.gov, WHO ICTRP, medRxiv, RetractionWatch), Web of Science (Science Citation Index Expanded and Emerging Sources Citation Index) and WHO COVID‐19 Global Global literature on coronavirus. The search term included different variants of HCW, SARS-CoV-2 and PCR. The detailed search strategies are available as additional material (Additional file 1).
Five reviewers conducted a title and abstract screening. In a second step reports potentially meeting the inclusion criteria were read in full-text to finally decide for inclusion.
Inclusion and exclusion criteria
Inclusion criteria were (i) any HCW of any age and gender, without symptoms working in hospitals settings, (ii) non-cause-related screening for SARS-CoV-2 conducted by reverse transcriptase polymerase chain reaction (RT-PCR) testing (additional rapid test/serology was possible/allowed).
Cause-related testing was not excluded per se, but recorded separately, although this was not explicitly sought. The same applies to studies reporting on screening programmes in nursing homes or homecare services, which are also described, but not included for further analysis.
Outcomes considered were (i) reduction of infected person-days of HCW, (ii) and/or number of positive tested HCW (overall, asymptomatic).
Included study types were (i) randomized controlled trials (RCTs), (ii) non-RCTs (including quasi RCTs using inappropriate strategies of randomly allocating interventions), cross-sectional studies, cohort studies, controlled before-and-after studies, interrupted time series and (iii) any type of evidence synthesis (e.g., systematic reviews) if primary data were available or for identifying relevant additional studies.
Exclusion criteria were (i) testing of non-medical staff, (ii) performance of exclusively rapid tests / serology, (iii) exclusively cause-related screening (contacts, symptoms) for SARS-CoV-2 and (iv) any type of modelling studies.
Data extraction
The following data were extracted independently by the reviewers: (i) key study characteristics (bibliographical data, study design, geographical area where data were collected, period of data collection, mean age, gender and number of included HCW); (ii) Number tested, number positive tested asymptomatic, Reduction of infected person days; (iii) Setting [level 1: Primary Care (Primary Care Physician, Family Physician or Public Health Clinic); level 2: Specialty Physician Care (Specialist Physician); level 3: Hospital Care (Acute Care General Hospital or Ambulatory Surgical Center); level 4: Specialty Hospital Care (Specialty Acute Care Hospital], ward (ICU, emergency, regular); (iiii) relevant exclusion criteria.
Missing results were reported, but not included in further analysis.
Data analyses
For the meta-analysis, the R package meta (Version 4.18-0) was used [17, 18]. Proportions were calculated with exact binomial 95% confidence intervals (CI) and visualized using a forest plot, including a 95%-prediction interval to depict the range of proportions across the available and potential future studies. Higgins’ I2 was used to describe the estimated proportion of variability due to heterogeneity between studies rather than random error [19]. If appropriate, proportions were pooled using a random intercept logistic regression model [20].
Risk of bias and representativeness
The risk of bias and the representativeness of the results was assessed considering pre-defined criteria which were developed by our group based on other epidemiological research [21]. Thereby, risk of bias assessment was based on the completeness of data, i.e., whether all recruited HCW (whole study sample) were considered when data were analysed (low risk of bias) or whether data were missing (e.g. due to drop-outs; high risk of bias). Data representativeness based on the characteristics of the study sample; i.e., when a selected sample (e.g. HCW from a high-risk region) was considered to derive estimates, representativeness was judged as “low”, whereas data representativeness was judged as “high” when the study included a broad-ranging sample reflecting HCW worldwide.
Of note, for both data extraction and the methodological assessments, we relied on information provided in the individual study reports. If no judgment could be made owing to missing information (poor reporting), the corresponding item for risk of bias or data representativeness was classified as “unclear”.
Results
Study selection process
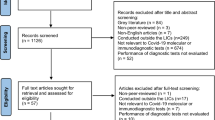
Figure 1 (PRISMA flowchart) presents the study selection process in detail [22].
The searches yielded 5218 records, of which 39 studies including 51,700 HCW met the inclusion criteria (reporting on non-cause-related screening of HCW).
In addition, we found eight studies that reported on cause-related testing, including 7.950 samples of HCW, which is described separately.
Study characteristics
Table 1 presents the details of the 39 included studies.
In short, data collection took place between January 2020 [47] and August 2020 [36] and sample sizes of PCR tested HCW ranged from 70 [32] to 9449 samples [30]. The studies were conducted in all six WHO defined regions (Africa, America, South-East Asia, Europe, Eastern Mediterranean and Western Pacific), most of the samples were taken in the USA (27,385 samples).
17 studies (n = 12,229) reported on mean age of HCW. Mean age ranged from 31.9 years [23] to 45.2 years [46] with an overall mean age of 40.6 years. 29 studies (n = 30,931) reported on gender distribution of tested HCW. Proportion of women ranged from 33 [24] to 84.2% [29], resulting in an overall proportion of 71.7%. Tested participants included doctors, nurses, allied health professionals, emergency first responders, healthcare assistants, physiotherapists, administrators, security guards, cleaning staff, food service workers and patient transporters. These were working in ICU, Emergency ward and Regular ward, 17 studies did not further report on the corresponding wards. 24 of the included studies used a cross-sectional design, 15 studies were based on cohorts (prospective cohort studies without control groups) and one study was a case series. RT-PCR testing was used in all studies. A total of 36 studies were conducted at acute care hospitals, three studies did not provide any information regarding the facilities’ level.
The studies on nursing homes, home care services and additional studies on cause-related testing are described in Tables 2 and 3, with no relevant differences in study characteristics compared to non-cause-related testing.
Outcomes reported
In total 1000 (1.9%) of 51,700 HCW were tested positive. Figure 2 presents a forest plot of the positive rate of asymptomatically tested HCW. We abstained from presenting a pooled estimate and a confidence interval because of the large between-study heterogeneity (I2 = 94.5% with 95% CI 93.3–95.5%).
Thereby, the proportion of positive test results of screened HCW ranged from 0.0% [24, 29, 32, 41, 42] to 14.3% [23] (Table 1). None of the studies reported infected person-days or reduction of these.
In the non-systematically considered studies reporting on cause-related testing of HCW, 782 of 7950 samples were positive, with the proportion of positive test results ranging from 1.9 to 34%. The four studies on screening of asymptomatic HCW in nursing homes and home care services reported on 77 positive test results in 14,857 tested individuals (0.5% in total, ranging from 0.002 to 13.3%).
Assessment of risk of bias and representativeness
The results of the respective assessments are shown in Table 4.
Discussion
This systematic review aimed to summarise the existing literature on routine SARS-CoV-2 screening of HCW in acute care hospitals. We identified 39 studies, which took place from January to August 2020 (first and second wave of the pandemic). A total of 1000 (1.9%) of 51,700 asymptomatic HCW tested positive for SARS-CoV-2. Individuals were positive in up to 14.3% of the tested individuals [23], the lowest detection rate was 0% [24, 29, 32, 41, 42].
The data on routine testing of HCW are heterogeneous and ambiguous, as the forest plot (Fig. 2) demonstrates. No underlying cause could be found, therefore pooling or subgroup analysis was not suitable. The varying numbers might be explained by regional differences in incidences and/or baseline features of the pandemic in the different countries. The SARS-CoV-2 pandemic has exhibited a substantial diachronous habit and therefore baseline features as well as measures such as lock-downs [15, 72], or in general surveillance efforts might have inflated or conversely deflated local incidence rates. The included studies collected their data from January 2020 during the first COVID-19 wave, until August 2020, hence effects of vaccination will not yet have impacted the results.
In general, higher positive rates among asymptomatic HCW can be expected if incidence increases in the overall population due to a higher probability of exposure to SARS-CoV-2 positive close contacts outside the hospital setting. This was confirmed by the study of Shields et al. in which the parallel determination of SARS-CoV-2 immunglobulin-G showed high rates of expired infections, contrasting very low detection rates of positives in RT-PCR [73]. But in the context of low circulation of the virus screening of asymptomatic HCW was poorly effective in the identification of virus-spreading HCW [74]. On the opposite, the highest proportion of asymptomatic patients is detectable in Egypt, which could be seen as representative for countries with younger demographic structures and a high incidence in the population [23]. In cases of such immensely high detection rates, early detection may be able to prevent a relevant proportion of transmissions, especially if high incidences are associated with a low hygiene adherence. In high-prevalence regions and situations, screening of asymptomatic HCW could therefore be a useful and recommendable additional measure to established prevention strategies. A modelling study concluded that weekly screening of asymptomatic staff in an emergency department could reduce new HCW and patient infections by 5.1% within 30 days (Assuming a constant 1.2 new infections per 10,000 persons) and by 21.1% within 30 days at higher incidences (Assuming a constant 3.7 new infections per 10,000 persons) [75]. The associated risk of transmission to vulnerable patient groups by HCW as well as the more severe course described for nosocomial transmissions should also be considered. While the stringent use of PPE not only protects the HCW but also close contact patients, this barrier is not unbreachable since in clinical practice adherence to the complex prevention bundle is not expected to reach 100% [76].
Regarding risk of bias assessment RT-PCR as an objective method and gold standard for the diagnosis of SARS-CoV-2 was used as an assessment tool of infection in all studies. Nevertheless, preanalytics, which can significantly reduce sensitivity of the test, must be considered [77]. These were not reported in detail in particular, neither transport routes nor the qualifications of the samplers were listed. Testing scenarios in level 3 and 4 facilities were predominant, thus limiting data representativeness of the entire global population and facilities of other levels, especially level 1 (primary care) and level 2 (specialist physician).
Additionally, we non-systematically found studies reporting on cause-related testing of HCW, showing higher detection rates (9.8% vs. 1.9%). Due to higher pre-test probability, those numbers are not surprising. However, given the fact our initial search for relevant literature did not focus on this population, our results lack representativeness. The same applies to our results on HCW in nursing homes and home care providers, showing a lower proportion of positive tested compared to HCW working in hospitals (0.5% vs. 1.9%).
At the time the included studies took place, no vaccine was yet available for widespread use.
Currently, the majority of HCW in developed countries are vaccinated against SARS-CoV-2. However, the benefits of screening regimens among asymptomatically vaccinated individuals are even more unclear due to the lower and shorter infectivity [78], but possibly an inverse effect through an increased feeling of safety, and lower prevalence of COVID-19 among vaccinated individuals [79]. Emerging variants of SARS-CoV-2 like Omicron with possibly reduced vaccine effectiveness [80], as well as the continued development of vaccines and test methods could influence the usefulness of those prevention and control strategies in the near future. Rapid PCR tests [81] and PCR mass tests [82] have been developed, but cannot be used on a regular and widespread base yet, because they require a high logistical effort.
At the time the systematic review was conducted, there was no evidence screening for HCW can lead to reduced transmission rates. However, asymptomatic SARS-CoV-2 carriers can lead to transmission [83, 84]. Thus, it is plausible that screening in vulnerable areas may subsequently lead to a reduction in infected person days. If unscreened asymptomatic SARS-CoV-2 positive HCW continue working, transmission to patients and staff could occur, resulting in relevant staff absences that may compromise medical care. The current state of evidence, however, does not firmly support unconditional HCW screening. From a public health perspective screening asymptomatic HCW e.g. several times a week is a costly exercise with unknown effect on transmission rates, in particular since standard infection control measures such as wearing medical masks—namely surgical masks or FFP2/KN95/N95 masks—were commonly implemented in hospital settings worldwide during the pandemic (Additional file 1).
In total, asymptomatic SARS-CoV-2 infections were detected in a relatively small proportion of HCW; accordingly, in times of low incidence strict trade-offs must be made in terms of feasibility and cost-effectiveness. Unfortunately, we did not find trials evaluating endpoints such as reduction in nosocomial infected person-days. In addition, up until completion of this review, no planned or ongoing trials with this outcome were registered at clinicaltrials.gov.
Currently there are two ongoing studies registered on clinicaltrials.gov investigating how COVID-19 spreads among HCW (ClinicalTrials.gov Identifier: NCT04574765, NCT04370119). We are looking forward to the results of these studies.
Conclusions
Asymptomatic infections of HCW and a possible associated risk of transmission to vulnerable patient populations may impact patient safety. Additionally, reducing nosocomial transmission between HCW is important in pandemic control since staff absences impact healthcare for all patients negatively and in particular SARS-CoV-2 patients needing mechanical ventilation. Our findings indicate that asymptomatic infections in HCW vary widely. Screening HCW for SARS-CoV-2 at regular intervals thus seems reasonable in times and regions of higher incidence. However, no certain incidence level can currently be determined for starting routine screening in a cost-effective way. Clinical studies investigating the reduction of infected person-days by routine screening are currently lacking. In particular since new variants of SARS-CoV-2 will continue to appear that might change transmission dynamics, implementing surveillance in critical structures such as the healthcare sector seems nevertheless appropriate.
Availability of data and materials
All the data generated and/or analysed are included in this published article and its additional information files.
Abbreviations
- HCW:
-
Healthcare worker
- RT-PCR:
-
Reverse transcriptase polymerase chain reaction
- PPE:
-
Personal protective equipment
- WHO:
-
World Health Organisation
- RCT:
-
Randomized controlled trial
- ICU:
-
Intensive care unit
References
Kramer A, Eggers M, Hübner N-O, Walger P, Steinmann E, Exner M. Virucidal gargling and virucidal nasal spray. GMS Hyg Infect Control. 2021;16: Doc02. https://doi.org/10.3205/dgkh000373.
Alhazzani W, Møller MH, Arabi YM, Loeb M, Gong MN, Fan E, et al. Surviving sepsis campaign: guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID-19). Crit Care Med. 2020;48:e440–69. https://doi.org/10.1097/CCM.0000000000004363.
Abbas M, Robalo Nunes T, Martischang R, Zingg W, Iten A, Pittet D, Harbarth S. Nosocomial transmission and outbreaks of coronavirus disease 2019: the need to protect both patients and healthcare workers. Antimicrob Resist Infect Control. 2021;10:7. https://doi.org/10.1186/s13756-020-00875-7.
Joseph B, Joseph M. The health of the healthcare workers. Indian J Occup Environ Med. 2016;20:71–2. https://doi.org/10.4103/0019-5278.197518.
Evans S, Agnew E, Vynnycky E, Robotham J. The impact of testing and infection prevention and control strategies on within-hospital transmission dynamics of COVID-19 in English hospitals. medRxiv. 2020. https://doi.org/10.1101/2020.05.12.20095562.
Iacobucci G. COVID-19: doctors sound alarm over hospital transmissions. BMJ. 2020;369:m2013. https://doi.org/10.1136/bmj.m2013.
McMichael TM, Currie DW, Clark S, Pogosjans S, Kay M, Schwartz NG, et al. Epidemiology of COVID-19 in a long-term care facility in King County, Washington. N Engl J Med. 2020;382:2005–11. https://doi.org/10.1056/NEJMoa2005412.
World Health Organisation. Report of the WHO-China joint mission on coronavirus disease 2019 (COVID-19); 2020.
He X, Lau EHY, Wu P, Deng X, Wang J, Hao X, et al. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat Med. 2020;26:672–5. https://doi.org/10.1038/s41591-020-0869-5.
Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–9. https://doi.org/10.1001/jama.2020.1585.
Lin A, He Z-B, Zhang S, Zhang J-G, Zhang X, Yan W-H. Early risk factors for the duration of severe acute respiratory syndrome coronavirus 2 viral positivity in patients with coronavirus disease 2019. Clin Infect Dis. 2020;71:2061–5. https://doi.org/10.1093/cid/ciaa490.
Zhou R, Li F, Chen F, Liu H, Zheng J, Lei C, Wu X. Viral dynamics in asymptomatic patients with COVID-19. Int J Infect Dis. 2020;96:288–90. https://doi.org/10.1016/j.ijid.2020.05.030.
McAlonan GM, Lee AM, Cheung V, Cheung C, Tsang KWT, Sham PC, et al. Immediate and sustained psychological impact of an emerging infectious disease outbreak on health care workers. Can J Psychiatry. 2007;52:241–7. https://doi.org/10.1177/070674370705200406.
Lazzerini M, Barbi E, Apicella A, Marchetti F, Cardinale F, Trobia G. Delayed access or provision of care in Italy resulting from fear of COVID-19. Lancet Child Adolesc Health. 2020;4:e10–1. https://doi.org/10.1016/S2352-4642(20)30108-5.
Rivett L, Sridhar S, Sparkes D, Routledge M, Jones NK, Forrest S, et al. Screening of healthcare workers for SARS-CoV-2 highlights the role of asymptomatic carriage in COVID-19 transmission. Elife. 2020. https://doi.org/10.7554/eLife.58728.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372: n71. https://doi.org/10.1136/bmj.n71.
R Core Team. R: a language and environment for statistical computing.
Balduzzi S, Rücker G, Schwarzer G. How to perform a meta-analysis with R: a practical tutorial. Evid Based Ment Health. 2019;22:153–60. https://doi.org/10.1136/ebmental-2019-300117.
Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–58. https://doi.org/10.1002/sim.1186.
Stijnen T, Hamza TH, Ozdemir P. Random effects meta-analysis of event outcome in the framework of the generalized linear mixed model with applications in sparse data. Stat Med. 2010;29:3046–67. https://doi.org/10.1002/sim.4040.
Munn Z, Moola S, Lisy K, Riitano D, Tufanaru C. Methodological guidance for systematic reviews of observational epidemiological studies reporting prevalence and cumulative incidence data. Int J Evid Based Healthc. 2015;13:147–53. https://doi.org/10.1097/XEB.0000000000000054.
Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6: e1000097. https://doi.org/10.1371/journal.pmed.1000097.
Abdelmoniem R, Fouad R, Shawky S, Amer K, Elnagdy T, Hassan WA, et al. SARS-CoV-2 infection among asymptomatic healthcare workers of the emergency department in a tertiary care facility. J Clin Virol. 2021;134: 104710. https://doi.org/10.1016/j.jcv.2020.104710.
Al-Zoubi NA, Obeidat BR, Al-Ghazo MA, Hayajneh WA, Alomari AH, Mazahreh TS, et al. Prevalence of positive COVID-19 among asymptomatic health care workers who care patients infected with the novel coronavirus: a retrospective study. Ann Med Surg (Lond). 2020;57:14–6. https://doi.org/10.1016/j.amsu.2020.06.038.
Armin S, Karbasian F, Hoseinialfatemi SM, Mansour Ghanaie R, Rafiei Tabatabaei S, Fahimzad SA, et al. Prevalence of SARS-CoV-2 specific antibodies in the staff of a Children’s Hospital, in Tehran, Iran. Jundishapur J Microbiol. 2020. https://doi.org/10.5812/jjm.108592.
Brown CS, Clare K, Chand M, Andrews J, Auckland C, Beshir S, et al. Snapshot PCR surveillance for SARS-CoV-2 in hospital staff in England. medRxiv. 2020. https://doi.org/10.1101/2020.06.14.20128876.
Campbell M, Datta R, Wyllie A, Casanovas-Massana A, Handoko R, Sewanan L, et al. 493. Clinical and epidemiological features of healthcare workers detected with coronavirus disease. Open Forum Infectious Dis. 2020;7:S313–S313. https://doi.org/10.1093/ofid/ofaa439.686.
Cavicchiolo ME, Trevisanuto D, Lolli E, Mardegan V, Saieva AM, Franchin E, et al. Universal screening of high-risk neonates, parents, and staff at a neonatal intensive care unit during the SARS-CoV-2 pandemic. Eur J Pediatr. 2020;179:1949–55. https://doi.org/10.1007/s00431-020-03765-7.
Demmer RT, Ulrich AK, Wiggen TD, Strickland A, Naumchik BM, Kulasingam S, et al. Severe acute respiratory coronavirus virus 2 (SARS-CoV-2) screening among symptom-free healthcare workers. Infect Control Hosp Epidemiol. 2021. https://doi.org/10.1017/ice.2021.81.
Dillner J, Elfström KM, Blomqvist J, Engstrand L, Uhlén M, Eklund C, et al. Screening for high amounts of SARS-CoV-2 identifies pre-symptomatic subjects among healthy healthcare workers. medRxiv. 2020. https://doi.org/10.1101/2020.12.13.20248122.
Fakhim H, Nasri E, Aboutalebian S, Gholipour S, Nikaeen M, Vaezi A, et al. Asymptomatic carriers of coronavirus disease 2019 among healthcare workers in Isfahan, Iran. Fut Virol. 2021;16:93–8. https://doi.org/10.2217/fvl-2020-0224.
Favara DM, Cooke A, Doffinger R, Houghton S, Budriunaite I, Bossingham S, et al. First results from the UK COVID-19 Serology in Oncology Staff Study (CSOS). medRxiv. 2020. https://doi.org/10.1101/2020.06.22.20136838.
Ferreira VH, Chruscinski A, Kulasingam V, Pugh TJ, Dus T, Wouters B, et al. Prospective observational study and serosurvey of SARS-CoV-2 infection in asymptomatic healthcare workers at a Canadian tertiary care center. PLoS ONE. 2021;16: e0247258. https://doi.org/10.1371/journal.pone.0247258.
Fusco FM, Pisaturo M, Iodice V, Bellopede R, Tambaro O, Parrella G, et al. COVID-19 among healthcare workers in a specialist infectious diseases setting in Naples, Southern Italy: results of a cross-sectional surveillance study. J Hosp Infect. 2020;105:596–600. https://doi.org/10.1016/j.jhin.2020.06.021.
Guery R, Delaye C, Brule N, Nael V, Castain L, Raffi F, de Decker L. Limited effectiveness of systematic screening by nasopharyngeal RT-PCR of medicalized nursing home staff after a first case of COVID-19 in a resident. Med Mal Infect. 2020;50:748–50. https://doi.org/10.1016/j.medmal.2020.04.020.
Halbrook M, Gadoth A, Martin-Blais R, Grey A, Contreras D, Kashani S, et al. Incidence of SARS-CoV-2 infection among asymptomatic frontline health workers in Los Angeles County, California. medRxiv. 2020. https://doi.org/10.1101/2020.11.18.20234211.
Handal N, Whitworth J, Blomfeldt A, Espvik HJ, Lysaker E, Berdal JE, Bakken JS. Comparison of SARS-CoV-2 infections in healthcare workers with high and low exposures to COVID-19 patients in a Norwegian University Hospital. Infect Dis (Lond). 2021;53:420–9. https://doi.org/10.1080/23744235.2021.1885734.
Hellewell J, Russell TW, Beale R, Kelly G, Houlihan C, Nastouli E, Kucharski AJ. Estimating the effectiveness of routine asymptomatic PCR testing at different frequencies for the detection of SARS-CoV-2 infections. BMC Med. 2021;19:106. https://doi.org/10.1186/s12916-021-01982-x.
Hidayat R, Aini N, Ilmi AFN, Azzahroh F, Giantini A. Test, trace, and treatment strategy to control COVID-19 infection among hospital staff in a COVID-19 referral hospital in Indonesia. Acta Med Indones. 2020;52:206–13.
Horton LE, Taplitz R, Torriani FJ, Abeles SR, Ikeda L, Ikeda T. 437. Asymptomatic healthcare worker COVID-19 Testing Program. Open Forum Infect Dis. 2020;7:S286–7. https://doi.org/10.1093/ofid/ofaa439.630.
Huang FS, Schaffzin JK, Simmons J, Goebel MJ, Thrasher T, Wong H, Macaluso M. 463. Random sampling of asymptomatic hospital employees: a period prevalence study. Open Forum Infect Dis. 2020;7:S298–9. https://doi.org/10.1093/ofid/ofaa439.656.
Jameson AP, Biersack MP, Sebastian TM, Jacques LR. SARS-CoV-2 screening of asymptomatic healthcare workers. Infect Control Hosp Epidemiol. 2020;41:1229–31. https://doi.org/10.1017/ice.2020.361.
Johnson CC, Coleman CM, Sitarik AR, Leon JE, Tibbetts RJ, Cook BC, et al. SARS-CoV-2 RT-PCR positivity and antibody prevalence among asymptomatic hospital-based health care workers. J Clin Virol. 2021;140: 104794. https://doi.org/10.1016/j.jcv.2021.104794.
Kantele A, Lääveri T, Kareinen L, Pakkanen SH, Blomgren K, Mero S, et al. SARS-CoV-2 infections among healthcare workers at Helsinki University Hospital, Finland, spring 2020: serosurvey, symptoms and risk factors. Travel Med Infect Dis. 2021;39: 101949. https://doi.org/10.1016/j.tmaid.2020.101949.
Kassem AM, Talaat H, Shawky S, Fouad R, Amer K, Elnagdy T, et al. SARS-CoV-2 infection among healthcare workers of a gastroenterological service in a tertiary care facility. Arab J Gastroenterol. 2020;21:151–5. https://doi.org/10.1016/j.ajg.2020.07.005.
Lahner E, Dilaghi E, Prestigiacomo C, Alessio G, Marcellini L, Simmaco M, et al. Prevalence of Sars-Cov-2 infection in health workers (HWs) and diagnostic test performance: the experience of a teaching hospital in Central Italy. Int J Environ Res Public Health. 2020. https://doi.org/10.3390/ijerph17124417.
Lai X, Wang M, Qin C, Tan L, Ran L, Chen D, et al. Coronavirus disease 2019 (COVID-2019) infection among health care workers and implications for prevention measures in a tertiary hospital in Wuhan, China. JAMA Netw Open. 2020;3: e209666. https://doi.org/10.1001/jamanetworkopen.2020.9666.
Lombardi A, Consonni D, Carugno M, Bozzi G, Mangioni D, Muscatello A, et al. Characteristics of 1,573 healthcare workers who underwent nasopharyngeal swab testing for SARS-CoV-2 in Milano, Lombardy, Italy. medRxiv. 2020. https://doi.org/10.1101/2020.05.07.20094276.
Martin C, Montesinos I, Dauby N, Gilles C, Dahma H, van den Wijngaert S, et al. Dynamics of SARS-CoV-2 RT-PCR positivity and seroprevalence among high-risk healthcare workers and hospital staff. J Hosp Infect. 2020;106:102–6. https://doi.org/10.1016/j.jhin.2020.06.028.
Mohanty S, Lakkireddy D, Trivedi C, MacDonald B, Quintero Mayedo A, Della Rocca DG, et al. Creating a safe workplace by universal testing of SARS-CoV-2 infection in asymptomatic patients and healthcare workers in the electrophysiology units: a multi-center experience. J Interv Card Electrophysiol. 2021;62:171–6. https://doi.org/10.1007/s10840-020-00886-9.
Moncunill G, Mayor A, Santano R, Jiménez A, Vidal M, Tortajada M, et al. SARS-CoV-2 seroprevalence and antibody kinetics among health care workers in a Spanish hospital after 3 months of follow-up. J Infect Dis. 2021;223:62–71. https://doi.org/10.1093/infdis/jiaa696.
Moolla MS, Parker A, Parker MA, Sithole S, Amien L, Chiecktey R, et al. Staff testing for COVID-19 via an online pre-registration form. S Afr J Infect Dis. 2021;36:232. https://doi.org/10.4102/sajid.v36i1.232.
Olalla J, Correa AM, Martín-Escalante MD, Hortas ML, Martín-Sendarrubias MJ, Fuentes V, et al. Search for asymptomatic carriers of SARS-CoV-2 in healthcare workers during the pandemic: a Spanish experience. QJM. 2020. https://doi.org/10.1093/qjmed/hcaa238.
Olmos C, Campaña G, Monreal V, Pidal P, Sanchez N, Airola C, et al. SARS-CoV-2 infection in asymptomatic healthcare workers at a clinic in Chile. PLoS ONE. 2021;16: e0245913. https://doi.org/10.1371/journal.pone.0245913.
Oster Y, Wolf DG, Olshtain-Pops K, Rotstein Z, Schwartz C, Benenson S. Proactive screening approach for SARS-CoV-2 among healthcare workers. Clin Microbiol Infect. 2021;27:155–6. https://doi.org/10.1016/j.cmi.2020.08.009.
Stock AD, Bader ER, Cezayirli P, Inocencio J, Chalmers SA, Yassari R, et al. COVID-19 infection among healthcare workers: serological findings supporting routine testing. Front Med (Lausanne). 2020;7:471. https://doi.org/10.3389/fmed.2020.00471.
Temkin E. Extremely low prevalence of asymptomatic COVID-19 among healthcare workers caring for COVID-19 patients in Israeli hospitals: a cross-sectional study. Clin Microbiol Infect. 2021;27:130.e1-130.e4. https://doi.org/10.1016/j.cmi.2020.09.040.
Treibel TA, Manisty C, Burton M, McKnight Á, Lambourne J, Augusto JB, et al. COVID-19: PCR screening of asymptomatic health-care workers at London hospital. Lancet. 2020;395:1608–10. https://doi.org/10.1016/S0140-6736(20)31100-4.
Vahidy FS, Sostmann DH, Bernard DW, Boom ML, Drews AL, Christensen P, et al. Prevalence of SARS-CoV-2 Infection Among Asymptomatic Healthcare Workers in the Greater Houston: a cross-sectional analysis of surveillance data from a large healthcare system. medRxiv. 2020. https://doi.org/10.1101/2020.05.21.20107581.
Bayle C, Cantin D, Vidal J-S, Sourdeau E, Slama L, Dumesges N, et al. Asymptomatic SARS COV-2 carriers among nursing home staff: a source of contamination for residents? Infect Dis Now. 2021;51:197–200. https://doi.org/10.1016/j.idnow.2020.11.008.
Hassan SS, Seigerud Å, Abdirahman R, Arroyo Mühr LS, Nordqvist Kleppe S, Pin E, et al. SARS-CoV-2 infections amongst personnel providing home care services for older persons in Stockholm, Sweden. J Intern Med. 2021;290:430–6. https://doi.org/10.1111/joim.13274.
McBee SM, Thomasson ED, Scott MA, Reed CL, Epstein L, Atkins A, Slemp CC. Notes from the field: universal statewide laboratory testing for SARS-CoV-2 in nursing homes—West Virginia, April 21-May 8, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1177–9. https://doi.org/10.15585/mmwr.mm6934a4.
van Buul LW, van den Besselaar JH, Koene FM, Buurman BM, Hertogh CM. Asymptomatic cases and limited transmission of SARS-CoV-2 in residents and healthcare workers in three dutch nursing homes. Gerontol Geriatr Med. 2020;6:2333721420982800. https://doi.org/10.1177/2333721420982800.
Borras-Bermejo B, Martínez-Gómez X, San Miguel MG, Esperalba J, Antón A, Martin E, et al. Asymptomatic SARS-CoV-2 infection in nursing homes, Barcelona, Spain, April 2020. Emerg Infect Dis. 2020. https://doi.org/10.3201/eid2609.202603.
Harada S, Uno S, Ando T, Iida M, Takano Y, Ishibashi Y, et al. Control of a nosocomial outbreak of COVID-19 in a University Hospital. Open Forum Infect Dis. 2020;7:ofaa512. https://doi.org/10.1093/ofid/ofaa512.
Khalil A, Hill R, Ladhani S, Pattisson K, O’Brien P. COVID-19 screening of health-care workers in a London maternity hospital. Lancet Infect Dis. 2021;21:23–4. https://doi.org/10.1016/S1473-3099(20)30403-5.
Rajme-López S, González-Lara MF, Ortiz-Brizuela E, Román-Montes CM, Santiago-Cruz J, Mendoza-Rojas MÁ, et al. Large-scale screening for severe acute respiratory coronavirus virus 2 (SARS-CoV-2) among healthcare workers: prevalence and risk factors for asymptomatic and pauci-symptomatic carriers, with emphasis on the use of personal protective equipment (PPE). Infect Control Hosp Epidemiol. 2021. https://doi.org/10.1017/ice.2021.68.
Rasmussen KMB, Andersen PA, Channir HI, Aanæs K, Knudsen JD, Kirkeby NS, et al. COVID-19 infection rate among tertiary referral center otorhinolaryngology healthcare workers. Eur Arch Otorhinolaryngol. 2021;278:3091–8. https://doi.org/10.1007/s00405-021-06615-w.
Sebastian P, Jorge P, Ariel G, Francisco S, Carolina M, Milton A, et al. Assesment of SARS-CoV-2 infection-in dentists and supporting staff at a university dental hospital in Argentina. J Oral Biol Craniofac Res. 2021;11:169–73. https://doi.org/10.1016/j.jobcr.2021.01.006.
Soltani-Zangbar MS, Aghebati-Maleki L, Hajivalili M, Haji-Fatahaliha M, Motavalli R, Mahmoodpoor A, et al. Application of newly developed SARS-CoV2 serology test along with real-time PCR for early detection in health care workers and on-time plasma donation. Gene Rep. 2021;23: 101140. https://doi.org/10.1016/j.genrep.2021.101140.
Zhao D, Wang M, Wang M, Zhao Y, Zheng Z, Li X, et al. Asymptomatic infection by SARS-CoV-2 in healthcare workers: a study in a large teaching hospital in Wuhan, China. Int J Infect Dis. 2020;99:219–25. https://doi.org/10.1016/j.ijid.2020.07.082.
Jones NK, Rivett L, Sparkes D, Forrest S, Sridhar S, Young J, et al. Effective control of SARS-CoV-2 transmission between healthcare workers during a period of diminished community prevalence of COVID-19. Elife. 2020. https://doi.org/10.7554/eLife.59391.
Shields A, Faustini SE, Perez-Toledo M, Jossi S, Aldera E, Allen JD, et al. SARS-CoV-2 seroprevalence and asymptomatic viral carriage in healthcare workers: a cross-sectional study. Thorax. 2020;75:1089–94. https://doi.org/10.1136/thoraxjnl-2020-215414.
Farfour E, Amiel C, Lecuru M, Zia-Chahabi S, Jolly E, Mazaux L, et al. SARS-CoV-2 screening of asymptomatic health care workers: experience of a General hospital. Ann Biol Clin (Paris). 2021;79:325–30. https://doi.org/10.1684/abc.2021.1664.
Zhang Y, Cheng S-R. Periodic COVID-19 testing in emergency department staff. medRxiv. 2020. https://doi.org/10.1101/2020.04.28.20084053.
Klompas M, Baker MA, Rhee C, Tucker R, Fiumara K, Griesbach D, et al. A SARS-CoV-2 cluster in an acute care hospital. Ann Intern Med. 2021;174:794–802. https://doi.org/10.7326/M20-7567.
Wang W, Xu Y, Gao R, Lu R, Han K, Wu G, Tan W. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA. 2020;323:1843–4. https://doi.org/10.1001/jama.2020.3786.
Regev-Yochay G, Amit S, Bergwerk M, Lipsitch M, Leshem E, Kahn R, et al. Decreased infectivity following BNT162b2 vaccination: a prospective cohort study in Israel. Lancet Reg Health Eur. 2021;7: 100150. https://doi.org/10.1016/j.lanepe.2021.100150.
Angel Y, Spitzer A, Henig O, Saiag E, Sprecher E, Padova H, Ben-Ami R. Association between vaccination with BNT162b2 and incidence of symptomatic and asymptomatic SARS-CoV-2 infections among health care workers. JAMA. 2021;325:2457–65. https://doi.org/10.1001/jama.2021.7152.
Wilhelm A, Widera M, Grikscheit K, Toptan T, Schenk B, Pallas C, et al. Reduced neutralization of SARS-CoV-2 omicron variant by vaccine sera and monoclonal antibodies. medRxiv. 2021. https://doi.org/10.1101/2021.12.07.21267432.
Brendish NJ, Poole S, Naidu VV, Mansbridge CT, Norton NJ, Wheeler H, et al. Clinical impact of molecular point-of-care testing for suspected COVID-19 in hospital (COV-19POC): a prospective, interventional, non-randomised, controlled study. Lancet Respir Med. 2020;8:1192–200. https://doi.org/10.1016/S2213-2600(20)30454-9.
Ludwig KU, Schmithausen RM, Li D, Jacobs ML, Hollstein R, Blumenstock K, et al. LAMP-Seq enables sensitive, multiplexed COVID-19 diagnostics using molecular barcoding. Nat Biotechnol. 2021;39:1556–62. https://doi.org/10.1038/s41587-021-00966-9.
Wilmes P, Zimmer J, Schulz J, Glod F, Veiber L, Mombaerts L, et al. SARS-CoV-2 transmission risk from asymptomatic carriers: results from a mass screening programme in Luxembourg. Lancet Reg Health Eur. 2021. https://doi.org/10.1016/j.lanepe.2021.100056.
Müller CP. Do asymptomatic carriers of SARS-COV-2 transmit the virus? Lancet Reg Health Eur. 2021;4:1–2. https://doi.org/10.1016/j.lanepe.2021.100082.
Acknowledgements
Not applicable.
Funding
Open Access funding enabled and organized by Projekt DEAL. This study was funded by the Federal Ministry of Education and Research, Germany (NaFoUniMedCovid19, funding number: 01KX2021; part of the project “CEOSys”, which was paid to the institution)”.
Author information
Authors and Affiliations
Contributions
JMJ was the lead investigator, provided the first draft of the manuscript and participated in literature screening and extraction. AS participated in literature extraction, evaluation of the results and provided the second draft of the manuscript. ADW participated in literature extraction, interpretation of the results and critically reviewed the manuscript. BG and JS took part in the screening of the literature. HG, VK, AK, JR, SS and SA participated in development of the study question, criteria development for the literature screening and performed critical revisions of the article. IM performed the literature search. FR and SE participated in screening and literature extraction. GR completed the data analysis, created the forest plot, and drafted the corresponding text passages. CS was involved in data extraction and interpretation, risk of bias assessment, and critical text revision of the article. NTM was instrumental in conceptualization, literature screening, and text development. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
Search term.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Jabs, J.M., Schwabe, A., Wollkopf, A.D. et al. The role of routine SARS-CoV-2 screening of healthcare-workers in acute care hospitals in 2020: a systematic review and meta-analysis. BMC Infect Dis 22, 587 (2022). https://doi.org/10.1186/s12879-022-07554-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-022-07554-5