Abstract
Background
Human papillomavirus (HPV) is the most common sexually transmitted infection worldwide, affecting about 80% of women up to the age of 50. The persistent infection of high risk-HPV types (HR-HPV) is the leading cause of cervical cancer, the fourth most common cancer of women. Therefore, we aimed to evaluate the frequency and typing of HPV in the genital lesions in the Iranian population.
Methods
This descriptive-analytic study was conducted on a population in the South-Khorasan province of Iran. All of the participants were sexually active and were checked for evident cervical warts. Biopsy samples were collected from various lesions, and all samples were tested for detection and genotyping of HPV using a reverse dot blot hybridization method (HPV direct flow CHIP).
Results
In overall, 370 samples were evaluated; 10 cases (2.7%) were male and the rest were female. The mean age of patients was 33.3 ± 8.5 years, of which 48.1% were in the age range from 25 to 36 years. Among the samples, 345 (93.2%) were positive for HPV-DNA; the low risk HPV types (LR-HPV) and HR-HPV were identified among 80.9% and 15.5% of tissue samples, respectively. Among the LR-HPV, HPV-6, 11, 42 and 54 were the most common genotypes, and HPV-16 and 39 were prevalent HR-HPV types detected. The number of pregnancies, marriage age, and partner infection were not significantly related to the HPV types. Types 42 had a declining pattern toward aging, and HPV-11 was increasing toward aging.
Conclusion
The number of samples with HR-HPV was rather high. Due to the greater frequency of infection in the age range of 25–35 years, it is advised that all individuals referred to gynecological clinics at gestational age be tested for HPV types.
Similar content being viewed by others
Introduction
Anogenital condyloma acuminata are genital lesions defined as wart caused by human papillomavirus (HPV) infections [1]. Genital warts include different types of flat or exophytic warts of the vagina and cervix in women, although, in men, condyloma acuminata could rise in the external genital such as the anogenital and penile area [2]. Usually, HPV infection is sub-clinical, and lesions spontaneously improve, resulting in mild disease. However, it may settle precancerous lesions that lead to an invasive form of cancer [3].
Cancers are the second leading cause of death in developed countries [4]. Hence, cervical cancer (CC) is the fourth leading cause of mortality among women globally and the first cause of death in the East, Middle, South, and West Africa [5]. Moreover, the vulvar and vaginal cancers account for about 4–7% of gynecologic cancers in women [6].
Oncogenic HPV-DNA is identified in many cancers such as the cervical, vulva, vaginal, vulvar intraepithelial neoplasia (VIN), and vaginal intraepithelial neoplasia (VAIN) [7,8,9,10]. Thus, it is nowadays a fact that the leading cause of these cancers worldwide is HPV [11]. This virus is a small double-stranded DNA virus belonging to the Papillomaviridae family [12]. HPV is commonly found in epithelial tissues and promotes proliferation of cells in the benefit of having E6 and E7 onco-proteins which have roles to disturb the tumor suppressors’ activity of p53 and pRB, respectively [13]. At present, more than 100 genotypes of HPVs are known [14], among which types 16 and 18 are the most common HPV types which were found in malignancies associated with this virus (notably Squamous Cell Carcinoma (SCC) [12, 15, 16]. Moreover, there are other HPV types which are high risk for CC including HPV-types 26, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 68, and 82 [17].
Today, the preventive measures of primary (vaccination against HPV) and secondary (screening and typing of HPV along with treatment of precancerous lesions) have main roles to control the carcinogenic effect of the virus [5].
However, unlike developed countries, developing countries still lack an efficient and regular screening program, which is responsible for the rising prevalence of CC in these countries during the last three decades [18,19,20,21,22]. Though, it is evident that prevention, early detection, and timely treatment have an obvious effect on reducing CC-related death rather than any other cancers [23].
Screening tests are one of the preventive measures for cervical cancer. Pap smear screening is employed for nearly half a century to identify the precancerous lesions in advanced countries [24]. There are various techniques to detect HPV-DNA in clinical samples. However, polymerase chain reaction (PCR), in situ hybridization, and Southern blotting techniques are routinely employed [14].
Reports on HPV infection and typing in genital warts are limited. Most previous studies declare HPV types 6 and 11 as leading causes [25]; however, there are huge numbers of inconsistent results and evidences of roles of HR-HPV types in warts. Given the scarcity of data on HPV prevalence and genotypes in diverse parts of Iran, including the South Khorasan Province, this research looked at the frequency and variety of HPV types among various genital warts.
Methods
Patients and samples
This was a cross-sectional study conducted from October 2018 to March 2021 in the East of Iran, South Khorasan province. The population of the study were women and men referred to different gynecology clinics in the city of Birjand. A gynecologist selected patients with visible genital lesions, including wart types; one sample was taken from each patient. Condyloma acuminata defines as genital warts which are benign cauliflower-shaped lesions. Biopsy samples were given from the vulva, vagina, cervix, and external warts. The specimens were taken by a gynecologist and archived at the laboratory after investigating by an expert pathologist. The Ethics Committee approved the provisions of the research of Birjand University of Medical Sciences (Ethics code: IR.BUMS. 1398,168).
Preparing samples
The tissue samples were fixed in formalin and passed via a tissue processor (Leica TP1020 Germany) and then embedded in paraffin. Afterwards, formalin-fixed paraffin embedded (FFPE) samples were subjected to sectioning by microtome (Leica RM2255 Germany). At first, 4 micron (4 µM) sections were undergone for Hemathoxilin and Eosin staining. Moreover, 10 other 4 µM sections were put into microfuge tubes for molecular assays. A separate blade was used for each sample, and necessary conditions and considerations were regarded to prevent carry over contamination of samples and/or sections.
Detection and typing of HPV
According to the kit’s instruction, detection and typing of HPV were performed to the benefit of using the HPV Direct Flow CHIP test (Master Diagnóstica, Granada, Spain). This kit works in a reverse dot blot hybridization setting for multiplex detection and genotyping of HPV. In brief, the workflow of the above-mentioned kit is as follows:
First, tissue sections were subjected to removal of paraffin using an ethanol/xylene approach. Then, DNA was extracted using a tissue genomic DNA isolation kit (DNeasy Blood & Tissue Kit, Qiagen). Then, the extracted DNA was mixed with the Multiplex master mix of the HPV PCR reaction which was given in the content of the kit (HPV Direct Flow CHIP test, Master Diagnóstica, Granada, Spain). PCR Program was started with 5 min at 98 °C, followed by 5 cycles of 98 °C–42 °C–72 °C, and then followed by another 45 cycles of 98 °C–60 °C–72 °C.
The hybridization stage was performed by a full automated e-BRID System® (Master Diagnostic Co, Spain). The microchip used in this method was blotted in 81 positions: 72 dot which is complementary with one single-strand DNA of each various 36 types of HPV, 5 dots for Blank or QC of chromogen, 2 for evaluation and control of correct extraction of DNA, and finally 2 dots for universal genotypes of HPV (Fig. 1).
Statistical methods
The statistical analyses was performed using the Statistical Package for Social Sciences software version 25 (SPSS Inc, Chicago, IL, USA). Descriptive analysis was used to determine frequencies and percentiles. Chi-square test and Fisher’s exact test were used to test association and to compare between genotypes and age. The statistical significance was set at P < 0.05.
Results
Baseline information of population
In total, 370 samples of genital warts were collected from 370 patients. Among which, 10 cases (2.7%) were male, and the rest were female. The mean age was 33.3 ± 8.5 years old, with no significant difference among the genders which ranged from 14 to 88 years old (Fig. 2). Most of the participants were college-educated, and housewives (67.68% and 48.48%, respectively). The mean age of marriage was 22.46 ± 4.15 years, and 49.5% were married from 22 to 29 years. According to the baseline demographic information, 76% of patients had a history of 1 to 3 pregnancies.
Prevalence and types of HPV
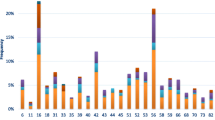
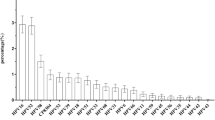
The result of the Direct Flow CHIP revealed 345 samples (93.2%) were positive for HPV-DNA, and the rest (6.7%) were defined as undetected HPV-DNA. The result of HPV genotyping showed that among the samples tested, type 6 (78.3%), type 54 (26.7%), type 11 (16.5%), and type 42 (12.8%) were the most prevalent LR-HPV types (Fig. 2). In total, HR-HPV types were identified among 53 samples (15.5%), of which type 16 (9%) and type 39 (3.5%) were the most prevalent. Single infections of HPV types 16 and 39 were detected among 5 and 11 samples, respectively. There was no statistically significant difference among the frequency of genotypes with the occupation, education level, number of pregnancies, age of marriage, and spouse infection of the participants (P > 0.05).
Prevalence of mix HPV infections
In general, 158 samples (45.8%) were infected with only one HPV type (mono-type HPV infection), although 53.7% had more than one type simultaneously (mix-type HPV infections); among which 47% had a mix of two types, 6.1% three types and 0.6% had 4 types coincidently. HPV type 6 was most prevalent HPV type as mono-type HPV infection (68.4%), then followed by HPV types 11 (13.3%), 16 (4.4%), 54 (7%), 42 (1.9%) and 31 (1.2%). There were two samples with mix of 4 HPV types (Mix of types 6–11–16–35 and 6–43–53–54). Among the triple mix infections, the mix of types 6–11–42, 6–42–54, and 6–11–54 were observed in 4, 3, and 2 samples, respectively. Although there were more other triple infections that observed just in one tissue sample. Among the double HPV infections, mix of HPV types 6–42, 6–11, 6–16, 6–40, 6–54 were most prevalent with percentage of 8.1%, 4.9, 3.8, 1.4 and 16.8%, respectively (Table 1).
HPV-types among different age groups
The mean age among participants with different HPV types was not statistically different (Table 2). Although, the pattern of some HPV infections was significantly different in regard to age groups. HPV types were considerably prevalent in the age group of 25–35 years, tough, the rate of HPV type 54 was growing in older ages and was more prevalent between 36 and 45 years. Moreover, HPV type 42 was more prevalent among ages lower than 25 years (P = 0.03). HPV type 6 was ubiquitously distributed in different age ranges, though, types 16 and 42 had a declining pattern toward aging, and HPV 11 was elevated in higher ages (Table 2).
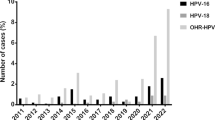
As sampling was followed per 3 continuous years, we compared the rate of different types among 3 years. It seems that the HPV type 6 is continually prevalent, type 11 is significantly declined and shifted to other low-risk types such as type 54 (P < 0.05) (Fig. 3).
Discussion
It is widely accepted that HPV-DNA testing and typing are useful for screening CC. Previous studies indicated the higher sensitivity of HPV testing rather than cytology experiments due to the evaluation of the high-grade cervical intraepithelial lesions and invasive cancer [26]. Therefore, the superiority of the HPV-DNA testing and cooperation with the Pap smear test should be more concerned and be used in clinical situations.
In this study, a reverse dot blot hybridization assay, named as HPV Direct Flow CHIP was used to detect and typing of HPV. The overall detection rate of HPV-DNA among the wart samples was 93.2%, so, 6.7% defined as undetected HPV. Most of the previous similar works have been reported in this range [27,28,29]. No detection of HPV from wart samples is partly due to sample type, pre-processing, and formalin fixation and/or methodology used for detection of HPV.
According to our findings, HPV types 6, 11, 42, and 54 were the most prevalent LR-HPV types; however, HPV types 16 and 39 were prevalent HR-HPV types detected. There are limited studies around Iran that worked on genital warts; almost most of them reported HPV-6 as the leading cause of genital warts [30, 31]. Although there were studies that report the dominance of type 11 [29, 32], the sample size and methodology affect these figures. In south Khorasan province, Mousavi et al. recently reported 40.7% of HPV type 6 among wart samples that it declined with aging; however, in the current study, type 6 was consistent in all ages with 78.3% overall prevalence [33]. Moreover, the pattern of some types was changing over 3 years; types 11 and 42 declined and shifted to increasing of type 54. This finding is reasonable with minor differences from the result of Mousavi et al. [31].
In total, in this project, HR-HPV types were found in 15.5% of samples (including 9% single infection with HR and 6.5% coinfection with an HR), which is a considerable rate in terms of follow-up and cancer preventive programs. This rate is consistent with most of the previous reports on genital warts [28, 30]. Although, studies have reported HR-HPV as low as 1.5% [2], or high from 40 to 58.7% among genital warts [1, 34]. This sharp difference is mainly explained by the population type, geographic region, and the prevalence of associated risk factors. Some differences could be related to the technical issues of HPV detection and typing; most of the above-mentioned studies have used conventional PCR methods and/or sequencing. According to the extent of nucleotide heterogenecity among HPV types, the amplification and detection rate of all common HPV types might be reduced using limited numbers of primer pairs.
Another significant finding was the high prevalence of HPV types 42 and 54 in the current study. This figure was rarely reported from Iranian studies and seemed to be recently introduced and circulated in the Iranian population as LR-HPV [28, 33, 35, 36]. Although it was proven that the HPV types 6 and 11 are the most prominent types among genital warts [37], nevertheless, HPV types 42 and 54 were reported from urogenital regions [34]. Whilst HPV type 42 and 54 are proven as low risk HPV types [38], type 42 has been recently reported in association to the pathogenesis of Seborrheic keratosis-like lesion of genital tract [39].
The detection of more than one HPV type in the same sample, which is considered mix HPV infection and/or coinfection, was seen in 53.7% of HPV positive samples of this study (mix of two, three, and 4 HPV types was seen among 47%, 6.1% and 0.6% of HPV positive samples, respectively). This figure is in line with a just recently published study with a similar setting [2]. In reports with a similar setting, mix infections were varied from 13.4% [3], 33.8% [4], to 54% which were mostly used blotting and hybridization methods such as INNO-LiPA® [5]. The high sensitivity of HC-2 and hybridization assays to detect HPV types were previously approved [6, 7]. The rate of coinfection has not been addressed in most of the previously reported studies [8, 9], and some reported it in very low levels [10,11,12], which were mostly used PCR-based assays.
The prevalence of genital HPV was significantly high in the age range from 25 to 35 years, which is in line with previous studies [40]. It was found that people over 25 years old have the highest rate of infection [41]. An investigation on 1000 women revealed the highest rate of HPV in cases aged 19–25 years [42]. However, Newall et al. found that the highest rate of HPV was in the age group of 30–39 years with the dominance of genotypes of 6, 16, and 18 [43]. As a result, the rising prevalence of HPV coincides with the onset of sexual activities, which may change somewhat between geographic locations. The above-mentioned statistic, as well as the relatively high occurrence of mono and coinfection with HR-HPV, demand that cancer-prevention programs pay greater attention. Moreover, HPV vaccine coverage in Iran seems reasonable, though, vaccination happens in later ages and is not completely adherent to the guidelines [13]. Besides, previous studies demonstrated that anal warts are often heterogeneously originated and could not be assumed to LSIL (Low-grade squamous intraepithelial lesion) [14]; hence, HPV typing is a useful tool to assign a precise classification and grading lesions.
Conclusion
This study presents beneficial information on typing of HPV among genital warts that should be considered in the transmission rate of different HPV types, cancer prevention, and designing HPV types in vaccines. Regarding the 15.5% rate of HR-HPV among genital warts as a benign lesion, this figure is relatively high and needs more consideration. Hence, screening women in sexually active age is requisite for controlling HPV infection, as well as HPV typing.
Availability of data and materials
The supporting data for the finding of current study are available from the corresponding author upon a reasonable request.
References
Boda D, Neagu M, Constantin C, Voinescu RN, Caruntu C, Zurac S, et al. HPV strain distribution in patients with genital warts in a female population sample. Oncol Lett. 2016;12(3):1779–82.
Ozaydin-Yavuz G, Bilgili SG, Guducuoglu H, Yavuz IH, Elibuyuk-Aksac S, Karadag AS. Determinants of high-risk human papillomavirus infection in anogenital warts. Postepy Dermatol Alergol. 2019;36(1):76–81.
Al-Awadhi R, Al-Mutairi N, Albatineh AN, Chehadeh W. Association of HPV genotypes with external anogenital warts: a cross sectional study. BMC Infect Dis. 2019;19(1):375.
Modesitt SC, van Nagell Jr JR. The impact of obesity on the incidence and treatment of gynecologic cancers: a review. Obstet Gynecol Surv. 2005;60(10):683–92.
Arbyn M, Weiderpass E, Bruni L, de Sanjosé S, Saraiya M, Ferlay J, et al. Estimates of incidence and mortality of cervical cancer in 2018: a worldwide analysis. Lancet Glob Health. 2020;8(2):e191–203.
Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57(1):43–66.
Schiffman M, Kjaer SK. Chapter 2: Natural history of anogenital human papillomavirus infection and neoplasia. JNCi monographs. 2003;2003(31):14–9.
Smith JS, Backes DM, Hoots BE, Kurman RJ, Pimenta JM. Human papillomavirus type-distribution in vulvar and vaginal cancers and their associated precursors. Obstet Gynecol. 2009;113(4):917–24.
Srodon M, Stoler MH, Baber GB, Kurman RJ. The distribution of low and high-risk HPV types in vulvar and vaginal intraepithelial neoplasia (VIN and VaIN). Am J Surg Pathol. 2006;30(12):1513–8.
Javanmard D, Behravan M, Ghannadkafi M, Salehabadi A, Ziaee M, Namaei MH. Detection of Chlamydia trachomatis in pap smear samples from South Khorasan Province of Iran. Int J Fertil Steril. 2018;12(1):31.
Roberts CC, Tadesse AS, Sands J, Halvorsen T, Schofield TL, Dalen A, et al. Detection of HPV in Norwegian cervical biopsy specimens with type-specific PCR and reverse line blot assays. J Clin Virol. 2006;36(4):277–82.
Abbas AK, Aster JC. Robbins basic pathology. Amsterdam: Elsevier/Saunders; 2013.
Narisawa-Saito M, Kiyono T. Basic mechanisms of high-risk human papillomavirus-induced carcinogenesis: roles of E6 and E7 proteins. Cancer Sci. 2007;98(10):1505–11.
Zare Mahmoud Abadi R, Saghafi S, Mohajertehran F, Rafiee S, Shokri M. Detection of Human Papillomavirus (HPV) in ameloblastoma using the polymerase chain reaction (PCR). J Mashhad Dent Sch. 2018;42(4):298–306.
Gravitt P, Peyton C, Alessi T, Wheeler C, Coutlee F, Hildesheim A, et al. Improved amplification of genital human papillomaviruses. J Clin Microbiol. 2000;38(1):357–61.
Serrano B, Alemany L, Tous S, Bruni L, Clifford GM, Weiss T, et al. Potential impact of a nine-valent vaccine in human papillomavirus related cervical disease. Infect Agents Cancer. 2012;7(1):1–13.
Niya MHK, Tameshkel FS, Panahi M, Salim FB, Monavari SHR, Keyvani H. Human papillomavirus investigation in head and neck squamous cell carcinoma: initial report from the low risk HPV types associations. Asian Pac J Cancer Prev. 2017;18(9):2573.
Curado M-P, Edwards B, Shin HR, Storm H, Ferlay J, Heanue M, et al. Cancer incidence in five continents, vol. IX. Lyon: IARC Press, International Agency for Research on Cancer; 2007.
Ferlay J. Cancer incidence, mortality and prevalence worldwide. GLOBOCAN2002. 2004.
Sankaranarayanan R, Nene BM, Shastri SS, Jayant K, Muwonge R, Budukh AM, et al. HPV screening for cervical cancer in rural India. N Engl J Med. 2009;360(14):1385–94.
Sankaranarayanan R, Budukh AM, Rajkumar R. Effective screening programmes for cervical cancer in low-and middle-income developing countries. Bull World Health Organ. 2001;79:954–62.
Haedicke J, Iftner T. Human papillomaviruses and cancer. Radiother Oncol. 2013;108(3):397–402.
van der Aa MA, Pukkala E, Coebergh JWW, Anttila A, Siesling S. Mass screening programmes and trends in cervical cancer in Finland and the Netherlands. Int J Cancer. 2008;122(8):1854–8.
Sasagawa T, Maehama T, Osaka Y, Sakamoto J, Shibata T, Fujita S, et al. Comparison of the digene hybrid capture 2 and Roche cobas 4800 HPV tests for detection of CIN2+ in a referral population in Japan. J Med Virol. 2018;90(5):972–80.
Chang L, Ci P, Shi J, Zhai K, Feng X, Colombara D, et al. Distribution of genital wart human papillomavirus genotypes in China: a multi-center study. J Med Virol. 2013;85(10):1765–74.
Jaberipour M, Momtahan M, Najib F, Amooei S, Saidifard F, Ghaderi A, et al. Detection of high-risk human papillomavirus types 16 and 18 but not 33 and 52 in external genital warts from Iranian females. Asian Pac J Cancer Prev. 2011;12(3):771–4.
Manyere N, Dube Mandishora R, Magwali T, Mtisi F, Mataruka K, Mtede B, et al. Human papillomavirus genotype distribution in genital warts among women in Harare-Zimbabwe. J Obstet Gynaecol. 2020;40(6):830–6.
Mehri M, Hosseinzadeh Kakroudi S, Askari FS, Mohebbi A, Tabarraei A. Prevalence of human papillomavirus genotypes in patients with genital warts in Gorgan. Iran J Clin Basic Res. 2020;4(3):3–9.
Ebrahimi A, Moradi MR, Rezaei M, Kavoussi H, Madani SH, Mohammadamini K, et al. Comparison of the risk factors and HPV types in males with anogenital warts with and without involvement of the urethral meatus in Western Iran. Acta Dermatovenerol Alpina, Pannonica et Adriatica. 2017;26(3):55–8.
Jamshidi M, Shekari M, Nejatizadeh AA, Malekzadeh K, Baghershiroodi M, Davudian P, et al. The impact of human papillomavirus (HPV) types 6, 11 in women with genital warts. Arch Gynecol Obstet. 2012;286(5):1261–7.
Moossavi M, Fereidouni M, Zardast M, Khazaei Z, Ghanbarzadeh N. Genotype distribution of human papilloma virus among women with genital warts biopsies in southern Khorasan, eastern Iran. Meta Gene. 2020;25:100720.
Yaghoobi R, Makvandi M, Afshar N, Pazyar N, Hamidifard M, Sharifpour C. High frequency of human papillomavirus genotype 16 among patients with anogenital warts. Jundishapur J Microbiol. 2015;8(11):e25882-e.
Salehi-Vaziri M, Sadeghi F, Hashemi FS, Haeri H, Bokharaei-Salim F, Monavari SH, et al. Distribution of human papillomavirus genotypes in Iranian women according to the severity of the cervical lesion. Iran Red Crescent Med J. 2016;18(4):e24458-e.
Kiwerska K, Jozefiak A, Markowska J, Kedzia W, Jackowska J, Wierzbicka M. Oral-genital human papillomavirus infection in Polish couples: frequent detection of HPV 42. BMC Infect Dis. 2019;19(1):122.
Hamkar R, Azad TM, Mahmoodi M, Seyedirashti S, Severini A, Nategh R. Prevalence of human papillomavirus in Mazandaran province, Islamic Republic of Iran. EMHJ-East Mediterr Health J. 2002;8(6):805–11.
Chalabiani S, Nazari MK, Shabani M, Davoodi NR, Sarafnejad A, Amirzargar AA. Retrospective analysis of prevalence of high-risk and low-risk Human Papillomavirus (HPV) genotypes in iranian women during 2013–2016. Asian Pac J Cancer Biol. 2017;2(4):85–90.
Yanofsky VR, Patel RV, Goldenberg G. Genital warts: a comprehensive review. J Clin Aesthet Dermatol. 2012;5(6):25–36.
Nasseri S, Monavari SH, Keyvani H, Nikkhoo B, Vahabpour Roudsari R, Khazeni M. The prevalence of Human Papilloma Virus (HPV) infection in the oligospermic and azoospermic men. Med J Islam Repub Iran. 2015;29:272.
Pujari R, Newman MR, Talia KL, Pendlebury A, Hawkes D, Ireland-Jenkin K, et al. Seborrheic keratosis-like lesion of the cervix: first report of the cytological features of a low-risk HPV 42-associated lesion. Acta Cytol. 2021;65(5):448–52.
Chen C-J, Viscidi RP, Chuang C-H, Huang Y-C, Chiu C-H, Lin T-Y. Seroprevalence of human papillomavirus types 16 and 18 in the general population in Taiwan: implication for optimal age of human papillomavirus vaccination. J Clin Virol. 2007;38(2):126–30.
Newall AT, Brotherton JM, Quinn HE, McIntyre PB, Backhouse J, Gilbert L, et al. Population seroprevalence of human papillomavirus types 6, 11, 16, and 18 in men, women, and children in Australia. Clin Infect Dis. 2008;46(11):1647–55.
Mohammadpour F, Mansouri A, Hadjibabaie M. Utilization evaluation of human papilloma virus vaccine (GARDASIL®) in Iran; a cross-sectional study. Iran J Pharm Res IJPR. 2020;19(1):68.
Clavero O, McCloskey J, Molina VM, Quirós B, Bravo IG, de Sanjosé S, et al. Squamous intraepithelial lesions of the anal squamocolumnar junction: histopathological classification and HPV genotyping. Papillomavirus Res. 2017;3:11–7.
Acknowledgements
This work was fully founded and supported by Birjand University of Medical Sciences (BUMS). We would like to express great thanks to the roles of Shafa Pathobiology laboratory staffs and Mr. Miri for their help in the process of this study.
Funding
This work was fully supported and funded by research deputy of Brjand University of Medical Sciences.
Author information
Authors and Affiliations
Contributions
NG was supervised the project and get the idea as well as drafting proposal and designing the work. MZ was implemented experiments and gathered information. MZ have roles in selecting samples, pathologic examinations and data collection. ANS, MNS and SG have equal roles in data collection and gathering samples. DJ was analyzed data, drafted and submitted the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
An informed consent was taken from all participants. All procedures were in accordance with the 1964 Helsinki Declaration, and the research were approved by the Ethics Committee of Birjand University of Medical Sciences (Ethic code: IR.BUMS. 1398,168).
Consent for publication
Not applicable.
Competing interests
All authors of the manuscript have stated to have no conflict of interest for publication of this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zare-Bidaki, M., Zardast, M., Nadjafi-Semnani, A. et al. Investigation of frequency and typing of human papillomavirus among genital warts using a reverse dot blot hybridization approach. BMC Infect Dis 22, 278 (2022). https://doi.org/10.1186/s12879-022-07276-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-022-07276-8