Abstract
Objectives
This study aims to investigate the association between CD4+ T cell count and combined antiretroviral therapy (cART) with the prevalence of anal human papillomavirus (HPV) infection among HIV-positive male cohort in China.
Methods
A survey was conducted in men from a HIV cohort in Taizhou, China between 2016 and 2019. A face-to-face questionnaire interview was administered, and an anal-canal swab was collected for HPV genotyping.
Results
A total of 766 HIV-positive men were recruited. The HPV prevalence was lower among those with increased CD4+ T cell count than those with decreased or unchanged (46.5 vs. 56.6%, p = 0.033) from baseline. In multivariable models, having the current CD4+ T cell count of 350–499 cells/µL (aOR 0.28, 95% CI 0.13–0.64), and of ≥ 500 cells/µL (aOR 0.26, 95% CI 0.11–0.60) were associated with lower prevalence of any type HPV infection compared with those with < 200 cells/µL. Having taken NVP + 3TC + AZT was inversely associated with any high-risk (HR)-HPV (aOR 0.47, 95% CI 0.25–0.90) and any low-risk (LR)-HPV infection (aOR 0.40, 95% CI 0.18–0.88), compared with those taking EFV + 3TC + TDF.
Conclusions
Increased CD4+ T cell count at follow-up was significantly associated with lower prevalence of anal HPV infection. Inverse associations between NVP + 3TC + AZT and HR-HPV or LR-HPV infecton were observed.
Similar content being viewed by others
Introduction
Human papillomavirus (HPV) infection is the most prevalent sexually transmitted infection (STI) in the world [1, 2]. Persistent high risk (HR)-HPV infection is the cause of almost all cervical cancer, and is a crucial oncogenic driver in other cancers including anogenital and head and neck cancers [3, 4]. Studies have shown that HIV infection is associated with higher HPV prevalence and incidence [5,6,7,8]. The prevalence of anal HPV infection is higher in HIV-positive populations than in HIV-negatives, especially among HIV-positive men who have sex with men (MSM) across the world, ranging from 82.7% to 95.0% in recent reports [9,10,11,12,13,14]. Shared transmission route for both viruses may explain part of the high HPV prevalence among HIV population, however, to what extent the impaired immune function plays a role in HPV infection remains a research question. Studies of the impact of CD4+ T cell count, as the commonly used proxy for immune function, on the natural history of HPV infection have generated inconclusive results. While some studies showed that increased CD4+ T cell count is associated with lower risk of HPV infection among HIV-positives [15,16,17], others had observed null associations [5, 6, 18]. On the other hand, cART is reported to affect the anal HPV prevalence, anal intraepithelial neoplasia, and anal cancer. A recent worldwide meta-analysis evidenced that effective cART use is associated with a decreased prevalence of anal HR-HPV infection in HIV- positives [19]. In addition, a longitudinal study observed a significant beneficial effect from cART against acquisition of anal HR-HPV infection in HIV positive MSM [20], while negative or null associations were also reported [21, 22]. Furthermore, few studies have looked into associations between specific cART drugs or treatments with HPV infection, although there have been hypotheses that protease inhibitors may have off-target effect on infections including HPV[23].
Development of strategies for the prevention and treatment of HPV-related cancers in the anogenital tract requires a better understanding of the risk factors and natural history of persistent HPV infection, especially given that HPV vaccine has not been widely available in China. A cross-sectional study was conducted in male participants from an ongoing HIV cohort to investigate HPV prevalence from anal canal swab samples. We hypothesized an inverse association between CD4+ T cell improvement and anal HPV infection, and hypothesized that cART treatments were correlated with HPV infection in the HIV-positive male population.
Materials and methods
Study design and subject
Participants in this study were those HIV infected men registered in and routinely followed-up by Taizhou City Center for Disease Control and Prevention (CDC) and four district-level CDCs in Taizhou, Zhejiang Province. A survey for anal HPV infection was conducted between 2016 to 2019. The eligible criteria included: (1) aged 18 years or older; (2) had baseline or current test result for CD4+ T cell count when the survey was conducted; (3) willing to have an anal swab sample collected. There were 1,304 HIV infected men being followed-up from the study sites, and 776 (58.7%) were willing to participate in this survey. Ten of them had neither the baseline nor the current CD4+ T cell test results and thus were excluded. A total of 766 participants were included in this analysis. The study was approved by the Institutional Review Board of School of Public Healh, Fudan University, and all the participants provided written informed consent.
Data collection
Most of the participants’ socio-demographic characteristics, HIV-related data including cART treatments and multiple CD4+ T cell counts were obtained from the Chinese HIV/AIDS Comprehensive Information Management System. For this survey, participants were face-to-face interviewed by trained local public health staff during the visit, and a questionnaire was used to obtain epidemiological data. Overall, the following information was obtained: age; level of education; marital status with women; sexual orientation; self-reported history of STIs (including gonorrhea, syphilis, chlamydia, condyloma acuminata and genital herpes); number of lifetime sex partners; condom use in the last six months; smoking status and alcohol consumption. Ever smoked was defined as having ever smoked more than 100 cigarettes lifetime [24]. Frequency of alcohol consumption in the past year was recorded.
cART treatment and CD4+ T cell counts
Of all 766 participants, 741 participants were under cART based on the recommendation from the manual of national free antiretroviral treatment program for HIV/AIDS in China [25]. Participants received one combination of two nucleoside reverse transcriptase inhibitors (NRTIs) and either a protease inhibitors (PIs) or a non-nucleoside reverse transcriptase inhibitors (NNRTIs), or a NRTIs/PI combination regimen plus another antiretroviral drug. In this study, participants mainly received the combination of two NRTIs plus one NNRTI. cART treatments were classified as four groups in the final analysis: EFV + 3TC + TDF (a combination of Efavirenz, Lamivudine and Tenofovir); EFV + 3TC + AZT (a combination of Efavirenz, Lamivudine and Zidovudine); NVP + 3TC + AZT (a combination of Nevirapine, Lamivudine and Zidovudine); and all other combined with limited number of people using them.
Baseline CD4+ T cell count was measured at the time of HIV diagnosis, and current CD4+ T cell count was measured at the follow-up which was most close to the time of HPV test, representing the baseline and recent immune status of the participants, respectively. The median time interval was 2.3 years (interquartile range [IQR], 0.7–5.0 years) between these two CD4 measurement. The change of CD4+ T cell counts was also calculated by current CD4 minus baseline CD4. If the result was positive, we defined those participants as having “increased CD4+ T cell counts”; and if the result was negative or zero, we defined those participants as having “decreased or unchanged CD4+ T cell counts”. Baseline and the current CD4+ T cell count were recorded from the system, which had been measured by local CDCs using flow cytometry (Becton, Dickinson and Company, USA) according to the manufacturer’s protocol.
Sample collection and laboratory tests
For each participant, an anal canal swab was collected by the trained staff. A nylon flocked swab was inserted into the anal canal for 2–3 cm in depth, rotated with gentle pressure against the canal wall while being slowly moved out. The whole process of swab sample collection was required to be no less than 2 min to gain enough cells. Then the swab was kept in a tube with 3 ml liquid medium using PBS as the main component to preserve the cells, and stored at − 20 °C waiting for HPV DNA extraction. HPV genotyping was performed using the HPV GenoArray Test Kit (Hybribio Ltd., Guangdong, China). The test kit detects 21 HPV genotypes using a L1 consensus primer-based polymerase chain reaction assay by the flow-through hybridisation technique with a TC-96/G/H6 HPV DNA Amplification Analyzer and an HMM-2 fast nucleic acid molecule hybridisation instrument. Among the 21 HPV genotypes, HPV 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59 are carcinogenic types, HPV68 is a probably carcinogenic type, and HPV53 and 66 are possibly carcinogenic types, defined by the International Agency for Research on Cancer (IARC) [26]. Here we define them all as the high-risk genotypes of HPV. And the other six are LR-HPV, including 6, 11, 42, 43, 44 and 81 [27]. Biotin control, internal control, positive control and negative control from the kit were used in each laboratory test as quality control, and only when both of the biotin control and internal control showed coloration, the test was defined as valid and the genotyping results could be read and reported.
Statistical analyses
Chi-Square test and Fisher exact test were used to compare the distribution of categorical variables where appropriate. Unconditional univariable and multivariable logistic regression models were used to estimate the odds ratios (OR) and 95% confidence intervals (CI) in determining the association between the prevalence of HPV infection and CD4+ T cell count or cART. Multivariable logistic regression models were adjusted for age (18–24, 25–34, 35–44, 45–54, 55–82 years), level of education (illiterate or primary school, middle school, high school, college or above), marital status (never married, currently married, widowed/divorced), smoking amount (pack year), alcohol consumption (drank≤ once/month, drank > once/month), sexual orientation (hetero-sexual, homo-sexual, bi-sexual or uncertain), self-reported history of STIs (no or yes), life-time multiple sex partners (no or yes), condom use in the last six months(no or yes). A two-sided p-value < 0.05 was considered as statistically significant. All statistical analyses were performed using SAS 9.4 statistical software (SAS Institute Inc., Cary, NC).
Results
A total of 766 HIV-positive male participants with median age of 46.9 years (range, 18.0–82.0 years) were included in this analysis. 741 participants were under cART (96.7%). Distribution of the socio-demographic and HIV-related characteristics, as well as the prevalence of HPV infection are shown in Table 1. The majority of the study population had a level of education of middle school or lower. One third of the participants were never married; 54.0% reported their sexual orientation as heterosexual, 35.0% homosexual, and 11.0% bisexual or uncertain. 26.5% of the participants self-reported a history of STIs. The median baseline CD4+ T cell count was 264 cells/µL (interquartile range [IQR], 152–344 cells/µL), and the median current CD4+ T cell count was 446 cells/µL (IQR, 282–588 cells/µL). About half of the participants were having EFV + 3TC + TDF for cART treatment, 25.1% were EFV + 3TC + AZT, 16.7% were NVP + 3TC + AZT, and all others combined accounted for 8.8% (Table 1).
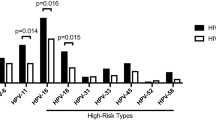
The prevalence of each genotype of anal HPV infection is shown in Fig. 1, with HPV-52 (11.2%), HPV-58 (10.2%) and HPV-16 (9.4%) being the top three for HR-HPV, and HPV-6 (12.3%), HPV-11 (8.9%) and HPV-44 (3.0%) being the top three for LR-HPV. There are 16.7% participants infected with only one genotype of HPV, and 29.0% participants infected with two or more than two genotypes (multiple infections). Overall, the prevalence of anal HPV infection was 48.7% for any HPV type, 40.3% for any HR-HPV, and 22.6% for any LR-HPV (Table 1). The prevalence of anal HPV infection decreased significantly with the increase of age, and was higher among those with higher level of education. The prevalence of any type of HPV infection was 39.4% for heterosexual, lower than the 59.7% for homosexual and 62.7% for bisexual or uncertain (p < 0.001). The HPV prevalence was lower among those with increased CD4+ T cell count than those with decreased or unchanged (46.5 vs. 56.6%, p = 0.033) comparing current to baseline tests. The prevalence of HPV was lower among those with longer duration on cART (p = 0.017). Similar differences were observed for HR-HPV and LR-HPV prevalence for most of the above-mentioned characteristics (Table 1).
The prevalence of each genotype of anal HPV infection among HIV-positive males in Taizhou, China. There are 16.7% participants infected with only one genotype of HPV, and 29.0% participants infected with two or more than two genotypes (multiple infections). The prevalence adds up to more than 100% because of multiple infection
Univariable (Table 2) and multivariable (Table 3) logistic regression models were conducted to examine the associations between CD4+ T cell counts, cART treatments, duration of cART and other characteristics with prevalence of HPV. In multivariable models, after adjusting for covariates, having the current CD4+ T cell count of 350–499 cells/µL (aOR 0.28, 95% CI 0.13–0.64), and of ≥ 500 cells/µL (aOR 0.26, 95% CI 0.11–0.60) were inversely associated with prevalence of any type HPV infection compared with those of < 200 cells/µL. Similar significant associations were observed for any HR-HPV infection, but not LR-HPV. Having taken NVP + 3TC + AZT compared with those taking EFV + 3TC + TDF was inversely associated with any HR-HPV infection (aOR 0.47, 95% CI 0.25–0.90), and inversely associated with any LR-HPV infection (aOR 0.40, 95% CI 0.18–0.88), but not any HPV infection. The association between duration of cART and any type of HPV infection had not been observed. The longer duration on cART was associated with lower HPV prevalence in univariable model, while the association was null in multivariable model (Tables 2 and 3).
Discussion
In this study, we report a lower prevalence of any HPV and any HR-HPV infection among people with increased CD4+ T cell count, and suggest an inverse association of NVP + 3TC + AZT of cART with HR-HPV and LR-HPV prevalence in a HIV-positive male population in China.
Our study found that the prevalence of anal HPV infection among HIV-positive males was high, especially for any HPV (48.7%) and any HR-HPV (40.3%), after expanding the sample size from the previous cross-sectional study [28]. HIV-positive men who have sex with men still showed the highest prevalence for anal HPV infection, which was concordant with observations all over the world [9,10,11, 29, 30].
We found that higher CD4+ T cell count was inversely associated with anal HPV infection in this HIV male population. Lower HPV and HR-HPV prevalence were not only seen among the participants from the higher current CD4+ T cell count groups, but also among those with an increased CD4+ T cell count from baseline. Yet the effect of CD4+ T cell counts on HPV infection is inconsistent from literature. Some studies have reported that increased CD4+ T cell count were associated with lower risk of HPV infection [15,16,17, 31, 32], but others have not observed a significant association [5, 6, 18]. We added some evidence that an improved immune function represented by CD4 might help preventing or clearing HPV infection. This also emphasized the importance of timely cART use for the prevention of HPV infection in people living with HIV.
Most studies analyzing the impact of cART on HPV infection reported beneficial effect [19, 20, 33,34,35]. A meta-analysis concluded that cART is associated with decreased prevalence of anal HR-HPV infection in HIV population [19]. A prospective study reported that cART reduces the risk of acquiring anal HR-HPV infection in HIV positive men who have sex with men (MSM) [20]. However, negative or null associations were also reported [21, 22]. The HIV-infected people are given cART immediately after HIV diagnoses in China since 2016. We observed that the duration on cART was marginally inversely associated with HPV infection. We also explored the associations between specific cART treatments and HPV infection, and found that participants taking NVP + 3TC + AZT showed the lowest prevalence of any HR-HPV and any LR-HPV infection compared with those taking EFV + 3TC + TDF or EFV + 3TC + AZT. Taking NVP + 3TC + AZT is also associated with a greater proportion gaining CD4 increase from baseline in the study population (data not shown), suggesting a potential indirect effect from the therapy through CD4 improvement on HPV infection. Both laboratory and human studies have looked into associations of HIV drugs, especially protease inhibitors, with HPV infection and HPV related disease progression [23]. Studies found that HIV protease inhibitor lopinavir inhibited the ability of HPV16 E6 in degrading p53 expression in vitro [36], lopinavir increased expression of antiviral protein in HPV positive cervical carcinoma cells [37], and ritonavir or saquinavir inhibited cervical intraepithelial neoplasia progression in either HIV-infected or uninfected women [38]. A small single-arm trial conducted in Kenya found that lopinavir/ritonavir is effective in clearance of HPV infection and regression of cervical leasions [39]. However, an observational study in HIV-positive women in the United States did not observe significant association between lopinavir and oncogenic HPV prevalence or clearance [40]. Although in the current study we did not have enough statistical power to examine the association of lopinavir/ritonavir (only 5% participants were using it), these interesting findings deserve further investigation in both population study on disease progression and laboratory exploration for underlying mechanism.
This study has several limitations. First, sexual behavior characteristic was based on self-reported by participants and may not completely represent actual exposure. Second, the recruitment of participants was based on a convenient sample instead of a random sample. It was possible that the participants differed in behavior or knowledge related to HPV infection from those who did not participate. Third, the testing kit for HPV used in this study was capable of detecting 21 genotypes of HPV, which did not cover all genotypes. Thus the prevalence of HPV might have been under-estimated. However, the kit included the most important genotypes of HR-HPV including 16 and 18, and the most prevalent LR-HPV 6 and 11, we think the measurement of outcome is sufficient in this study. Fourth, this study only included HIV-positive males from four CDCs of Taizhou. Hence, it is necessary to be cautious to generalize the results to all HIV-positive males in China.
Conclusion
In conclusion, we observed lower prevalence of anal HPV infection among those HIV-infected men with increased CD4, and those taking NVP + 3TC + AZT. Given the high prevalence of HPV infection among HIV-positive males, a large-scale prospective study on natural history of HPV infection should be conducted in the future to confirm the associations. Besides the impact of CD4 and specific antiretroviral treatments, other host and environmental factors on the persistent HPV infection are of interest for further investigation.
Availability of data and materials
The datasets generated and/or analysed during the current study are not publicly available due to the privacy but are available from the corresponding author on reasonable request.
Abbreviations
- 3TC:
-
Lamivudine
- aOR:
-
Adjusted odds ratio
- AZT:
-
Zidovudine
- cART:
-
Combined antiretroviral therapy
- CD4:
-
Cluster of differentiation 4
- CDC:
-
Center for Disease Control and Prevention
- CI:
-
Confidence interval
- DNA:
-
Deoxyribonucleic acid
- EFV:
-
Efavirenz
- HIV/AIDS:
-
Human immunodeficiency virus/acquired immunodeficiency syndrome
- HPV:
-
Human papillomavirus
- HR:
-
High risk
- IQR:
-
Interquartile range
- LR:
-
Low risk
- MSM:
-
Men who have sex with men
- NNRTIs:
-
Non-nucleoside reverse transcriptase inhibitors
- NRTIs:
-
Nucleoside reverse transcriptase inhibitors
- NVP:
-
Nevirapine
- OR:
-
Odds ratios
- PBS:
-
Phosphate Buffer Saline
- PIs:
-
Protease inhibitors
- STI:
-
Sexual transmitted infection
- TDF:
-
Tenofovir
References
Bosch FX, Manos MM, Muñoz N, Sherman M, Jansen AM, Peto J, et al. Prevalence of human papillomavirus in cervical cancer: a worldwide perspective. International biological study on cervical cancer (IBSCC) Study Group. J Natl Cancer Inst. 1995;87:796–802.
de Sanjosé S, Diaz M, Castellsagué X, Clifford G, Bruni L, Muñoz N, et al. Worldwide prevalence and genotype distribution of cervical human papillomavirus DNA in women with normal cytology: a meta-analysis. Lancet Infect Dis. 2007;7:453–9.
Spence T, Bruce J, Yip KW, Liu F-F. HPV associated head and neck cancer. Cancers. 2016. https://doi.org/10.3390/cancers8080075.
de Martel C, Plummer M, Vignat J, Franceschi S. Worldwide burden of cancer attributable to HPV by site, country and HPV type. Int J Cancer. 2017;141:664–70.
Mooij SH, van Santen DK, Geskus RB, van der Sande MAB, Coutinho RA, Stolte IG, et al. The effect of HIV infection on anal and penile human papillomavirus incidence and clearance: a cohort study among MSM. AIDS Lond Engl. 2016;30:121–32.
Critchlow CW, Hawes SE, Kuypers JM, Goldbaum GM, Holmes KK, Surawicz CM, et al. Effect of HIV infection on the natural history of anal human papillomavirus infection. AIDS. 1998;12:1177–84.
Silverberg MJ, Lau B, Justice AC, Engels E, Gill MJ, Goedert JJ, et al. Risk of anal cancer in HIV-infected and HIV-uninfected individuals in North America. Clin Infect Dis Off Publ Infect Dis Soc Am. 2012;54:1026–34.
Piketty C, Selinger-Leneman H, Grabar S, Duvivier C, Bonmarchand M, Abramowitz L, et al. Marked increase in the incidence of invasive anal cancer among HIV-infected patients despite treatment with combination antiretroviral therapy. AIDS Lond Engl. 2008;22:1203–11.
Supindham T, Chariyalertsak S, Utaipat U, Miura T, Ruanpeng D, Chotirosniramit N, et al. High prevalence and genotype diversity of anal HPV infection among MSM in Northern Thailand. PLoS ONE. 2015;10:e0124499.
Hernandez AL, Karthik R, Sivasubramanian M, Raghavendran A, Gnanamony M, Lensing S, et al. Prevalence of anal HPV infection among HIV-positive men who have sex with men in India. J Acquir Immune Defic Syndr. 1999;2016(71):437–43.
Blas MM, Brown B, Menacho L, Alva IE, Silva-Santisteban A, Carcamo C. HPV prevalence in multiple anatomical sites among men who have sex with men in peru. PLoS ONE. 2015;10:e0139524.
Ablanedo-Terrazas Y, Romero-Mora K, Gómez-Palacio M, Alvarado-de la Barrera C, Ruiz-Cruz M, Hernández-Juan R, et al. Prevalence and risk factors for oral human papillomavirus infection in Mexican HIV-infected men. Salud Publica Mex. 2018;60:653–7.
Cranston RD, Carballo-Diéguez A, Gundacker H, Richardson BA, Giguere R, Dolezal C, et al. Prevalence and determinants of anal human papillomavirus infection in men who have sex with men and transgender women. Int J STD AIDS. 2019;30:154–62.
Cheng S-H, Chu F-Y, Lin Y-S, Hsueh Y-M. Influence of age and CD4+ T cell counts on the prevalence of genital human papillomavirus infection among HIV-seropositive men who have sex with men in Taiwan. J Med Virol. 2012;84:1876–83.
Schwartz LM, Castle PE, Follansbee S, Borgonovo S, Fetterman B, Tokugawa D, et al. Risk factors for anal HPV infection and anal precancer in HIV-infected men who have sex with men. J Infect Dis. 2013;208:1768–75.
Bertisch B, Franceschi S, Lise M, Vernazza P, Keiser O, Schöni-Affolter F, et al. Risk factors for anal cancer in persons infected with HIV: a nested case-control study in the Swiss HIV Cohort Study. Am J Epidemiol. 2013;178:877–84.
Torres-Ibarra L, Conde-Glez CJ, Salmerón J, Palefsky J, Hernández-Nevares P, Sánchez-Alemán MA, et al. Risk factors for anal HPV-16/18 infection in Mexican HIV-infected men who have sex with men. Prev Med. 2014;69:157–64.
Chambuso R, Ramesar R, Kaambo E, Murahwa AT, Abdallah MOE, De Sousa M, et al. Age, absolute CD4 count, and CD4 percentage in relation to HPV infection and the stage of cervical disease in HIV-1-positive women. Medicine (Baltimore). 2020;99:e19273.
Kelly H, Chikandiwa A, Alemany Vilches L, Palefsky JM, de Sanjose S, Mayaud P. Association of antiretroviral therapy with anal high-risk human papillomavirus, anal intraepithelial neoplasia, and anal cancer in people living with HIV: a systematic review and meta-analysis. Lancet HIV. 2020;7:e262–78.
Donà MG, Giuliani M, Rollo F, Vescio MF, Benevolo M, Giglio A, et al. Incidence and clearance of anal high-risk Human Papillomavirus infection and their risk factors in men who have sex with men living with HIV. Sci Rep. 2022;12:184.
Del Mistro A, Bertorelle R, Franzetti M, Cattelan A, Torrisi A, Giordani MT, et al. Antiretroviral therapy and the clinical evolution of human papillomavirus-associated genital lesions in HIV-positive women. Clin Infect Dis Off Publ Infect Dis Soc Am. 2004;38:737–42.
Shrestha S, Sudenga SL, Smith JS, Bachmann LH, Wilson CM, Kempf MC. The impact of highly active antiretroviral therapy on prevalence and incidence of cervical human papillomavirus infections in HIV-positive adolescents. BMC Infect Dis. 2010;10:295.
Hampson L, Oliver AW, Hampson IN. Using HIV drugs to target human papilloma virus. Expert Rev Anti Infect Ther. 2014;12:1021–3.
CDCTobaccoFree. Burden of Tobacco Use in the U.S. Centers for Disease Control and Prevention. 2021. https://www.cdc.gov/tobacco/campaign/tips/resources/data/cigarette-smoking-in-united-states.html. Accessed 28 Feb 2022.
National Center for AIDS/STD Control and Prevention, ChinaCDC. Manual of National free antiretroviral treatment(ART)program for HIV/AIDS in China. 4th ed. Beijing: People’s Health Publishing House; 2016.
List of Classifications – IARC Monographs on the Identification of Carcinogenic Hazards to Humans. https://monographs.iarc.who.int/list-of-classifications. Accessed 28 Feb 2022.
Muñoz N, Bosch FX, de Sanjosé S, Herrero R, Castellsagué X, Shah KV, et al. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med. 2003;348:518–27.
Liu X, Lin H, Chen X, Shen W, Ye X, Lin Y, et al. Prevalence and genotypes of anal human papillomavirus infection among HIV-positive vs. HIV-negative men in Taizhou China. Epidemiol Infect. 2019;147:e117.
Jin Z-Y, Liu X, Ding Y-Y, Zhang Z-F, He N. Cancer risk factors among people living with HIV/AIDS in China: a systematic review and meta-analysis. Sci Rep. 2017;7:4890.
Ma X, Wang Q, Ong JJ, Fairley CK, Su S, Peng P, et al. Prevalence of human papillomavirus by geographical regions, sexual orientation and HIV status in China: a systematic review and meta-analysis. Sex Transm Infect. 2018;94:434–42.
Konopnicki D, Manigart Y, Gilles C, Barlow P, de Marchin J, Feoli F, et al. Sustained viral suppression and higher CD4(+) T-cell count reduces the risk of persistent cervical high-risk human papillomavirus infection in hiv-positive women. J Infect Dis. 2013;207:1723–9.
Jong E, van Gorp ECM, Mulder JW, Tol A, Smits PHM. Effect of HIV viral load, CD4 cell count and antiretroviral therapy on human papillomavirus prevalence in urine samples of HIV-infected men. Int J Std Aids. 2009;20:262–4.
van der Snoek EM, van der Ende ME, den Hollander JC, Schutten M, Neumann H a. M, van Doornum GJJ. Use of highly active antiretroviral therapy is associated with lower prevalence of anal intraepithelial neoplastic lesions and lower prevalence of human papillomavirus in HIV-infected men who have sex with men. Sex Transm Dis. 2012;39:495–500.
Paramsothy P, Jamieson DJ, Heilig CM, Schuman PC, Klein RS, Shah KV, et al. The effect of highly active antiretroviral therapy on human papillomavirus clearance and cervical cytology. Obstet Gynecol. 2009;113:26–31.
Wang Q, Ma X, Zhang X, Ong JJ, Jing J, Zhang L, et al. Human papillomavirus infection and associated factors for cervical intraepithelial neoplasia in women living with HIV in China: a cross-sectional study. Sex Transm Infect. 2019;95:140–4.
Hampson L, Kitchener HC, Hampson IN. Specific HIV protease inhibitors inhibit the ability of HPV16 E6 to degrade p53 and selectively kill E6-dependent cervical carcinoma cells in vitro. Antivir Ther. 2006;11:813–25.
Batman G, Oliver AW, Zehbe I, Richard C, Hampson L, Hampson IN. Lopinavir up-regulates expression of the antiviral protein ribonuclease L in human papillomavirus-positive cervical carcinoma cells. Antivir Ther. 2011;16:515–25.
Barillari G, Iovane A, Bacigalupo I, Palladino C, Bellino S, Leone P, et al. Ritonavir or saquinavir impairs the invasion of cervical intraepithelial neoplasia cells via a reduction of MMP expression and activity. AIDS Lond Engl. 2012;26:909–19.
Hampson L, Maranga IO, Masinde MS, Oliver AW, Batman G, He X, et al. A single-arm, proof-of-concept trial of lopimune (lopinavir/ritonavir) as a treatment for HPV-related pre-invasive cervical disease. PLoS ONE. 2016;11:e0147917.
Lahiri CD, Dugan KB, Xie X, Reimers L, Burk RD, Anastos K, et al. Oral Lopinavir Use and Human Papillomavirus Infection in HIV-Positive Women. J Acquir Immune Defic Syndr. 1999;2015(70):e63-66.
Acknowledgements
We gratefully acknowledge all participants for their contribution to the study.
Funding
This work was supported by the National Natural Science Foundation of China (81803291), Shanghai Municipal Health and Family Planning Commission (20164Y0232), Fudan University (JJF201041, JIF201104) and Taizhou Program of Planning for Science and Technology (21YWA68).
Author information
Authors and Affiliations
Contributions
XL, NH and HL designed and supervised the overall study, and contributed to data analyses and interpretation of the data. JZ analyzed and interpreted the data, and drafted the manuscript. XC, WS and HL organized the survey and sample collection. XY, YL, ZL and ST conducted the survey and collected the samples. JZ, YW cleaned and analyzed the data. YD provided help in laboratory test and data analysis. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The procedures followed for human subjects were in accordance with the ethical standards of the Helsinki Declaration of the World Medical Association. The study was approved by the Institutional Review Board of Fudan University with IRB#2018-TYSQ-03-12, and all the participants provided written informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhang, J., Chen, X., Ye, Y. et al. Increased CD4+ T cell count is associated with lower anal human papillomavirus prevalence among HIV-positive male cohort in Taizhou, China: a cross-sectional study. BMC Infect Dis 22, 250 (2022). https://doi.org/10.1186/s12879-022-07251-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-022-07251-3