Abstract
Background
Many studies have reported high efficacy and safety of artesunate-amodiaquine (AS-AQ) and artemether-lumefantrine (AL) when administered under direct observation in Cameroon. There is paucity of data to support their continuous use in home-based treatment of uncomplicated Plasmodium falciparum malaria in Cameroon. Hence, this study aimed to assess the effectiveness and safety of AS-AQ versus AL for home-based treatment of uncomplicated P. falciparum malaria among children 6–120 months in Yaoundé, Cameroon.
Methods
A two-arm, open-label, randomized, controlled trial comparing the equivalence of AS-AQ (experimental group) and AL (control group) was carried out from May 2019 to April 2020 at two secondary hospitals in Yaoundé. Participants were randomized to receive either AS-AQ or AL. After the first dose, antimalarial drugs were given at home, rather than under direct observation by a study staff. The conventional on-treatment and post-treatment laboratory and clinical evaluations were not done until day 3 of the full antimalarial treatment course. The evaluation of effectiveness was mainly based on per protocol polymerase chain reaction adjusted adequate clinical and parasitological response (PP PCR adjusted ACPR) on day 28 post-treatment. Safety was based on assessment of adverse events (AEs) and severe adverse events (SAEs) from day 1 to day 28.
Results
A total of 242 children were randomized to receive AS-AQ (n = 114) and AL (n = 128). The PP PCR adjusted day 28 cure rates were [AS-AQ = 96.9% (95% CI, 91.2–99.4) versus AL = 95.5% (95% CI, 89.9–98.5), P = 0.797]. Expected mild to moderate adverse events were reported in both arms [AS-AQ = 83 (84.7%) versus AL = 99 (86.1%), P = 0.774]. The most common adverse events included: transient changes of hematologic indices and fever.
Conclusions
This study demonstrated that AS-AQ and AL are effective and safe for home management of malaria in Yaoundé. The evidence from this study supports the parallel use of the two drugs in routine practice. However, the findings from this study do not describe the likely duration of antimalarial effectiveness in holoendemic areas where multiple courses of treatment might be required.
Trial registration: This study is a randomized controlled trial and it was retrospectively registered on 23/09/2020 at ClinicalTrials.gov with registration number NCT04565184.
Similar content being viewed by others
Background
Malaria remains a major public health challenge in sub-Saharan Africa, Southeast Asia and Latin America despite the deployment and scaling up of control measures [1]. In 2020, the disease accounted for 241 million cases and 627,000 related deaths worldwide [1]. Since April 2001, the World Health Organization (WHO) has recommended the swift change of policy from monotherapies to artemisinin-based combination therapies (ACTs) in the treatment of uncomplicated P. falciparum malaria due to rapid emergence and dispersal of drug resistant parasites [2]. Clinical trials are the mainstay for monitoring the efficacy of ACTs in vivo [3]. WHO has approved six ACTs: artesunate-amodiaquine (AS-AQ), artemether-lumefantrine (AL), dihydroartemisinin-piperaquine (DHA-PPQ), artesunate-mefloquine (AS-MQ), artesunate + sulfadoxine-pyrimethamine (AS + SP) and artesunate-pyronaridine (AS-PY) [4, 5]. The sixth ACT (AS-PY) was recently adopted due to its high efficacy and safety profile despite initial concerns raised in previous studies [6, 7]. Cameroon adopted the use of AS-AQ and AL in 2004 and 2006 respectively for the treatment of P. falciparum malaria [8]. AS-AQ and AL have been in continuous use since their introduction in 2004 and 2006. Both AS-AQ and AL are used in the Southern regions, but in the North and Far North regions, AL is primarily used for treatment and sulfadoxine-pyrimethamine (SP-AQ) is used for seasonal malaria prophylaxis among children 3 to 59 months [9].
Antimalarial drug effectiveness is a composite assessment that encompasses clinical efficacy (the performance of the medicine under controlled conditions) and real-world clinical practice (less optimal conditions) [10]. Several ACTs have been shown to be highly efficacious [11,12,13,14,15] and effective [16, 17] in the treatment of malaria in different endemic countries across the world. The ACTs already adopted by the WHO are safe with no major adverse events reported [18,19,20,21].
Presently, the ACTs are under threat due to the identification of single nucleotide polymorphisms (SNPs) in the P. falciparum kelch 13 propeller gene conferring resistance to the artemisinins [22]. The prevalence of the mutations (F446I, N458Y, N458Y, Y493H, R539T, I543T, P553L, R561H, P574L, C580Y) has been well documented in the Greater Mekong sub-region in Southeast Asia [23,24,25]. The occurrence of the C469Y [26], R561H [27,28,29], P574L [27,28,29] and A675V [26] polymorphisms has also been recently reported in Africa. This may negatively affect the gains already achieved in the drive towards malaria elimination in endemic countries if measures to curb the threat are not urgently put in place. Furthermore, it has been shown that the emergence of drug resistant parasites could be delayed by using multiple first line ACTs [30, 31].
The WHO recommends the change of treatment lines if the efficacy of ACTs is less than the minimum benchmark of 90%. Many studies have reported high efficacy and safety of artesunate-amodiaquine (AS-AQ) and artemether-lumefantrine (AL) under strict drug administration in Cameroon [13, 32, 33]. There is paucity of data to support their continuous use in home-based treatment of uncomplicated P. falciparum malaria Cameroon. Hence, this study aimed to assess the effectiveness and safety of AS-AQ versus AL for home-based treatment of uncomplicated P. falciparum malaria among children 6–120 months in Yaoundé, Cameroon.
Methods
Study sites
The study was coordinated at the District Hospital, Cité Verte and District Medical Center, Minkoa-Meyos located in the Health Districts of Cité Verte and Nkolbisson, respectively in Yaoundé, Cameroon. The town of Yaoundé (3°50′N 11°31′E) in the Center Region is found at an elevation of 760 m (2493 feet) above sea level [34]. The average temperature is 23.8 °C while the annual rainfall is 1628.3 mm [34]. It has four distinct seasons: two rainy seasons (March–May/June, September–November) and two dry seasons (December–February, June/July–August). Malaria transmission is holoendemic with peak transmission taking place during and immediately following the two rainy seasons with an entomological inoculation rate that varies from 0–90.0 infective bites per person per year [35].
Study design
This study was a two-arm, equivalence, open-label, randomized controlled trial comparing the effectiveness and safety of AS-AQ and AL among children age 6 to 120 months over a 28 days period of follow-up. The experimental group was AS-AQ while the control group was AL.
Study period
The study was conducted from May 2019 to April 2020. This corresponded with the period during which the first participant was enrolled and the last child successfully followed-up.
Sample size determination
The data regarding the efficacy of the two ACTs were derived from previous studies conducted in Yaoundé. The cure rates were 96.8% for AS-AQ in 2014 [12] and 100% for AL in 2009 [32]. The sample size for equivalence was determined using the WHO acceptable cure rate of 95% [3] for AS-AQ within 95% CI, a power of 90% and assuming a difference of 10% between the AS-AQ and AL. The following sample size determination formula was employed [36, 37]:
where: n = sample size; Zα/2 = Z value at two-tailed alpha (0.05) at 95% CI = 1.96, Zβ = Z value of 1-β = 1.28, P1 = proportion of successes on AS-AQ = 95.0%; d = difference between the 2 drugs
A minimum sample of 100 patients was required for the study. With a 14% increase to allow for loss to follow-up and withdrawals during the 28-day follow-up period, 114 patients were allocated to each of the two study arms. Considering a non-compliance rate of 14% to the treatment guideline due to the number of doses (6 doses) administered and timing of doses, 14 additional participants were allocated to the AL arm giving a total of 128.
Study procedures
The study procedure was nearly identical to the standard WHO 28-day efficacy protocol [3]. The main exceptions were that only the first doses of antimalarial drugs were supervised (by clinical staff) and there was no planned clinical or laboratory evaluation until day 3.
Screening, eligibility and enrolment
Patients who fulfilled all the following criteria were included in the study: (i) Children of either gender, aged 6 months to 120 months were recruited; (ii) Uncomplicated P. falciparum malaria confirmed by microscopy using Giemsa-stained thick film with an asexual parasite density within the range > 0 to \(\le\) 200,000 parasites/μl with a slight modification of the WHO recommended guideline [3]; (iii) Presenting with fever (axillary temperature ≥ 37.5 °C) or having a history of fever in the preceding 24 h; (iv) Able to ingest tablets orally (either suspended in water or uncrushed with food); (v) Willingness to participate in the study with written assent from parent/guardian; (vi) Willing and able to attend the clinic on stipulated regular follow-up visits.
The final decision for enrollment depended on the parents or guardians willingness to supervise home-based treatment and to facilitate post-treatment clinical and laboratory evaluations. The parents and guardians always gave written consent in addition to written assent for their children to participate in the study.
Patients who presented with any one of the following criteria were excluded from participating in the study: (i) Mixed or mono-infection with another Plasmodium species detected by microscopy; (ii) Children who were currently suffering or had the following within the last 2 months: tuberculosis, HIV, schistosomiasis, diabetes mellitus, cardiovascular disease, gout, rheumatoid arthritis, underlying chronic hepatic or renal disease, hypoglycemia, jaundice, respiratory distress, and other inflammatory related diseases. (iii) Signs/symptoms indicating severe/complicated malaria according to WHO criteria (WHO definition) such as: parasitemia ≥ 5% of red blood cell count, not able to drink or breast feed, persistent vomiting (> 2 episodes within previous 24 h), convulsions (> 1 episode within previous 24 h), lethargic/unconscious, severe anemia (hemoglobin < 5 g/dl) and axillary temperature > 40 ºC; (iv) Serious gastrointestinal disease; v) Presence of severe malnutrition defined as a child aged between 6–60 months whose weight-for-height is below –3 z-score (W/H < 70%) or has symmetrical edema involving at least the feet or has a mid-upper arm circumference < 115 mm; (vi) Regular medication which may interfere with antimalarial pharmacokinetics; (vii) History of hypersensitivity reactions or contraindications to any of the medicine (s) being tested or used as alternative treatment (s); (viii) Individuals who took part in antimalarial efficacy and safety studies 3 months before the start of the study; (ix) Participants who took antimalarial drugs in the past one month.
Randomization of study participants
A randomization list was produced according to a randomization allocation schedule generated by a computer-based randomization program in blocks of 20 s [38]. The allocation of participants was concealed in opaque envelopes that were opened sequentially by the study pharmacist once assent was provided and enrollment validated by the study physician. The children were randomized to receive either AS-AQ or AL. The randomization codes were recorded on the case report forms against the study identification numbers.
Trial drug administration and follow-up
Artesunate-amodiaquine (AS-AQ, Winthrop, Sanofi, Paris, France, lot numbers: M0001501-01, M0001502-01, M0001503-01) oral tablets supplied were fixed-dosed combinations and available in three dosage forms. Each prescription was based on age groups and weights. The drug, AS-AQ was administered for three days (Additional file 1).
Artemether-lumefantrine (AL, Dispersible Coartem®: Novartis, Basel, Switzerland, lot numbers: KX673, KT866) oral tablets were also fixed-dose combinations provided in blister packs of different doses. Each tablet contained 20 mg artemether and 120 mg lumefantrine. The prescription was based on the weight of the child in line with the manufacturer’s recommended guidelines. The drug, AL was given to each participant for three days (Additional file 1).
A replacement dose was given to any child if vomiting occurred less than 30 min after ingestion. The participant was given half the dose if vomiting took place after 30 min of ingestion. Participants who had more than one episode of vomiting within one hour after drug intake were excluded from the study.
Drug intake was supervised by study staff for the first dose; subsequent doses were supervised by participants’ parents or guardians. Parents/guardians of participants were advised on the time and mode of administration for the 3 days treatment unobserved at home. Antipyretic medications such as paracetamol syrup or tablets were administered together with the study drugs.
Follow-up visits were carried out on days 3, 7, 14, 21, 28 or any other day that the child felt unwell in order to evaluate clinical and parasitological resolution of their malaria episodes as well as adverse drug events. Community health workers were also used to locate the houses of the study participants and to ensure compliance with follow-up schedules.
The rescue treatments administered to participants who developed severe malaria during follow-up included: injectable artesunate, injectable artemether and quinine infusion. These treatments were administered in line with the WHO 2015 guidelines [38].
Clinical evaluation
A standard physical examination was done for body weight, axillary temperature, blood pressure (systolic/diastolic), respiratory rate, and pulse rate (heart rate) at baseline (day 0 before dosing) and on days 3, 7, 14, 21, 28 and during unscheduled visits.
Laboratory analyses
Detection and quantification of the malaria parasite
The presence of malaria parasite was detected in capillary blood or venous blood using the SD BIOLINE Malaria Ag P. falciparum (HRP2/pLDH) (Standard Diagnostics, Incorporation, South Korea) and CareStart™ Malaria HRP2/pLDH (Pf/PAN) Combo (Access Bio, Incorporation, Somerset, New Jersey, United State of America) rapid diagnostic test kits before proceeding with microscopy. Thick and thin blood films for parasite counts and speciation of non-falciparum species were obtained and examined at screening on day 0 to confirm adherence to the inclusion and exclusion criteria [39]. Thick blood films were also examined on days 3, 7, 14, 21, and 28 or on any other day if the patient returned spontaneously and parasitological reassessment was required. The slide for each patient was prepared in duplicates. Thick and thin blood films were dried and stained with 10% Giemsa for at least 15–20 min. Giemsa-stained thick and thin blood films were examined at a magnification of 1000 × to identify the parasite species and to determine the parasite density. At least 200 white blood cells (WBCs) were counted. Parasite density was calculated by multiplying the number of parasites counted per microscopic field by a factor of 40 on the assumption that the average count of WBC is 8000 per µl. If on counting 200 WBCs, the number of parasites was not up to 100, the count continued till 500 WBCs and calculations made to get the estimated parasitemia of the patient. Parasitemia was reported in parasites/μl. A microscopy slide was considered negative when examination of 1000 white blood cells or 100 fields containing at least 10 white blood cells per field revealed no asexual parasites. The presence of gametocytes on an enrollment or follow-up slide was also recorded. To detect the presence of gametocyte, at least 1000 white blood cells were counted. Two qualified microscopists read all the slides independently, and parasite densities were calculated by averaging the two counts. Blood smears with discordant results (differences between the two microscopists in species diagnosis, in parasite density of > 50% or in the presence of parasites) were re-examined by a third, independent microscopist, and parasite density was calculated by averaging the two closest counts. Additionally, 10% of all the slides were randomly selected and read by a senior microscopist for quality control. Enrollment into the study depended on a positive malaria rapid diagnostic test and microscopy.
Measurement of hematology and biochemistry parameters
Hemoglobin level was measured from finger prick blood sample using portable Hemocue 201 and Hemocue 301 spectrophotometers (HemoCue®, Ängelholm, Sweden). Whole blood was collected in ethylene diamine tetra acetic acid (EDTA) tubes and dry tubes for hematology and biochemistry analyses respectively. The samples in EDTA tubes were properly mixed to avoid blood clot before laboratory analyses. Hematological parameters were analyzed with URIT 3000 hematology analyzer (URIT Medical Electronic Co., Limited, Guangxi, China) and Mindray 3000 Plus (Shenzen Mindray Bio-medical Electronic Co., Limited, Shenzen, China). Blood biochemistry parameters were analyzed using the URIT 810 semi-automated Chemistry analyzer (URIT Medical Electronic Co., Limited, Guangxi, China). Measurements were carried out on days 0, 7, 14, 28 and during unplanned visits. Quality control was carried out daily to validate each test run. Each parameter was evaluated by comparing it values with the established reference ranges (Additional file 2).
Malaria parasite DNA extraction
The malaria parasite deoxyribonucleic acid (DNA) was extracted from whole blood collected in EDTA tubes and dried blood spots (DBS) on Whatman® No 3 mm filter papers using the EZNA Biotek method according to the manufacturer’s guidelines. DNA was extracted from the whole blood in EDTA tubes/DBS on day 0 (before treatment) and during recurrence of parasitemia on day 7 onwards (cases of treatment failure).
Genotyping of malaria parasites to differentiate recrudescence from reinfection
Genotyping was done in order to differentiate a recrudescence (same parasite strain) from a newly acquired infection (different parasite strain). The P. falciparum merozoite surface protein 1 (Pfmsp-1), P. falciparum merozoite surface protein 2 (Pfmsp-2) and P. falciparum glutamate-rich protein (Pfglurp) genes were used to discriminate reinfections from recrudescence as previously described [40, 41]. Summarily, DNA fragments obtained from amplification of baseline samples (day 0) and on the day of recurrent parasitemia were compared according to band size and number, considering the 3 families of Pfmsp-1 (MAD20, RO33, K1), the 2 families of Pfmsp-2 (FC27, 3D7/IC) and single family type of Pfglurp. Cases were categorized as new infections when there were no common bands between day 0 and the day of recurrent parasitemia. However, when there was at least one common band between baseline sample and that of the day of parasite reappearance for any of the 3 markers (even if there were additional bands on day 0) the case was recrudescence. Malaria parasite infected cases were considered not to be clinical failures if their recurrent parasitemia were classified as new infections rather than recrudescent infections. The gene amplification was done using the Biometra T3 Thermocycler (United Kingdom).
Assessment of caregivers’ compliance to treatment schedules
Adherence to prescribed treatment schedules was assessed through interviews (self-reporting) on day 3 post-treatment about the timing of treatment, the number of doses administered and the number of days over which drug was given. The interviews were conducted at the health facility. Used packs of drugs were inspected where available. Participants who had persistent vomiting (vomited more than once within one hour) after the first dose was administered, were enrolled without meeting all the inclusion criteria, withdrew consent after day 0 and did not show up on day 3 post-treatment were excluded from the assessment. Adherence was classified as follows: poor adherence (adherence rate < 95%) and good or perfect adherence (adherence rate ≥ 95%).
Evaluation of effectiveness
Treatment effectiveness was evaluated based on clinical and parasitological outcomes. This assessment was done according to the WHO 2009 guideline for monitoring therapeutic efficacy studies [3]. Based on this guideline, treatment outcome was classified as treatment failure and treatment success. Treatment failure is defined as early treatment failure (ETF), late clinical failure (LCF) and late parasitological failure (LPF). Treatment success is defined as adequate clinical and parasitological response (ACPR).
Assessment of safety
Safety was based on assessment of adverse events (AEs) and severe adverse events (SAEs) on day 1 to day 28. A physician from the study team was designated to monitor the safety of ACTs. Adverse events were recorded through interviews or self-reporting about previous symptoms and about symptoms that have emerged since the previous follow-up visit. A clinical examination was performed to determine any adverse event. In addition to clinical assessment, hematological parameters, liver function and renal function were evaluated for abnormal values. An adverse event (AE) is defined according to the International Conference on Harmonization guidelines as any untoward medical occurrences in a patient administered a pharmaceutical product and which does not necessarily have a causal relationship with the treatment of interest [42]. A severe adverse event (SAE) is considered as an untoward medical occurrence that at any dose results in death, is life threatening, requires or prolongs hospitalization, results in persistent and significant disability, is a congenital anomaly/birth defect or is another medically important condition [42].
Study outcomes
-
1)
The primary outcome was the proportion of patients with PP PCR-corrected cure rates for AS-AQ in comparison with AL after a follow-up period of 28 days.
-
2)
The secondary outcome was proportion of participants with adverse events and severe adverse events for AS-AQ in comparison with AL after a follow-up period of 28 days.
-
3)
The other exploratory outcomes investigated were: i) The proportion of patients with PP PCR-corrected cure rates for AS-AQ in comparison with AL on day 7 and day14. ii) The number of children in the two drug groups with parasitemia on day 3, fever on day 3 and gametocyte carriage on days 0, 3, 7, 14, 21 and/or 28. iii) The proportion of children who adhered to AS-AQ and AL treatment guidelines. iv) The evolution of biological parameters between day 0 and day 7.
Data management
The Microsoft Excel 2010 (Microsoft Corporation, Redmond, Washington, United States of America) was used to design the data extraction sheet. Data was double entered by two independent data clerks. Discrepancies were resolved by mutual consent after discussion and independent review from the third data clerk. This was done in order to improve on the quality and acceptability of data generated. The database in Microsoft Excel was piloted and validated before completion of the process.
Statistical analysis
The International Business Machines Statistical Software Package for Social Sciences (IBM SPSS) version 20.0 software package (IBM Corporation, Armonk, New York, United States of America) was used for data analysis. The Pearson chi-square test (χ2) or Fisher’s exact test was used to determine the association between qualitative variables depending on the percentage of cells having an expected frequency < 5. The Shapiro–Wilk test was use to check the normality of quantitative variables. The mean differences for quantitative variables were estimated using the Student’s t test for independent samples if they complied with the normal distribution hypothesis and the Mann–Whitney U test with median when non-normally distributed. Normal data was represented as mean \(\pm\) standard deviation while non-normal data was summarized as median (interquartile range).
The effectiveness of AS-AQ vs. AL was assessed based on unadjusted and adjusted PCR ACPR. The following parameters were used: intention-to-treat (ITT) population, per-protocol (PP) population and Kaplan–Meier (K-M) survival estimates. The significance of observed differences between the treatment arms based on ITT and PP analyses were assessed using the Pearson chi-square test or Fisher’s exact test. The log rank (Mantel-Cox) chi-square test was used to investigate the difference between the drug groups based on K-M survival estimates. The significance of observed differences between the safety parameters of AS-AQ and AL were also evaluated using the Pearson chi-square test or Fisher’s exact test.
The assessment of equivalence of the two drug groups was based on: P < 0.05 (not equivalent) or P > 0.05 (equivalent).
All P-values were two-tailed, and values less than 0.05 were considered to be statistically significant at 95% confidence interval.
Ethical considerations
This study was reviewed and approved by the Center Regional Ethics Committee (CE Nº 05859/CRERSHC/2019) and National Ethics Committee (Nº 2018/07/1091/CE/CNERSH/SP) for Human Health Research, Ministry of Public Health, Yaoundé, Cameroon. Administrative authorizations were also sought from the Director of District Hospital Cité Verte, Director of District Medical Center Minkoa-Meyos, Center Regional Delegate of Public Health and the Minister of Public Health. Parents/guardians of participants were explained the full scope of the study and the right of their children to participate or not without any prejudice; participation could be stopped at any time without any explanation. Written consent and assent in French or English were obtained from all parents or guardians of eligible children before enrollment into the study. Subjects with malaria were treated in accordance with the National Malaria Control Program guideline. The clinical trial was registered retrospectively and posted at ClinicalTrials.gov on 23/09/2020 with registration number: NCT04565184 (https://clinicaltrials.gov/ct2/show/NCT04565184).
Results
Trial profile on enrollment, allocation, intervention, follow-up and data analysis
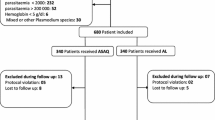
Out of 987 participants screened for eligibility, 242 met the inclusion criteria and were enrolled into the study while 745 children who did not meet the inclusion criteria were excluded from the study. A total of 242 participants were randomized to receive either artesunate-amodiaquine (AS-AQ) or artemether-lumefantrine (AL) in the ratio of 114:128. In the AS-AQ group, 16 children were excluded due to protocol violation (n = 6) and failure to appear on day 3/other days of follow-up (n = 10). Similarly, in the AL group, 13 children were excluded because of the following reasons: withdrawal of consent (n = 1), protocol violation (n = 3) and failure to appear on day 3/other days of follow-up (n = 8).
In the AS-AQ arm, 114 participants were included in the intention-to-treat (ITT) analysis while 98 children were included in the per protocol (PP) analysis after the exclusion of 16 children. Likewise, in the AL arm, 128 participants were included in the intention-to-treat (ITT) analysis while 115 children were included in the per protocol (PP) analysis after the exclusion of 14 children. The 28 days period of follow-up was adopted in both treatment arms (Fig. 1).
Trial profile of effectiveness and safety study on artesunate-amodiaquine (AS-AQ) and artemether-lumefantrine (AL) in Yaoundé, Cameroon. *Enrollment violation (1 participant had baseline parasitaemia > 200,000 parasites/µl, 2 had only positive results by malaria rapid diagnostic test and 2 had signs of severe malaria); Treatment violation (1 did not comply with the treatment procedure). **Enrollment violation (1 participant had baseline parasitaemia > 200,000 parasites/µl and 1 had only positive result by malaria rapid diagnostic test); Treatment violation (1 did not comply with the treatment procedure)
Baseline characteristics of the study participants treated with artesunate-amodiaquine (AS-AQ) and artemether-lumefantrine (AL) using the ITT population
Out of 114 participants who received AS-AQ, there were more males than females (68:46). Likewise, out of 128 children assigned to the AL arm, there were more males than females (72:56). In both drug arms, majority of the study participants were enrolled in the age group 60–120 months [AS-AQ 58 (50.9%) versus AL 73 (57.0%)]. The median age was 58.5 months in the AS-AQ arm while in the AL arm it was 64.5 months.
Furthermore, there were no statistically significant differences observed in all the categorical and quantitative variables evaluated in the two comparable independent treatment arms (P > 0.05). This permitted the comparison between the two drug groups at baseline (Table 1).
Primary outcome evaluation of in vivo effectiveness of AS-AQ and AL for the treatment of uncomplicated Plasmodium falciparum malaria on day 28 (intention-to-treat and per protocol analyses)
The in vivo effectiveness on day 28 using unadjusted PCR intention-to-treat (ITT) analyses were 82.5% (95% CI, 74.2–88.9) for AS-AQ and 83.6% (95% CI, 76.0–89.6) for AL. The ITT cure rates adjusted by PCR were 83.2% (95% CI, 75.0–89.6) for AS-AQ and 85.6% (95% CI, 78.2–91.2) for AL. Conversely, the PCR unadjusted in vivo effectiveness obtained from per protocol (PP) analyses were 95.9% (95% CI, 89.9–98.9) for AS-AQ and 93.0% (95% CI, 86.8–97.0) for AL. The PCR adjusted ACPR derived from PP analyses were 96.9% (95% CI, 91.2–99.4) for AS-AQ and 95.5% (95% CI, 89.9–98.5) for AL. The ITT and PP (PCR uncorrected and corrected) cure rates were not significantly different across arms (P > 0.05) (Table 2).
Treatment failures for the two drugs were due to early treatment failure (ETF), late clinical failure (LCF) and late parasitological failure (LPF) unadjusted and adjusted by PCR. There were 8 cases of treatment failures (3 for AS-AQ and 5 for AL) when adjusted by PCR. Recrudescence accounted for only one case of LPF in the AL arm after PCR correction. There were four cases of reinfections (1 for AS-AQ arm and 3 for AL arm) registered during the period of follow-up (Table 2).
Assessment of in vivo effectiveness on D7 and D14 based on per-protocol analysis
The adjusted PCR adequate clinical and parasitological response (Adjusted PCR ACPR) rates on day 7 and day 14 were: 96.9% for AS-AQ and 96.5% for AL. The cure rates did not differ when compared on day 7 and/or day 14 for either arm (P = 1.000) (Table 3).
Kaplan–Meier survival estimates for in vivo effectiveness of AS-AQ versus AL (PP without and with PCR correction)
The Kaplan–Meier survival cure rates without PCR correction were 96.5% (95% CI, 93.2–99.8) for AS-AQ and 93.8% (95% CI, 89.1–98.5) for AL. The two groups were not statistically different when compared (P = 0.363) (Table 4 and Additional file 3).
The Kaplan–Meier survival cure rates with PCR correction were 97.4% (95% CI, 94.1–100.0) for AS-AQ and 96.1% (95% CI, 92.4–99.8) for AL. Similarly, the two groups were not statistically different when compared (P = 0.605) (Table 4 and Additional file 3).
Evaluation of fever, parasitemia clearance and adherence to treatment guideline on D3 of the study
It was observed that 93 (92.1%) of study participants in the AS-AQ arm and 99 (86.1%) in the AL arm of study participants did not have fever on day 3 (P = 0.162). The proportion of children who cleared their parasites on day 3 were 70 (69.3%) for AS-AQ and 85 (73.9%) for AL (P = 0.453) (Table 5).
Good adherence to treatment guidelines were recorded in the AS-AQ [100/101 (adherence rate = 99.0%, 95% CI, 94.6–100.0)] and AL [115/116 (adherence rate = 99.1%, 95% CI, 95.3–100.0)] drug arms. The adherence rate was not statistically different between the two treatment groups [P = 1.000] (Table 5).
Proportion of gametocyte carriage at enrollment and post-treatment
The total number of the study participants carrying gametocytes on day 0, 3, 7, 14, 21 and/or 28 by microscopy were 5 (4.5%) for AS-AQ and 5 (4.0%) for AL (P = 1.000). In the AS-AQ arm, the highest proportion of gametocyte (n = 2, 2.0%) was recorded on day 3 and day 7 of follow-up while the lowest proportion was documented on day 0 (n = 1, 0.9%). In contrast, in the AL arm the highest prevalence of gametocyte (n = 2, 1.6%) was registered on day 0 while the lowest rate of (n = 1, 0.9%) was recorded on day 3, day 7, and day 14. There was complete gametocyte clearance on day 21 and 28 in both drug arms. None of the study participants in the two drug arms had more than one episode of gametocyte carriage post-treatment.
Safety and tolerability endpoints in the study arms of AS-AQ versus AL from day 1 to day 28 (per protocol analysis)
A total of 83 (84.7%) participants in the AS-AQ arm and 99 (86.1%) participants in the AL arm had mild to moderate adverse events from day 1 to day 28 based on per protocol analysis. Most of the study participants in both drug arms had at least two or more adverse events due to multiple responses. Out of 44 adverse events reported, the most common were: leucocytosis [AS-AQ = 14 (14.3%) versus AL = 20 (17.4%), P = 0.537], lymphocytosis percentage [AS-AQ = 19 (19.4%) versus AL = 16 (13.9%), P = 0.283], granulocytopenia percentage [AS-AQ = 20 (20.4%) versus AL = 22 (19.1%), P = 0.815], lymphocytosis number [AS-AQ = 20 (20.4%) versus AL = 17 (14.8%), P = 0.280], thrombocytosis [AS-AQ = 27 (27.6%) versus AL = 38 (33.0%), P = 0.386] and fever [AS-AQ = 17 (16.3%) versus AL = 28 (24.3), P = 0.150].
Only two types of adverse events had a statistically significant difference between the 2 drug arms: eosinophilia + basophilia-% [AS-AQ = 17 (17.3%) versus AL = 7 (6.1%), P = 0.010] and low diastolic blood pressure [AS-AQ = 0 (0.0%) versus AL = 6 (5.2%), P = 0.032]. All the adverse events resolved post-treatment (Table 6).
Evolution of biological parameters among study participants treated with AS-AQ and AL (Day 0 and Day 7) (PP)
A total of 22 parameters were evaluated in the 2 drug arms between day 0 and day 7. Majority of these parameters 13 (59.0%) did not significantly change over time. However, 9 biological parameters had a significant change between day 0 and day 7. A total of 53 (58.9%) and 66 (63.5%) of participants had abnormal values in the AS-AQ and AL groups respectively. Out of the 9 parameters, the following had significant increase between day 0 and day 7: white blood cell count-WBC (× 109/L) [median (IQR)] [AS-AQ, day 0 = 7.75 (6.20) versus day 7 = 9.75 (5.70), P = 0.006; AL, day 0 = 8.50 (5.1) versus day 7 = 9.30 (5.5), P = 0.029] and platelet count-PLT (× 109/L) [median (IQR)] [AS-AQ, day 0 = 247 (158) versus day 7 = 336 (226), P < 0.0001, AL, day 0 = 198 (157) versus day 7 = 334 (197), P < 0.0001] (Additional file 4).
Discussion
The present study had as primary objective to determine the effectiveness and safety of artesunate-amodiaquine (AS-AQ) versus artemether-lumefantrine (AL) for a period of 28 days among children infected with uncomplicated P. falciparum malaria in Yaoundé, Cameroon. The effectiveness of the two drugs was assessed under less optimal conditions to mimic what happens in the communities 17 years after their adoption and implementation in Cameroon. The approach adopted for this study is a modification of the standard procedure recommended by WHO for monitoring the efficacy of ACTs [3]. The standard method describes what is expected in health care but not what is observed.
The evaluation of in vivo effectiveness using per protocol (PP) analysis gave an adjusted PCR-ACPR value above the WHO benchmark of 90% for AS-AQ. Similar adjusted PCR cure rates for AS-AQ have been recorded before in Cameroon [12] and Burkina Faso [15]. The adjusted PCR cure rate for AS-AQ is higher than the range of 60.0% to 91.7% obtained from studies conducted at the slope of Mount Cameroon [43] and other countries [16, 44,45,46,47,48]. Conversely, the adjusted PCR-ACPR of the present study is lower than range of 98.1%-100.0% registered in supervised clinical trials conducted in Cameroon [13], Burkina Faso [14, 15], Ghana [49] and Senegal [50]. The assessment of in vivo effectiveness using PP analysis also registered a corrected PCR cure rate above 90% for AL. This finding is in agreement with the adjusted PCR cure rate of 95.1% registered in rural Tanzania in 2011 [17]. The adjusted PCR-ACPR is lower than the rates of 96.0–100.0% reported in malaria endemic countries in sub-Saharan Africa [13, 49,50,51], Southeast Asia [11] and South America [52]. A systematic review and meta-analysis on the therapeutic efficacy of AL (Coartem®) for the treatment of P. falciparum malaria in Africa gave a pooled PCR adjusted 97.0% [53]. The adjusted PCR cure rate for AL is higher than the range of 77.7–94.0% previously documented in several antimalarial drug effectiveness [16, 44, 46, 48] and efficacy [15] studies conducted elsewhere. The lower limit of the confidence interval for AL success, as reported, was less than 90% (the WHO benchmark), while the lower limit of the confidence interval for AS-AQ was slightly above this benchmark (91.2%). The differences observed in the effectiveness rates of AS-AQ and AL may be due to heterogeneity of study settings, participants, malaria transmission patterns, sample sizes, and development of resistance against artemisinin partner drugs. Even though, mutations conferring resistance to the artemisinins have not been reported in Cameroon, there is a need to regularly monitor the effectiveness and efficacy of antimalarial drugs as recommended by the WHO.
The comparison of in vivo effectiveness on day 28 did not show any significant difference between AS-AQ and AL. This is in agreement with the findings of the comparative effectiveness and efficacy studies in which the two drugs were used [13, 44, 48]. The insignificant difference in the in vivo effectiveness of AS-AQ versus AL disagrees with the results reported in Burkina Faso [15, 46]. These findings confirm the parallel use of AS-AQ and AL for the treatment of uncomplicated P. falciparum malaria.
Furthermore, the present study registered very few cases of reinfection 14 days post-treatment. This observation tallies with those of other studies that showed an increase in the effectiveness [16, 43, 44, 46, 48, 54] and efficacy [11,12,13,14,15] of AS-AQ and AL after correction with PCR. The AL drug arm documented more cases of reinfections after 14 day of follow-ups when compared with the AS-AQ drug arm [16, 44, 46, 48, 54]. The lack of direct supervision during drug administration did not seem to lower the effectiveness of the ACTs below the acceptable WHO benchmark of 90%. This could be due to perfect adherence by the caregivers to the dosing regimens. There are conflicting claims on the impact of adherence on antimalarial drug efficacy and effectiveness. Some studies did not find a difference in the PCR cure rates between ACT dispensed supervised and unsupervised [17, 45, 54]. This is inconsistent with the findings from another study that reported a significant difference between supervised and unsupervised therapeutic responses [55]. The difference observed may be due insufficient patient adherence. It has been revealed that antimalarial drug efficacy and effectiveness is influenced by a plethora of factors. These factors include: delayed access to drugs, bioavailability, drug quality, drug storage conditions, age [17, 56, 57], multiplicity of infection [58, 59], malaria parasite density [56, 60], immunity [61], pharmacogenomics [62, 63] and nutrition [64].
In both drug arms, fever and parasite disappeared gradually by day 3. Rapid fever and parasite clearance rates have been reported before in Senegal/Ivory Coast [65, 66], Cameroon [13], and Togo [67]. The presence of gametocytes at enrollment and during follow-up days was found to be higher in the AS-AQ group when compared to the AL group. These observations are in harmony with those reported in Senegal/Ivory coast [65, 66] and Togo [67]. A meta-analysis from individual patient data conducted by the Worldwide Antimalarial Resistance Network (WWARN) Gametocyte Study Group confirmed that AL is more effective than the AS-AQ fixed dose combination in preventing gametocyte carriage shortly after treatment [68]. The authors suggested that the non-artemisinin partner drugs and the timing of artemisinin dosing are important determinants responsible for post-treatment gametocyte dynamics [68]. Information on gametocyte carriage reported during this study is important to guide Cameroon government’s policy on malaria control and elimination especially in regions where transmission occur throughout the year.
In addition, expected mild to moderated adverse events were documented in the two drug arms that resolved during follow-up. There was no severe adverse event registered among the study participants. Previous pharmacovigilance studies on AS-AQ and AL have mostly reported the presence of common adverse events associated with the gastrointestinal tract and neuropsychiatric systems [13, 18, 19]. This approach of monitoring adverse events relies on self-reporting by the patients, parents or guardians. A major setback of this method is recall bias. Some parents or guardians may actually have over-reported perceived adverse events because of anxiety about the possibility of adverse events.
The study drugs had a statistical significant effect on 9 biological parameters on day 0 and day 7 in the AS-AQ and AL arms. It was also shown that AS-AQ and AL drugs had varying effects on alanine aminotransferase (ALAT) and creatinine (CREA) levels on day 0 and day 7. These results corroborate with those realized in Senegal and Ivory Coast [65]. These observations also confirm the findings of the study on the efficacy and safety of ACTs in Garoua and Mutengene, Cameroon reported by Nji and colleagues in 2015 [13]. The resolution of abnormal values during follow-up is an indication that AS-AQ and AL are well tolerated and safe.
Strengths and limitations of the study
The major strengths of this study are: (1) This work filled significant gaps in knowledge about the performance of AS-AQ and AL in Cameroon, which should provide important support for policy-making. (2) Both regimens were studied concurrently in the town of Yaoundé in Cameroon seventeen years after policy implementation. The existence of data from previous efficacy trials of the same regimens in the same region was also important. (3) The fact that the study was performed in a holoendemic region in Cameroon is actually important, because the national malaria control program needs data from diverse transmission settings in order to devise rational policy. (4) The design was intended to "mimic the routine standard of care" by omitting clinical supervision of treatment, clinical evaluation, and laboratory testing after day zero and before day three. This is actually a strength given the study team's emphasis on effectiveness.
The major limitations of this study are: (1) There was no direct clinical observation of antimalarial drug dosing (after dose 1), and standard clinical and laboratory evaluations were not conducted after day zero and before day 3. (2) The study was conducted in urban and peri-urban settings of Yaoundé. These areas are characterized with holoendemic malaria transmission. This may not present the reality of what happens in the other malaria transmission settings in Cameroon and globally. (3) The estimated sample size may have been inadequate to establish the equivalence of the two drugs. (4) Families who were willing to be adherent to medication and clinical/laboratory evaluation schedules may not have been representative of the entire population from which the subjects were drawn. Hence, study results may not be generalizable.
Conclusions
This study demonstrated that AS-AQ and AL are effective and well tolerated for home management of uncomplicated P. falciparum malaria among children in Yaoundé, Cameroon. The PP cure rates adjusted by PCR and safety parameters for the two drugs were not statistically different and are said to be substantively the same when compared. The two regimens seem to have retained their effectiveness and safety profiles for over 17 years, in spite of frequent administration likely driven by the intensity of malaria transmission. The evidence from this study supports the government’s policy on the parallel use of AS-AQ and AL in routine practice in the Southern regions of Cameroon. However, the findings from this study do not describe the likely duration of antimalarial effectiveness in holoendemic areas where multiple courses of treatment might be required. It was also reported that the lower limit of confidence interval was slightly below or above the WHO threshold of 90% for AL and AS-AQ respectively. Thus, there is a need to continuously monitor the effectiveness and safety profile of AS-AQ and AL in Cameroon.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ACT:
-
Artemisinin-based combination therapy
- ACPR:
-
Adequate clinical and parasitological response
- AL:
-
Artemether-lumefantrine
- ALT:
-
Alanine aminotransferase
- AS-AQ:
-
Artesunate-amodiaquine
- AST:
-
Aspartate aminotransferase
- CREA:
-
Creatinine
- ETF:
-
Early treatment failure
- LCF:
-
Late clinical failure
- LPF:
-
Late parasitological failure
- WHO:
-
World Health Organization
- WWARN:
-
Worldwide Antimalarial Resistance Network
References
WHO. World Malaria Report 2021. Geneva, World Health Organization. https://www.who.int/publications/i/item/9789240040496. Accessed on 26 Jan2022.
WHO briefing on Malaria Treatment Guidelines and artemisinin monotherapies. https://www.who.int/malaria/publications/atoz/meeting_briefing19april.pdf. Accessed 20 Jul 2021.
WHO. Geneva, World Health Organization 2009. Methods for surveillance of antimalarial drug efficacy. https://www.who.int/malaria/publications/atoz/meeting_briefing19april.pdf. Accessed 12 Feb 2019.
WHO. Geneva, World Health Organization 2015. Guidelines for the treatment of malaria. Third edition. https://www.who.int/malaria/publications/atoz/9789241549127/en/. Accessed15 Jul 2020.
WHO. Geneva, World Health Organization. Tackling antimalarial drug resistance. https://www.who.int/publications/m/item/WHO-UCN-GMP-2020.07. Accessed 3 May 2021.
Sagara I, Beavogui AH, Zongo I, Soulama I, Borghini-Fuhrer I, Fofana B, et al. Pyronaridine–artesunate or dihydroartemisinin–piperaquine versus current first-line therapies for repeated treatment of uncomplicated malaria: a randomised, multicentre, open-label, longitudinal, controlled, phase 3b/4 trial. Lancet. 2018;391(10128):1378–90.
Han KT, Lin K, Han ZY, Myint MK, Aye KH, Thi A, et al. Efficacy and safety of pyronaridine-artesunate for the treatment of uncomplicated Plasmodium falciparum and Plasmodium vivax malaria in Myanmar. Am J Trop Med Hyg. 2020;103(3):1088–93. https://doi.org/10.4269/ajtmh.20-0185.
Cameroon National Malaria Control Program (NMCP) annual report of activities 2006.
Cameroon National Malaria Control Program (NMCP) Annual Report of Activities 2016.
Rathmes G, Rumisha SF, Lucas TCD, Twohig KA, Python A, Nguyen M, et al. Global estimation of anti-malarial drug effectiveness for the treatment of uncomplicated Plasmodium falciparum malaria 1991–2019. Malar J. 2020;19(1):374. https://doi.org/10.1186/s12936-020-03446-8.
van der Pluijm RW, Tripura R, Hoglund RM, Pyae Phyo A, Lek D, ul Islam A, et al. Triple artemisinin-based combination therapies versus artemisinin-based combination therapies for uncomplicated Plasmodium falciparum malaria: a multicentre, open-label, randomised clinical trial. Lancet. 2020;395(10233):1345–60.
Tahar R, Almelli T, Debue C, Foumane Ngane V, Djaman Allico J, Whegang Youdom S, et al. Randomized trial of artesunate-amodiaquine, atovaquone-proguanil, and artesunate-atovaquone-proguanil for the treatment of uncomplicated falciparum malaria in children. J Infect Dis. 2014;210(12):1962–71. https://doi.org/10.1093/infdis/jiu341.
Nji AM, Ali IM, Moyeh MN, Ngongang E-O, Ekollo AM, Chedjou J-P, et al. Randomized non-inferiority and safety trial of dihydroartemisin-piperaquine and artesunate-amodiaquine versus artemether-lumefantrine in the treatment of uncomplicated Plasmodium falciparum malaria in Cameroonian children. Malar J. 2015;14:27.
Lingani M, Bonkian LN, Yerbanga I, Kazienga A, Valéa I, Sorgho H, et al. In vivo/ex vivo efficacy of artemether-lumefantrine and artesunate-amodiaquine as first-line treatment for uncomplicated falciparum malaria in children: an open label randomized controlled trial in Burkina Faso. Malar J. 2020;19(1):8–8.
Zongo I, Compaoré YD, Nikiéma F, Zongo M, Barry N, Somé FA, et al. Efficacy of artemether-lumefantrine and artesunate-amodiaquine as first line therapy of uncomplicated malaria in Burkina Faso 11 years after policy change. Pan Afr Med J. 2020. https://doi.org/10.11604/pamj.2020.35.68.20849.
Faucher J, Aubouy A, Adeothy A, Cottrell G, Doritchamou J, Gourmel B, et al. Comparison of sulfadoxine-pyrimethamine, unsupervised artemether-lumefantrine, and unsupervised artesunate-amodiaquine fixed-dose formulation for uncomplicated Plasmodium falciparum Malaria in Benin: a randomized effectiveness noninferiority trial. J Infect Dis. 2009;200(1):57–65. https://doi.org/10.1086/599378.
Ngasala BE, Malmberg M, Carlsson AM, Ferreira PE, Petzold MG, Blessborn D, et al. Efficacy and Effectiveness of Artemether-Lumefantrine after Initial and Repeated Treatment in Children <5 Years of Age with Acute Uncomplicated Plasmodium falciparum Malaria in Rural Tanzania A Randomized Trial. Clin Infect Dis. 2011;52(7):873–82. https://doi.org/10.1093/cid/cir066.
Chatio S, Aborigo R, Adongo PB, Anyorigiya T, Dalinjong PA, Akweongo P, et al. Factors influencing adverse events reporting within the health care system: the case of artemisinin-based combination treatments in northern Ghana. Malar J. 2016;15(1):125. https://doi.org/10.1186/s12936-016-1172-2.
Ndagije HB, Nambasa V, Manirakiza L, Kusemererwa D, Kajungu D, Olsson S, et al. The burden of adverse drug reactions due to artemisinin-based antimalarial treatment in selected Ugandan health facilities: an active follow-up study. Drug Saf. 2018;41(8):753–65. https://doi.org/10.1007/s40264-018-0659-x.
Gasasira AF, Kamya MR, Achan J, Mebrahtu T, Kalyango JN, Ruel T, et al. High risk of neutropenia in HIV-infected children following treatment with artesunate plus amodiaquine for uncomplicated malaria in Uganda. Clin Infect Dis. 2008;46(7):985–91. https://doi.org/10.1086/529192.
Zwang J, D’Alessandro U, Ndiaye J-L, Djimdé AA, Dorsey G, Mårtensson AA, et al. Haemoglobin changes and risk of anaemia following treatment for uncomplicated falciparum malaria in sub-Saharan Africa. BMC Infect Dis. 2017;17(1):443–443.
Ariey F, Witkowski B, Amaratunga C, Beghain J, Langlois A-C, Khim N, et al. A molecular marker of artemisinin-resistant Plasmodium falciparum malaria. Nature. 2014;505(7481):50–5.
Ménard D, Khim N, Beghain J, Adegnika AA, Shafiul-Alam M, Amodu O, et al. A worldwide map of Plasmodium falciparum K13-propeller polymorphisms. N Engl J Med. 2016;374(25):2453–64.
Ocan M, Akena D, Nsobya S, Kamya MR, Senono R, Kinengyere AA, et al. K13-propeller gene polymorphisms in Plasmodium falciparum parasite population in malaria affected countries: a systematic review of prevalence and risk factors. Malar J. 2019;18(1):60. https://doi.org/10.1186/s12936-019-2701-6.
WHO. Geneva, World Health Organization. Report on antimalarial drug efficacy, resistance and response: 10 years of surveillance (2010–2019). https://www.who.int/publications/i/item/9789240012813. Accessed 3 May 2021.
Balikagala B, Fukuda N, Ikeda M, Katuro OT, Tachibana S-I, Yamauchi M, et al. Evidence of artemisinin-resistant malaria in Africa. N Engl J Med. 2021;385(13):1163–71. https://doi.org/10.1056/NEJMoa2101746.
Bwire GM, Ngasala B, Mikomangwa WP, Kilonzi M, Kamuhabwa AAR. Detection of mutations associated with artemisinin resistance at k13-propeller gene and a near complete return of chloroquine susceptible falciparum malaria in Southeast of Tanzania. Sci Rep. 2020;10(1):3500. https://doi.org/10.1038/s41598-020-60549-7.
Uwimana A, Legrand E, Stokes BH, Ndikumana J-LM, Warsame M, Umulisa N, et al. Emergence and clonal expansion of in vitro artemisinin-resistant Plasmodium falciparum kelch13 R561H mutant parasites in Rwanda. Nat Med. 2020. https://doi.org/10.1038/s41591-020-1005-2.
Uwimana A, Umulisa N, Venkatesan M, Svigel SS, Zhou Z, Munyaneza T, et al. Association of Plasmodium falciparum kelch13 R561H genotypes with delayed parasite clearance in Rwanda: an open-label, single-arm, multicentre, therapeutic efficacy study. Lancet Infect Dis. 2021. https://doi.org/10.1016/S1473-3099(21)00142-0.
Boni MF, Smith DL, Laxminarayan R. Benefits of using multiple first-line therapies against malaria. Proc Natl Acad Sci U S A. 2008;105(37):14216–21.
Smith DL, Klein EY, McKenzie FE, Laxminarayan R. Prospective strategies to delay the evolution of anti-malarial drug resistance: weighing the uncertainty. Malar J. 2010;9(1):217. https://doi.org/10.1186/1475-2875-9-217.
Ndiaye JL, Randrianarivelojosia M, Sagara I, Brasseur P, Ndiaye I, Faye B, et al. Randomized, multicentre assessment of the efficacy and safety of ASAQ—a fixed-dose artesunate-amodiaquine combination therapy in the treatment of uncomplicated Plasmodium falciparum malaria. Malar J. 2009;8:125–125.
Faye B, Kuété T, Kiki-Barro CP, Tine RC, Nkoa T, Ndiaye JLA, et al. Multicentre study evaluating the non-inferiority of the new paediatric formulation of artesunate/amodiaquine versus artemether/lumefantrine for the management of uncomplicated Plasmodium falciparum malaria in children in Cameroon Ivory Coast and Senegal. Malar J. 2012;11:433–433.
Yaounde Climate and Temperature. http://www.yaounde.climatemps.com/index.php. Accessed 18 May 2021.
Antonio-Nkondjio C, Ndo C, Njiokou F, Bigoga JD, Awono-Ambene P, Etang J, et al. Review of malaria situation in Cameroon: technical viewpoint on challenges and prospects for disease elimination. Parasit Vectors. 2019;12(1):501. https://doi.org/10.1186/s13071-019-3753-8.
Sathian B, Sreedharan J, Baboo SN, Sharan K, Abhilash ES, Rajesh E. Relevance of sample size determination in medical research. Nepal J Epidemiol. 1970;1(1):4–10.
Zhong B. How to calculate sample size in randomized controlled trial? J Thorac Dis. 2009;1(1):51–4.
Sealed Envelope Ltd. 2019. Create a blocked randomisation list. https://www.sealedenvelope.com/simple-randomiser/v1/lists. Accessed 7 May 2019.
World Health Organization. UNICEF/UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases. Microscopy for the detection, identification and quantification of malaria parasites on stained thick and thin blood films in research settings (version 1.0): procedure: methods manual. Geneva: World Health Organization; 2015.
Snounou G. Genotyping of Plasmodium spp.: Nested PCR. In: Doolan DL, editor. Malaria Methods and Protocols. New Jersey: Humana Press; 2002. p. 103–16. https://doi.org/10.1385/1-59259-271-6:103.
Mohd Abd Razak MR, Sastu UR, Norahmad NA, Abdul-Karim A, Muhammad A, Muniandy PK, et al. Genetic diversity of Plasmodium falciparum populations in malaria declining areas of Sabah, East Malaysia. PLoS ONE. 2016. https://doi.org/10.1371/journal.pone.0152415.
WHO. Geneva. World Health Organization 2008. A practical handbook on the pharmacovigilance of antimalarial medicines. https://www.who.int/malaria/publications/atoz/9789241547499/en/. Accessed 29 May 2020.
Apinjoh T, Anchang-Kimbi J, Ajonina M, Njonguo E, Njua-Yafi C, Ngwai A, et al. In vivo efficacy of artesunate/sulphadoxine-pyrimethamine versus artesunate/amodiaquine in the treatment of uncomplicated P. falciparium malaria in children around the slope of mount cameroon: a randomized controlled trial. Biomedicines. 2016;4(1):5.
Kobbe R, Klein P, Adjei S, Amemasor S, Thompson WN, Heidemann H, et al. A randomized trial on effectiveness of artemether-lumefantrine versus artesunate plus amodiaquine for unsupervised treatment of uncomplicated Plasmodium falciparum malaria in Ghanaian children. Malar J. 2008;7:261–261.
Mutabingwa TK, Anthony D, Heller A, Hallett R, Ahmed J, Drakeley C, et al. Amodiaquine alone, amodiaquine+sulfadoxine-pyrimethamine, amodiaquine+artesunate, and artemether-lumefantrine for outpatient treatment of malaria in Tanzanian children: a four-arm randomised effectiveness trial. Lancet. 2005;365(9469):1474–80.
Sondo P, Derra K, Diallo-Nakanabo S, Tarnagda Z, Zampa O, Kazienga A, et al. Effectiveness and safety of artemether-lumefantrine versus artesunate-amodiaquine for unsupervised treatment of uncomplicated falciparum malaria in patients of all age groups in Nanoro Burkina Faso: a randomized open label trial. Malar J. 2015;14(325):325.
Ajayi IO, Browne EN, Bateganya F, Yar D, Happi C, Falade CO, et al. Effectiveness of artemisinin-based combination therapy used in the context of home management of malaria: a report from three study sites in sub-Saharan Africa. Malar J. 2008;7(1):190. https://doi.org/10.1186/1475-2875-7-190.
Tinto H, Diallo S, Zongo I, Guiraud I, Valea I, Kazienga A, et al. Effectiveness of artesunate-amodiaquine vs. artemether-lumefantrine for the treatment of uncomplicated falciparum malaria in Nanoro, Burkina Faso: a non-inferiority randomised trial. Trop Med Int Health. 2014;19(4):469–75. https://doi.org/10.1111/tmi.12274.
Abuaku B, Duah-Quashie NO, Quaye L, Matrevi SA, Quashie N, Gyasi A, et al. Therapeutic efficacy of artesunate–amodiaquine and artemether–lumefantrine combinations for uncomplicated malaria in 10 sentinel sites across Ghana: 2015–2017. Malar J. 2019;18(1):206. https://doi.org/10.1186/s12936-019-2848-1.
Diallo MA, Yade MS, Ndiaye YD, Diallo I, Diongue K, Sy SA, et al. Efficacy and safety of artemisinin-based combination therapy and the implications of Pfkelch13 and Pfcoronin molecular markers in treatment failure in Senegal. Sci Rep. 2020;10(1):8907. https://doi.org/10.1038/s41598-020-65553-5.
Ishengoma DS, Mandara CI, Francis F, Talundzic E, Lucchi NW, Ngasala B, et al. Efficacy and safety of artemether-lumefantrine for the treatment of uncomplicated malaria and prevalence of Pfk13 and Pfmdr1 polymorphisms after a decade of using artemisinin-based combination therapy in mainland Tanzania. Malar J. 2019;18(1):88. https://doi.org/10.1186/s12936-019-2730-1.
Olivera MJ, Guerra AP, Cortes LJ, Horth RZ, Padilla J, Novoa J, et al. Artemether-lumefantrine efficacy for the treatment of uncomplicated Plasmodium falciparum malaria in choco, colombia after 8 years as first-line treatment. Am J Trop Med Hyg. 2020;102(5):1056–63. https://doi.org/10.4269/ajtmh.19-0954.
Derbie A, Mekonnen D, Adugna M, Yeshitela B, Woldeamanuel Y, Abebe T. Therapeutic efficacy of artemether-lumefantrine (Coartem®) for the treatment of uncomplicated falciparum malaria in africa: a systematic review. J Parasitol Res. 2020. https://doi.org/10.1155/2020/7371681.
Piola P, Fogg C, Bajunirwe F, Biraro S, Grandesso F, Ruzagira E, et al. Supervised versus unsupervised intake of six-dose artemether-lumefantrine for treatment of acute, uncomplicated Plasmodium falciparum malaria in Mbarara, Uganda: a randomised trial. Lancet. 2005;365(9469):1467–73.
Depoortere E, Guthmann J-P, Presse J, Sipilanyambe N, Nkandu E, Balkan S, et al. Efficacy and effectiveness of the combination of sulfadoxine/pyrimethamine and a 3-day course of artesunate for the treatment of uncomplicated falciparum malaria in a refugee settlement in Zambia. Trop Med Int Health. 2005;10(2):139–45. https://doi.org/10.1111/j.1365-3156.2004.01363.x.
Dorsey G, Gasasira AF, Machekano R, Kamya MR, Staedke SG, Hubbard A. The impact of age, temperature, and parasite density on treatment outcomes from antimalarial clinical trials in Kampala. Uganda Am J Trop Med Hyg. 2004;71(5):531–6.
Sangowawa AO, Amodu OK, Olaniyan SA, Amodu FA, Olumese PE, Omotade OO. Factors Associated with a Poor Treatment Outcome among Children Treated for Malaria in Ibadan, Southwest Nigeria. Wilson ML, editor. Epidemiol Res Int. 2014. https://doi.org/10.1155/2014/974693.
Kyabayinze DJ, Karamagi C, Kiggundu M, Kamya MR, Wabwire-Mangen F, Kironde F, et al. Multiplicity of Plasmodium falciparum infection predicts antimalarial treatment outcome in Ugandan children. Afr Health Sci. 2008;8(4):200–5.
Muhindo Mavoko H, Kalabuanga M, Delgado-Ratto C, Maketa V, Mukele R, Fungula B, et al. Uncomplicated clinical malaria features, the efficacy of artesunate-amodiaquine and their relation with multiplicity of infection in the democratic republic of congo. PLoS ONE. 2016;11(6):e0157074–e0157074.
Borrmann S, Matsiegui P-B, Missinou MA, Kremsner PG. Effects of Plasmodium falciparum parasite population size and patient age on early and late parasitological outcomes of antimalarial treatment in children. Antimicrob Agents Chemother. 2008;52(5):1799–805.
Enevold A, Nkya WM, Theisen M, Vestergaard LS, Jensen AT, Staalsoe T, et al. Potential impact of host immunity on malaria treatment outcome in Tanzanian children infected with Plasmodium falciparum. Malar J. 2007;6(1):153. https://doi.org/10.1186/1475-2875-6-153.
Mutagonda RF, Kamuhabwa AAR, Minzi OMS, Massawe SN, Asghar M, Homann MV, et al. Effect of pharmacogenetics on plasma lumefantrine pharmacokinetics and malaria treatment outcome in pregnant women. Malar J. 2017;16(1):267. https://doi.org/10.1186/s12936-017-1914-9.
St Jean PL, Koh GCKW, Breton JJ, Espino FEJ, Hien TT, Krudsood S, et al. Pharmacogenetic assessment of tafenoquine efficacy in patients with Plasmodium vivax malaria. Pharmacogenet Genomics. 2020;30(7):161–5. https://doi.org/10.1097/FPC.0000000000000407.
Borrmann S, Sallas WM, Machevo S, González R, Björkman A, Mårtensson A, et al. The effect of food consumption on lumefantrine bioavailability in African children receiving artemether-lumefantrine crushed or dispersible tablets (Coartem ® ) for acute uncomplicated Plasmodium falciparum malaria. Trop Med Int Health. 2010. https://doi.org/10.1111/j.1365-3156.2010.02477.x.
Faye B, Offianan AT, Ndiaye JL, Tine RC, Touré W, Djoman K, et al. Efficacy and tolerability of artesunate-amodiaquine (Camoquin plus®) versus artemether-lumefantrine (Coartem®) against uncomplicated Plasmodium falciparum malaria: multisite trial in Senegal and Ivory Coast. Trop Med Int Health. 2010. https://doi.org/10.1111/j.1365-3156.2010.02487.x.
Yavo W, Konaté A, Kassi FK, Djohan V, Angora EK, Kiki-Barro PC, et al. Efficacy and safety of artesunate-amodiaquine versus artemether-lumefantrine in the treatment of uncomplicated Plasmodium falciparum malaria in sentinel sites across Côte d’Ivoire, Krishna S, editor. Malar Res Treat. 2015. https://doi.org/10.1155/2015/878132.
Dorkenoo AM, Yehadji D, Agbo YM, Layibo Y, Agbeko F, Adjeloh P, et al. Therapeutic efficacy trial of artemisinin-based combination therapy for the treatment of uncomplicated malaria and investigation of mutations in k13 propeller domain in Togo, 2012–2013. Malar J. 2016;15(1):331. https://doi.org/10.1186/s12936-016-1381-8.
WWARN Gametocyte Study Group. Gametocyte carriage in uncomplicated Plasmodium falciparum malaria following treatment with artemisinin combination therapy: a systematic review and meta-analysis of individual patient data. BMC Med. 2016;14:79–79.
Acknowledgements
The authors sincerely thank all those who accepted to participate in the study.
Funding
WFM, PTNN, AMN, IMA and MA are supported by the Malaria Research Capacity Development in West and Central Africa (MARCAD) Consortium through the Developing Excellence in Leadership, Training and Science (DELTAS) Africa Initiative (Grant # DEL-15-010) to the University of Yaoundé I. The DELTAS Africa Initiative is an independent funding scheme of the African Academy of Sciences (AAS)’s Alliance for Accelerating Excellence in Science in Africa (AESA) and supported by the New Partnership for Africa’s Development Planning and Coordinating Agency (NEPAD Agency) with funding from the Wellcome Trust (Grant # 107741/A/15/Z) and the United Kingdom (UK) government. The funding agencies were not involved in the design, conduct and publication of the study.
Author information
Authors and Affiliations
Contributions
WFM, MA, PTNN, JDB, AMN and IMA conceived the study and coordinated the research work. PTNN, LFA, CHD, JPKC, CTF, WDN, OLAO, JAN, AJL, PMA, MCEA, PAMO, GBK, LMA, DRAN, AD, ZA, MSE, JTT, FMT and GD conducted the field sample collection, processing and analyses. PTNN, CHD and AASN curated the data. PTNN and AMN conducted the formal analysis. PTNN and AMN drafted the manuscript, critically reviewed the manuscript, and wrote the final manuscript. The authors WFM, MA, IMA, LFA, CHD, JPKC, CTF, WDN, OLAO, AASN, MNM, JAN, AJL, PMA, MCEA, PAMO, GBK, LMA, DRAN, AD, ZA, MSE, JTT, FMT, GD, VNN, DAF and JDB proof read the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was reviewed and approved by the Center Regional Ethics Committee (CE Nº 05859/CRERSHC/2019) and National Ethics Committee (Nº 2018/07/1091/CE/CNERSH/SP) for Human Health Research, Ministry of Public Health, Yaoundé, Cameroon. Administrative authorizations were also sought from the Director of District Hospital Cité Verte, Director of District Medical Center Minkoa-Meyos, Center Regional Delegate of Public Health and the Minister of Public Health. In addition, the study was explained using the participant information sheet (PIS) and written assent in French or English was duly obtained from the parents/guardians of each participant before enrolment and collection of samples.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
Study treatment
Additional file 2:
Reference ranges of heart rate, systolic blood pressure, diastolic blood pressure, respiratory rate, hematology parameters and biochemistry parameters
Additional file 3:
Kaplan–Meier survival curve for the effectiveness of AS-AQ versus AL (PP without and with PCR correction)
Additional file 4:
Evolution of biological parameters among study participants treated with AS-AQ and AL (Day 0 and Day 7) (PP)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Niba, P.T.N., Nji, A.M., Ali, I.M. et al. Effectiveness and safety of artesunate–amodiaquine versus artemether–lumefantrine for home-based treatment of uncomplicated Plasmodium falciparum malaria among children 6–120 months in Yaoundé, Cameroon: a randomized trial. BMC Infect Dis 22, 166 (2022). https://doi.org/10.1186/s12879-022-07101-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-022-07101-2