Abstract
Background
Leishmaniasis is one of the most neglected tropical diseases in the world and remains endemic in some underdeveloped regions, including western China. The phylogeny and classification of Chinese Leishmania has not been completely clarified to date, especially within the Leishmania (L.) donovani complex, although phylogenetic analyses based on a series of gene markers have been performed. More analytic methods and data are still needed. Random amplified polymorphic DNA (RAPD) technology can sensitively identify slight intraspecific differences, and it is a powerful tool to seek species-specific markers. This work attempted to identify Chinese Leishmania isolates from diverse geographic regions at the genomic level. Meanwhile, specific markers of the L. donovani complex were also developed by RAPD.
Methods
RAPD was applied to 14 Chinese Leishmania isolates from diverse geographic regions and 3 WHO reference strains. The polymorphic sites of amplification were transformed into a data matrix, based on which genetic similarity was calculated, and a UPGMA dendrogram was constructed to analyse the genetic diversity of these Leishmania isolates. Meanwhile, the specific amplification loci of the L. donovani complex were TA-cloned, sequenced and converted into sequence characterized amplified region (SCAR) markers, which were validated preliminarily in 17 available Leishmania strains in this study and analysed by bioinformatics.
Results
The cluster analyses showed that the three Leishmania sp. isolates SC10H2, SD and GL clustered together and apart from others, the strains of the L. donovani complex clearly divided into two clades, and the three isolates Cy, WenChuan and 801 formed a subclade. Three specific SCAR markers of the L. donovani complex, i.e., 1-AD17, 2-A816 and 3-O13, were successfully obtained and validated on 17 available Leishmania strains in this study. Through bioinformatic analyses, Marker 1-AD17 may have more specificity for PCR detection of VL, and Marker 3-O13 has the potential to encode a protein.
Conclusions
The RAPD results verified that the undescribed Leishmania species causing visceral leishmaniasis (VL) in China was a unique clade distinguished from L. donovani and revealed that there was genetic differentiation among Chinese L. donovani. The identification of L. donovani-specific markers may help to provide a foundation for future research attempting to develop new specific diagnostic markers of VL and identify specific gene functions.
Similar content being viewed by others
Background
Leishmaniasis is a tropical disease caused by the obligate intracellular protozoan genus Leishmania and is transmitted through bites of the genus Phlebotomus, which threatens 350 million people over 98 countries, primarily in developing countries [1]. There are four main forms of leishmaniasis according to different clinical syndromes, i.e., visceral leishmaniasis (VL, kala-azar), post-kala-azar dermal leishmaniasis (PKDL), cutaneous leishmaniasis (CL) and mucocutaneous leishmaniasis (MCL), which are caused by different species of Leishmania. There are more than 64 species of the genus Leishmania consisting of the subgenera Euleishmania, Paraleishmania and Sauroleishmania [2], among which 20 species are considered infectious to humans [1].
Visceral leishmaniasis, which is acute and fatal if left untreated, is the main form of leishmaniasis that prevails in China. Although Chinese VL has been effectively restricted since the 1950s, there are still localized and sporadic outbreaks now, mostly in the Xinjiang Uygur Autonomous Region, Sichuan, and Gansu province [3]. Moreover, China is still one of 14 high-burden VL countries [4]. Over the past few decades, epidemiological characteristics, kinetoplasts and chromosomal DNA have been applied successively to Chinese VL typing [5,6,7]. In recent years, a series of gene markers, such as internal transcribed spacer 1 (ITS1), cytochrome oxidase II (COX II), cytochrome b (cyt b) and HSP70, have been applied to establish phylogenetic trees, and the L. donovani complex (including L. donovani and L. infantum), Leishmania gerbilli, Leishmania tropica and Leishmania turanica have been identified, along with an undescribed Leishmania species that has clustered with lizard Leishmania [8,9,10,11]. However, the species classification and pathogen identification of Chinese leishmaniasis is far from complete, especially within the L. donovani complex. Our previous phylogenetic analyses on HSP70 indicated a clear relationship within the L. donovani complex [11]. This finding indicated that L. infantum, which is one of the causative agents of VL, is primarily distributed in western mountainous areas and plains of northwestern China, including Sichuan, Gansu, and Xinjiang provinces. The identification of MHOM/CN/80/801 isolates from VL patients in Kashi, Xinjiang, was different than the results of a study using ITS1 sequences [12]. Moreover, the analysis of HSP70 concluded that L. donovani is the pathogen of CL in Karamay of Xinjian, and Phlebotomus major wui is the vector, which challenges the previous determination that L. infantum is the pathogen of CL in Karamay based on gene hybridization and animal inoculation [13, 14]. Thus, the application of more diverse analytical methods and more classified data is needed to deepen our knowledge of the genetic relationship of Chinese Leishmania isolates.
Rapid species identification is essential for the early diagnosis of leishmaniasis and is conducive to accurate treatments. Multilocus enzyme electrophoresis (MLEE) is still the golden standard in the identification of Leishmania species [15], but it is rarely used now because of its time-consuming procedure. DNA markers have been widely applied for phylogenetic research in Leishmania [16]. The phylogenetic trees of these markers provide much evidence for the taxonomy of the main Leishmania complex, but the relatively slow evolutionary rates of these genes are insufficient to solve the species relationship within the complex [17]. Furthermore, the discrimination capability among markers is diverse, which in turn makes the identification of species and subspecies sometimes inconsistent [18]. Random amplification polymorphic DNA (RAPD) is a technique that can be used for polymorphic analysis of unknown genomes on the basis of PCR [19, 20]. This technique is easy and sensitive and has always been applied for species identification [21] and correlation analysis of population differentiation with geographical origins in Leishmania [22]. Additionally, RAPD has advantages in taxonomy at the subgeneric level [23] and species level [24]. Meanwhile, through the selection of DNA markers among differentially amplified bands, the specific genetic markers of one species can be developed to perform species identification or assist with diagnosis via the creation of a probe. Therefore, RAPD has proved to be an effective method to obtain genetic markers for the development of relevant Leishmania DNA assays [25, 26].
In this study, RAPD was applied to Chinese Leishmania isolates from diverse geographic regions, which could help us more thoroughly understand the genetic differences of these isolates, especially the L. donovani complex. Meanwhile, the specific amplified bands of the L. donovani complex were screened and converted into L. donovani complex specific sequence characterized amplified regions (SCAR) markers. These SCAR markers were validated preliminarily in 17 available Leishmania strains in this study and analysed by bioinformatics, which may provide a foundation for research on specific gene functions and the development of new diagnostic markers of VL.
Methods
Leishmania strains and DNA extraction
Fourteen Chinese Leishmania isolates and three WHO reference strains were used in this study and are listed in Table 1. The 14 Chinese Leishmania isolates were collected from plain, hill, and desert foci in China. These parasites were cultured in Medium 199 supplemented with 15% heat-inactivated foetal bovine serum, 100 U/mL penicillin (Sigma) and 100 μg/mL streptomycin (Sigma) at 26 °C. The promastigotes were collected at logarithmic phase and centrifuged at 3300×g for 10 min. Total DNA was extracted using a commercially available DNA extraction kit (TianGen Cell DNA Kit). The concentrations of 17 DNA samples were detected (Thermo Scientific™ NanoDrop™ One) and adjusted to the same level before subsequent RAPD amplification.
RAPD-PCR
Twenty decamer primers were selected according to previous studies [27, 28] and commercially synthesized (Invitrogen). All primers were prepared as 10 μM (10 pmol/μl) working solutions. These primers were first screened through three independent RAPD amplifications on DNA of the same L. donovani isolate, and then 10 primers that presented polymorphic, reproducible and clear amplification profiles were selected (Table 2). RAPD amplification was performed in 50 μl reactions containing 0.8 μM primer, 10 ng of genomic DNA sample, 25 μl of 2 × TaqMater Mix (Tsingke, China) and PCR-grade distilled water. The PCR procedure was as follows: initial denaturation at 94 °C for 5 min followed by 45 cycles of 94 °C for 1 min, 36 °C for 1 min, 72 °C for 2 min, and a final extension at 72 °C for 8 min. The PCR products were separated using 1.5% agarose gel electrophoresis. Each PCR and electrophoresis separation were performed three times with the same protocol and operator to assure reproducibility.
Phenetic analysis of RAPD results
The bands of all polymorphic RAPD gels were marked as “0” for absent and “1” for present. The relative intensity among all bands was disregarded. A 0/1 data matrix was created in Microsoft Excel 2013 and analysed using the Numerical Taxonomy and Multivariate Analysis System (NTSYS) [29]. The similarity module was used to calculate the similarity matrix. The SAHN function of the clustering module was employed for clustering analysis, and the phenetic dendrogram was output under the unweighted pair-group method with arithmetic means (UPGMA).
Cloning, sequencing and verification of SCAR markers
The L. donovani and L. infantum strain-specific bands that were stably reproduced by gel electrophoresis were considered potential SCAR markers of the L. donovani complex for the following extraction. These gel blocks were purified and then cloned into the pGM-T vector (Tiangen, China) overnight. Recombined products were mixed with DH5α competent E. coli and then screened by blue-white selection. The white colonies were picked and identified by colony PCR, and then the positive samples were cultured in liquid LB medium and collected for DNA sequencing (Tsingke, China). The specific primer pairs of the obtained SCAR marker sequences were designed using Primer 5.0.
Then, PCR was performed on 17 currently available Leishmania strains to verify the specificity of these markers for the L. donovani complex in this study. For the PCR conditions, annealing temperature was tested by gradient for different primers of each SCAR marker.
Bioinformatic analysis of SCAR markers
The obtained SCAR markers sequences were submitted to BLAST online for homology analyses in the NCBI database, and the base component was analysed using Lasergene EditSeq. The open reading frames (ORFs) were predicted and located using NCBI-ORF Finder. To further determine whether the ORFs contained in the sequences have the potential to express proteins, the promoter binding sites were analysed and predicted online using Promotor Scan. Using Lasergene, the ORFs that demonstrated potential protein expression were translated, and the components of these presumed proteins were analysed. The secondary structures were predicted by the Chou-Fasman loading method. The hydrophobic regions were calculated using the Kyte-Doolittle method. The antigenic determinants were analysed through the JamesonWolf method, and the surface probability was assessed using the Emini method.
Results
RAPD analysis of 17 Leishmania strains
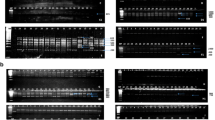
Through RAPD-PCR of the 17 Leishmania strains, a total of 121 RAPD bands were observed, of which 120 bands were polymorphic (99.17%). An average of 12.1 bands were amplified by each primer, and segments ranged from 200 to 3000 bp. According to the gel photographs, there were differences among the 17 Leishmania isolates. Figure 1 shows a gel photo with an example of polymorphism. The same species tended to form similar band models. The genetic similarity of 17 Leishmania strains ranged from 0.4393 to 1.0000 with an average of 0.6758 (Table S1, see Additional file 1), which indicated considerable genetic differentiation among these isolates. The UPGMA dendrogram established based on the similarity matrix is shown in Fig. 2. The isolates SC10H2, SD and GL clustered into Clade I with a high average similarity index of 0.9715, indicating a distant genetic relationship with the others. Other strains formed Clade II, which further consisted of Clades A, B and C. The isolate EJNI-154 clustered with the L. gerbilli WHO reference strain MRHO/CN/60/GERBILLI and formed Clade C. The strains that were previously identified as L. donovani complex did not cluster as one clade but two clades, Clades A and B, instead. The isolates Cy, WenChuan and 801 clustered with L. donovani reference strain DD8 as Clade A, whose average similarity index was 0.9439. The other L. donovani complex strains clustered as Clade B, in which genetic differences still existed. Within Clade B, the similarity index of SC6 from Sichuan Province with others was 0.9019, which was significantly lower than the average index of Clade B (0.9537), so SC6 was separated into an independent clade.
Cloning, sequencing and verification of SCAR markers
A total of four fragments that appeared only in all L. donovani complex strains were successfully T-cloned and sequenced. The obtained fragment sequences were named partly after their primers, i.e., 1-AD17, 2-A816, 3-O13 and 4–09. To evaluate the species specificity, an 18 base pair primer was designed for each potential marker. By PCR amplification of the 17 strains in this study, three of the four markers manifested strict species-specific single bands at their corresponding loci and were tentatively converted to potential SCAR markers of the L. donovani complex (Fig. 3). The primers and annealing temperature of the three SCAR markers are shown in Table 3. The DNA sequences are shown in Table S2 (see Additional file 2).
Bioinformatic analysis of SCAR markers
All three markers were subjected to BLAST in NCBI, the query coverage was 99%, and the identity percent was greater than 98% with L. donovani/infantum reference sequences. In addition, the E-valves were close to ‘0’. All results indicated that the three marker sequences had very high homology with L. donovani/infantum reference sequences. According to the distribution of BLAST hits of SCAR markers, except for the first two L. donovani/infantum reference sequences, marker 1-AD17 had a query coverage less than 90% and had no matching primer binding sites with other Leishmania species sequences (Fig. 4a). Markers 2-A816 and 3-O13 had more matching primer binding sites with other Leishmania species sequences except L. donovani/infantum reference sequences (Fig. 4b, c). Therefore, the primers for marker 1-AD17 have a greater specificity for amplification of the L. donovani complex.
The results for sequence components, chromosomal assignments, ORFs and promotor prediction for the three markers are listed in Table 4. There were 4 to 7 ORFs in these markers, of which only 3-O13 had two potential promotors, located at 611–816 bp of the sense strand and 1056–806 bp of the antisense strand. The distance between predicted promotor sites and ORFs implied that ORF-4 has the potential to encode proteins. The gene sequence was then translated into protein and analysed by Lasergene. The protein sequence of ORF-4 contains 7 strong basic amino acids, 6 strong acid amino acids, 19 hydrophobic residues and 10 polar residues, and the putative isoelectric point is 8.835. The structural prediction of the ORF-4 protein sequence by Lasergene Protean is shown in Fig. 5. The results showed that a clear structure of one hydrophilic β turn region (19–36 residues) was flanked by two hydrophobic α helices (1–18 and 37–50 residues), which implied that the α helices might be located in the interior of the protein and that β turns might be located on the surface. This was confirmed by surface probability analysis (Emini method). In addition, the β region has a higher antigen index. Furthermore, there is no homologous protein according to BLAST in GenBank.
Discussion
Generally, DNA markers are now the most widely used method in the identification and classification of Leishmania since they are both effective and efficient. Different evolutionary rates of diverse gene markers may lead to different classification results. Thus, to help us understand interspecific relationships more comprehensively, more dissimilar identification methods would be necessary. RAPD has been widely used in genetic map construction, breeding line identification and gene marker screening and in the genetic evolution of parasites such as trypanosome, schistosome and trichinella spiralis [30,31,32]. As we all know, RAPD has innate drawbacks in terms of the stability and repeatability of bands due to the highly random hybrid sites with template DNA. Therefore, to obtain stable bands and repeatable results with RAPD, we used PCR amplification conditions whose stability and repeatability have been already reported [27, 28] and validated the results before formal experiments. Meanwhile, the usage of commercial Taq DNA Mix from the same batch replaced the addition of dNTPs, Mg2+ and Taq DNA polymerase one by one, which also improved the reaction stability in this study. Theoretically, the larger the number of RAPD polymorphic sites, the more reliable the genetic relationship. In this study, 10 of 20 random primers produced 121 polymorphic bands, with a proportion of polymorphic bands greater than 99%, which could credibly reflect genetic diversity among these isolates.
In this study, the isolates SC10H2, SD and GL, which were identified as L. (SauroLeishmania) sp. previously [11], had a lower genetic similarity with other strains and first clustered as Clade I. This result from the genome perspective adds to the evidence suggesting the existence of an undescribed Leishmania species in China, which is a distinct branch that has low homology with Chinese L. donovani strains [9, 33, 34]. In particular, this RAPD result also demonstrated discrimination and differentiation in the relationship among subspecies of L. donovani complex. Three isolates, Cy, WenChuan and 801, which were isolated from Gansu, Sichuan and Xinjiang, respectively, were clustered together and separated from other L. donovani isolates. This result confirmed that genetic differentiation truly existed in Chinese L. donovani. Combined with the results of previous studies [11, 35], it can be concluded that Cy, WenChuan and 801 should be identified as L. infantum, which is the causative agent of canine leishmaniasis (CanL) in Sichuan and Gansu. Accordingly, it could be inferred that the VL in Sichuan, Gansu and Xinjiang was caused by L. infantum. This conclusion is also in accordance with a previous report [5]. From the UPGMA tree, the isolates KXG-918 and KXG-927 were identified as L. donovani in this study, which confirmed again that L. donovani was the pathogen of CL in Karamay of Xinjiang. Generally, CL was not the main prevalent type in China, and most were imported. Extensive investigation into indigenous CL in China is needed to obtain solid conclusions regarding the causative agent. In addition, the UPGMA dendrogram showed that L. donovani reference strain DD8 did not cluster with KXG-XU, KXG-LIU, 9044, KXG-65 and SC6, which were previously identified as L. donovani. This result indicated that there were differences between these Chinese L. donovani strains and the L. donovani reference strain from India at the genomic level, which was inconsistent with the phylogenetic analysis results obtained with gene markers [8, 34]. As a gene marker only contains partial genome information, and the selective pressure varies among different genes, intraspecific genetic differentiation probably cannot be reflected fully. On the other hand, notable genetic variation is often generated between species or genera with RAPD amplification, so using an individual to represent a species may cause deviation of the phylogenetic results. Consequently, the divergence in this study needs to be further verified by enlarging the sample size or combining it with other methods. In addition, the cluster dendrogram showed that clade B divided into two small branches: Sichuan isolate SC6 from hill foci, Shandong isolate 9044 from plain foci and five other Xinjiang isolates from desert foci. There were still differences among VL isolates from hills, plains and deserts in China, which corroborated the previous report [36]. Although RAPD technology has gradually waned, it is very sensitive for identifying slight intraspecific differences, making it suitable for the differentiation of sibling species.
For the species-specific segments of RAPD, further bioinformatic analysis is beneficial for the exploration of genetic information and the development of specific genetic markers. In this study, three L. donovani complex species-specific DNA markers were obtained and preliminarily verified. However, according to their distribution of BLAST hits in NCBI, only the primers of marker 1-AD17 may have a greater specificity for amplification of the L. donovani complex. We considered that the differences in primer binding sites or annealing sites of amplification were the cause of the generation of differential DNA fragments of diverse species in RAPD, which was proposed in a previous report [25]. Thus, the SCAR marker 1-AD17 has the potential to be developed into a rapid diagnostic marker of kala-azar, as it was able to separate L. donovani and L. infantum from the other species in this study. Admittedly, the L. donovani complex-specific DNA marker in this study still has certain limits because the species of Leishmania are multifarious, and less genomic information is available. Therefore, more parasite samples and patient specimens would be needed to test the specificity.
Through bioinformatic analysis, the three markers were all located on large chromosomes instead of kinetoplasts, which was similar to some other reports [28, 37, 38]. This may be related to the fact that multicopy genes are found preferentially on disomic chromosomes [39], which would increase the probability of random primers binding to them. As genome sequences of different Leishmania species are highly conserved [39], the amplification loci of RAPD are frequently located in variable regions. In this study, although there were 4 to 7 ORFs in the three markers, only 3-O13 had two potential promotors, and ORF-4 had the potential to encode proteins. The following protein prediction analysis showed that the hypothetical protein had a higher antigenic index and surface probability. Nevertheless, all of these findings need further experiments for verification.
Conclusions
Our results verified that the undescribed Leishmania species causing VL in China was a unique clade distinguished from L. donovani and revealed that there was genetic differentiation among Chinese L. donovani isolates at the genome level. Three L. donovani complex species-specific DNA markers in 17 available Leishmania strains were developed and analysed preliminarily through BLAST and bioinformatics, which may provide a foundation for developing new specific diagnostic markers of VL and performing research on specific gene functions. Nevertheless, the collection of more strains from different origins and patient specimens would be necessary to achieve more accurate intraspecific classification of Chinese L. donovani and effective verification of these specific SCAR markers.
Availability of data and materials
The datasets used and analysed during the current study are available from the corresponding author upon reasonable request.
Abbreviations
- RAPD:
-
Random amplified polymorphic DNA
- SCAR:
-
sequence characterized amplified regions
- VL:
-
visceral leishmaniasis
- CanL:
-
canine leishmaniasis
- cyt b :
-
cytochrome b
- HSP70:
-
heat shock protein 70
- NTSYS:
-
Numerical Taxonomy and Multivariate Analysis System
- UPGMA:
-
unweighted pair-group method with arithmetic means
References
World Health Organization. Control of the leishmaniases. World Health Organ Tech Rep Ser. 2010;(949): xii-xiii, 1–186, back cover.
Akhoundi M, Kuhls K, Cannet A, Votýpka J, Marty P, Delaunay P, et al. A historical overview of the classification, evolution, and dispersion of Leishmania parasites and sandflies. PLoS Negl Trop Dis. 2016;10(3):e0004349. https://doi.org/10.1371/journal.pntd.0004349.
Guan LR. Present situation of visceral Leishmaniasis and Prospect for its control in China. Chin J Parasitol Parasit Dis. 2009;27(5):394–7.
WHO. Leishmaniasis in high-burden countries: an epidemiological update based on data reported in 2014. In: The Weekly Epidemiological Record (WER). WHO. 2014. http://www.who.int/leishmaniasis/resources/who_wer9122/en/. Accessed 10 June 2020.
Lun ZR, Wu MS, Chen YF, Wang JY, Zhou XN, Liao LF, et al. Visceral Leishmaniasis in China: an endemic disease under control. Clin Microbiol Rev. 2015;28(4):987–1004. https://doi.org/10.1128/CMR.00080-14.
Hu X, Lu H, Luo P, Wang Z, Hu X, Yi T. Identification of Leishmania donovani isolates from different kala-azar foci in China by kDNA hybridization. Chin Med Sci J. 1992;7(2):63–6 (in Chinese with English abstract).
Lu HG, Zhong L, Guan LR, Qu JQ, Hu XS, Chai JJ, et al. Separation of Chinese Leishmania isolates into five genotypes by kinetoplast and chromosomal DNA heterogeneity. Am J Trop Med Hyg. 1994;50(6):763–70. https://doi.org/10.4269/ajtmh.1994.50.763.
Yang BB, Guo XG, Hu XS, Zhang JG, Liao L, Chen DL, et al. Species discrimination and phylogenetic inference of 17 Chinese Leishmania isolates based on internal transcribed spacer 1 (ITS1) sequences. Parasitol Res. 2010;107(5):1049–65. https://doi.org/10.1007/s00436-010-1969-9.
Cao DP, Guo XG, Chen DL, Chen JP. Species delimitation and phylogenetic relationships of Chinese Leishmania isolates reexamined using kinetoplast cytochrome oxidase II gene sequences. Parasitol Res. 2011;109(1):163–73. https://doi.org/10.1007/s00436-010-2239-6.
Yang BB, Chen DL, Chen JP, Liao L, Hu XS, Xu JN. Analysis of kinetoplast cytochrome b gene of 16 Leishmania isolates from different foci of China: different species of Leishmania in China and their phylogenetic inference. Parasit Vectors. 2013;6(1):32. https://doi.org/10.1186/1756-3305-6-32.
Yuan DM, Qin HX, Zhang JG, Liao L, Chen QW, Chen DL, et al. Phylogenetic analysis of HSP70 and cyt b gene sequences for Chinese Leishmania isolates and ultrastructural characteristics of Chinese Leishmania sp. Parasitol Res. 2017;116(2):693–702. https://doi.org/10.1007/s00436-016-5335-4.
Wang JY, Gao CH, Yang YT, Chen HT, Zhu XH, Lu S, et al. An outbreak of the desert sub-type of zoonotic visceral leishmaniasis in Jiashi, Xinjiang Uygur autonomous region. People’s Republic of China Parasitol Int. 2010;59(59):331–7.
Guan LR, Yang YQ, Xu YX, Qu JQ, Zuo XP, Wang G, et al. Leishmaniasis in Karamay XIV. Identification of promastigote isolates from naturally infected phlebotomus major wui. Chin J Parasitol Paras Dis. 1994;12(4):257–61 (In Chinese with English abstract).
Yang YQ, Guan LR, Wu JT. Study on the parasitic character of Leishmania infantum in monkey in Karamay area. Endemic Diseases Bulletin. 1997;12(1):8–11 (in Chinese with English abstract).
Rioux JA, Lanotte G, Serres E, Pratlong F, Bastien P, Perieres J. Taxonomy of Leishmania. Use of isoenzymes. Suggestions for a new classification. Ann Parasitol Hum Comp. 1990;65(3):111–25. https://doi.org/10.1051/parasite/1990653111.
Van der Auwera G, Dujardin JC. Species typing in dermal leishmaniasis. Clin Microbiol Rev. 2015;28(2):265–94. https://doi.org/10.1128/CMR.00104-14.
Stevens JR, Noyes HA, Schofield CJ, Gibson W. The molecular evolution of Trypanosomatidae. Adv Parasitol. 2001;48:1–56. https://doi.org/10.1016/S0065-308X(01)48003-1.
Akhoundi M, Downing T, Votýpka J, Kuhls K, Lukeš J, Cannet A, et al. Leishmania infections: molecular targets and diagnosis. Mol Asp Med. 2017;57:1–29. https://doi.org/10.1016/j.mam.2016.11.012.
Williams JG, Kubelik AR, Livak KJ, Rafalski JA, Tingey SV. DNA polymorphisms amplified by arbitrary primers are useful as genetic markers. Nucleic Acids Res. 1990;18(22):6531–5. https://doi.org/10.1093/nar/18.22.6531.
Babu KN, Rajesh MK, Samsudeen K, Minoo D, Suraby EJ, Anupama K, et al. Randomly amplified polymorphic DNA (RAPD) and derived techniques. Methods Mol Biol. 2014;1115:191–209. https://doi.org/10.1007/978-1-62703-767-9_10.
Ikram G, Dellagi K, Ismaïl RB. Random amplified polymorphic DNA technique for identification and differentiation of old world Leishmania species. Am J Trop Med Hyg. 2002;66(2):152–6. https://doi.org/10.4269/ajtmh.2002.66.152.
Yazidi R, Bettaieb J, Ghawar W, Jaouadi K, Châabane S, Zaatour A, et al. RAPD-PCR reveals genetic polymorphism among Leishmania major strains from Tunisian patients. BMC Infect Dis. 2015;15:269.
Adams RP, Demeke T. Systematic relationships in Juniperus based on random amplified polymorphic DNA. Taxon. 1993;42(3):553–71. https://doi.org/10.2307/1222534.
Wilkie SE, Issac PG, Slater RJ. Random amplified polymorphic DNA (RAPD) markers for genetic analysis in Allium. Theor Appl Genet. 1993;86(4):497–504. https://doi.org/10.1007/BF00838566.
Hanafi R, Barhoumi M, Ali SB, Guizani I. Molecular analyses of Old World Leishmania RAPD markers and development of a PCR assay selective for parasites of the Leishmania donovani species complex. Exp Parasitol. 2001;98(2):90–9. https://doi.org/10.1006/expr.2001.4617.
Martinez E, Alonso V, Quispe A, Thomas MC, Alonso R, Piñero JE, et al. RAPD method useful for distinguishing Leishmania species: design of specific primers for L. braziliensis. Parasitology. 2003;127(Pt 6):513–7. https://doi.org/10.1017/S0031182003004104.
Lu DM, Hu XS, Qiao ZD. Analysis of Leishmania species and strains from China by RAPD technique. Chin J Parasitol Paras Dis. 2001;19(5):290–3 (in Chinese with English abstract).
Mkada-Driss I, Lahmadi R, Chakroun AS, Talbi C, Guerbouj S, Driss M, et al. Screening and characterization of RAPD markers in viscerotropic Leishmania parasites. PLoS One. 2014;9(10):e109773.
Rohlf FJ. NTSYS-pc - Numerical Taxonomy and Multivariate Analysis System. Applied Biostatistics Inc. New York. 1988;2:1.
Lun ZR, Desser SS. Analysis of isolates within species of anuran trypanosomes using random amplified polymorphic DNA. Parasitol Res. 1996;82(1):22–7. https://doi.org/10.1007/s004360050062.
Barral V, Morand S, Pointier JP, Théron A. Distribution of schistosome genetic diversity within naturally infected Rattus rattus detected by RAPD markers. Parasitology. 1996;113(Pt 6):511–7. https://doi.org/10.1017/S003118200006755X.
Bandi C, La Rosa G, Bardin MG, Damiani G, Comincini S, Tasciotti L, et al. Random amplified polymorphic DNA fingerprints of the eight taxa of Trichinella and their comparison with allozyme analysis. Parasitology. 1995;110(Pt 4):401–7. https://doi.org/10.1017/S003118200006474X.
Guan W, Cao DP, Sun K, Xu JN, Zhang JR, Chen DL, et al. Phylogenic analysis of Chinese Leishmania isolates based on small subunit ribosomal RNA (SSU rRNA) and 7 spliced leader RNA (7SL RNA). Acta Parasitol. 2012;57(2):101–13. https://doi.org/10.2478/s11686-012-0022-9.
Sun K, Guan W, Zhang JG, Wang YJ, Tian Y, Liao L, et al. Prevalence of canine leishmaniasis in Beichuan County, Sichuan, China and phylogenetic evidence for an undescribed Leishmania sp. in China based on 7SL RNA. Parasite Vector. 2012;5:75.
Alam MZ, Nakao R, Sakurai T, Kato H, Qu JQ, Chai JJ, et al. Genetic diversity of Leishmania donovani/infantum complex in China through microsatellite analysis. Infect Genet Evol. 2014;22:112–9. https://doi.org/10.1016/j.meegid.2014.01.019.
Hu X, Bu L, Ma Y, Wang Y, Jing B, Yi T. Difference in DNA sequences in SSU rDNA variable regions among pathogens isolated from different epidemic foci of visceral leishmaniasis in China. Chin Med J. 2002;115(10):1457–9.
Van Eys GJ, Guizani I, Ligthart GS, Dellagi K. A nuclear DNA probe for the identification of strains within the Leishmania donovani complex. Exp Parasitol. 1991;72(4):459–63. https://doi.org/10.1016/0014-4894(91)90092-b.
Lewin S, Schönian G, El Tai N, Oskam L, Bastien P, Presber W. Strain typing in Leishmania donovani by using sequence-confirmed amplified region analysis. Int J Parasitol. 2002;32(10):1267–76. https://doi.org/10.1016/S0020-7519(02)00091-7.
Rogers MB, Hilley JD, Dickens NJ, Wilkes J, Bates PA, Depledge DP, et al. Chromosome and gene copy number variation allow major structural change between species and strains of Leishmania. Genome Res. 2011;21(12):2129–42. https://doi.org/10.1101/gr.122945.111.
Acknowledgements
We thank Dr. Lin Liao from the Department of Parasitology, West China College of Preclinical and Forensic Medicine, Sichuan University, for cultivating the parasites used in this work.
Funding
This work was supported by the National Natural Science Foundation of China to Dongmei Yuan (81802034) and Jianping Chen (81672048) and the Fundamental Research Funds for the Central Universities to Dongmei Yuan (2019SCU12014).
The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
YD carried out the RAPD studies and drafted the manuscript, QH performed the statistical analysis and edited the manuscript, CD participated in discussions about the analysis, and CJ conceived and coordinated the study. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
Genetic similarity matrix among the 17 Leishmania strains by NTSYS.
Additional file 2: Table S2.
Gene sequences of the converted L. donovani complex specific SCAR markers.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Yuan, D., Qin, H., Chen, D. et al. Genetic diversity analysis of Chinese Leishmania isolates and development of L. donovani complex-specific markers by RAPD. BMC Infect Dis 21, 464 (2021). https://doi.org/10.1186/s12879-021-06163-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-021-06163-y