Abstract
Background
HIV-associated chronic lung disease (CLD) is common among children living with HIV (CLWH) in sub-Saharan Africa, including those on antiretroviral therapy (ART). However, the pathogenesis of CLD and its possible association with microbial determinants remain poorly understood. We investigated the prevalence, and antibiotic susceptibility of Streptococcus pneumoniae (SP), Staphylococcus aureus (SA), Haemophilus influenzae (HI), and Moraxella catarrhalis (MC) among CLWH (established on ART) who had CLD (CLD+), or not (CLD-) in Zimbabwe and Malawi.
Methods
Nasopharyngeal swabs (NP) and sputa were collected from CLD+ CLWH (defined as forced-expiratory volume per second z-score < − 1 without reversibility post-bronchodilation with salbutamol), at enrolment as part of a randomised, placebo-controlled trial of azithromycin (BREATHE trial - NCT02426112), and from age- and sex-matched CLD- CLWH. Samples were cultured, and antibiotic susceptibility testing was conducted using disk diffusion. Risk factors for bacterial carriage were identified using questionnaires and analysed using multivariate logistic regression.
Results
A total of 410 participants (336 CLD+, 74 CLD-) were enrolled (median age, 15 years [IQR = 13–18]). SP and MC carriage in NP were higher in CLD+ than in CLD- children: 46% (154/336) vs. 26% (19/74), p = 0.008; and 14% (49/336) vs. 3% (2/74), p = 0.012, respectively. SP isolates from the NP of CLD+ children were more likely to be non-susceptible to penicillin than those from CLD- children (36% [53/144] vs 11% [2/18], p = 0.036). Methicillin-resistant SA was uncommon [4% (7/195)]. In multivariate analysis, key factors associated with NP bacterial carriage included having CLD (SP: adjusted odds ratio (aOR) 2 [95% CI 1.1–3.9]), younger age (SP: aOR 3.2 [1.8–5.8]), viral load suppression (SP: aOR 0.6 [0.4–1.0], SA: 0.5 [0.3–0.9]), stunting (SP: aOR 1.6 [1.1–2.6]) and male sex (SA: aOR 1.7 [1.0–2.9]). Sputum bacterial carriage was similar in both groups (50%) and was associated with Zimbabwean site (SP: aOR 3.1 [1.4–7.3], SA: 2.1 [1.1–4.2]), being on ART for a longer period (SP: aOR 0.3 [0.1–0.8]), and hot compared to rainy season (SP: aOR 2.3 [1.2–4.4]).
Conclusions
CLD+ CLWH were more likely to be colonised by MC and SP, including penicillin-non-susceptible SP strains, than CLD- CLWH. The role of these bacteria in CLD pathogenesis, including the risk of acute exacerbations, should be further studied.
Similar content being viewed by others
Background
More than 85% of the 3.8 million children living with HIV (CLWH) worldwide reside in Sub-Saharan Africa [1]. Substantial scale-up of antiretroviral therapy (ART) programmes and increased access to cotrimoxazole prophylaxis have remarkably improved the survival of perinatally HIV-infected children, many of whom would have died in infancy in the absence of these interventions [2]. As these children grow older, complications of long-standing HIV infection are becoming increasingly evident including delayed growth and cardiorespiratory diseases [3].
Respiratory diseases, in particular, are responsible for more than 50% of all HIV-related mortality in sub-Saharan Africa [4]. Recent studies in Malawi [5], Zimbabwe [6,7,8] and South Africa [9] have estimated a 30% prevalence of chronic lung disease (CLD) among CLWH. The typical clinical picture is that of hypoxia, tachypnoea, chronic cough, reduced exercise tolerance and impaired lung function [6, 10]; high resolution computed tomography findings are consistent with constrictive obliterative bronchiolitis [11].
Although the pathogenesis of HIV-associated CLD is incompletely understood, we and others have postulated that it may be a sequela of recurrent respiratory tract infections and dysregulated inflammation associated with HIV infection [12, 13]. Bacteria previously implicated in forms of HIV-associated CLDs such as bronchiectasis in individuals living with HIV include Streptococcus pneumoniae (SP), Staphylococcus aureus (SA), Haemophilus influenzae (HI) and Moraxella catarrhalis (MC) [14, 15]; however, their role in HIV-associated CLD pathogenesis is unclear.
We investigated the prevalence, bacterial load and antibiotic susceptibility of SP, SA, HI, MC and other Gram-negative bacilli (GNB) recovered from the nasopharyngeal (NP) swabs and sputa of CLWH (established on ART) who had CLD (CLD+) or not (CLD-). We also investigated risk factors for carriage of these bacteria.
Methods
Study characteristics
This is a case-control study nested within the BREATHe study; a multi-site, double-blind, placebo-controlled, individually-randomised trial that investigated the efficacy of azithromycin therapy in CLD (ClinicalTrials.gov, NCT02426112) [12]. The study setting, population and procedures of the trial are described elsewhere [10, 16]. Briefly, perinatally HIV-infected children aged 6–19 years with CLD (CLD+) who had been receiving ART for a minimum of six months were enrolled from outpatient HIV clinics in Blantyre, Malawi, and Harare, Zimbabwe, from June 2016 through August 2018. CLD was defined as forced expiratory volume in 1 s (FEV1) z-score less than − 1.0 with no reversibility (< 12% improvement in FEV1 after 200 μg of inhaled salbutamol). The justification for this definition is provided elsewhere [10]. A comparison group (CLD-) of perinatally HIV-infected children without CLD (FEV1 z-score > 0) was recruited at the same time as enrolment of trial participants using frequency matching for site, age (6–12 and 13–19 years) and duration of ART use (6 months to < 2 years and two or more years). These children had no heart or apparent lung disease and reported no respiratory symptoms. Socio-demographic data and clinical history were obtained through an interviewer-administered questionnaire.
Sample collection, transportation and processing
NP swabs were obtained at enrolment using nylon flocked swabs (Copan Italia, Brescia, Italy) by study staff. Sputum was subsequently obtained (expectorated or induced where necessary). Samples were immediately stored in 1 ml of skim milk, tryptone, glucose, and glycerine (STGG) medium, placed on ice for a maximum of 1 h and then frozen down to at -80 °C. The samples were transported on dry ice to Cape Town, South Africa, for batch processing.
Bacterial culture, identification and antimicrobial susceptibility testing (AST)
NP and sputum samples were thawed to 22 °C and vortexed for 30 s. A 10 μl volume of each sample was inoculated onto Bacitracin-heated blood agar (BHB), Columbia with Gentamicin agar (Colgent), Mannitol salt agar (MSA) and 2% sheep blood agar (BA)(NHLS Greenpoint Media, Cape Town, South Africa). The BHB, Colgent and BA were incubated in 5% carbon dioxide at 37 °C for 48 h whilst the MSA was incubated in ambient air at 37 °C for 48 h. Alpha haemolytic colonies on Colgent which were susceptible to optochin were presumptively identified as SP. Colourless, medium-sized colonies on BHB with a strict requirement for factor X (Hemin) and/or V (NAD+) were presumed to be HI or Haemophilus parainfluenzae, respectively. Non-haemolytic grey to white colonies on BA that tested positive for the push test were identified as MC. DNase-positive colonies from MSA were identified as SA. Colonies, other than MC and HI, from BA and BHB that grew on MacConkey agar were presumptively identified as ‘other’ gram-negative bacilli (GNB) by colony morphology and Gram staining. The species identities of these GNBs were confirmed using Matrix-Assisted Laser Desorption/Ionization-Time-of-Flight mass spectrometry (MALDI-TOF MS). Bacterial load was semi-quantitatively assessed as follows: an aliquot of each sample was streaked for single colonies. Growth in one, two, three or all quadrants of a culture plate was assigned the labels 1, 2, 3 and 4, respectively.
AST was conducted using the Kirby-Bauer disk diffusion method. The antibiotics tested for each pathogen were as follows: SP (oxacillin, cotrimoxazole, azithromycin and tetracycline), SA (cotrimoxazole, azithromycin, tetracycline, clindamycin, penicillin, and cefoxitin), HI (cotrimoxazole, azithromycin, tetracycline, ampicillin, amoxicillin-clavulanate, and cefuroxime) and MC (amoxicillin-clavulanate, cotrimoxazole, azithromycin and tetracycline). SA susceptibility to cefoxitin was tested as a surrogate for methicillin-resistance. AST was conducted and interpreted according to the 2018 Clinical and Laboratory Standards Institute guidelines and breakpoints, respectively [17].
Statistical analysis
R version 3.6.0 was used to conduct statistical analyses. The 1990 British growth reference curves [18] were used to generate height-for-age and weight-for-age z-scores. The lung function z-score were calculated using global lung function initiative GLI/ERS reference equations and the African American module [19]. Our team validated this among children in Zimbabwe and findings are published elsewhere [20]. Comparison of categorical data including semi-quantitative bacterial load distribution was performed by Fisher’s exact test or Chi-square test where appropriate. Univariate and multivariate analyses of association with carriage of each individual bacterial species were performed using logistic regression and presented as odds ratios (OR) and adjusted ORs with a 95% confidence interval (CI), respectively. The following variables were selected a priori to investigate the risk factors for carriage of each bacterium: CLD, sex, age category, study site (Zimbabwe or Malawi), the season of sample collection (Rainy season: December to April, Cool season: May to August, Hot season: September to November), HIV viral load, previous TB treatment and ART regimen and duration. Any other variables identified as independent predictors of carriage in univariate analysis (p < 0.25) were also included in the adjusted model for that species. The following were excluded from the multivariate model because of co-linearity: Enrolment BMI-for-age z-score and weight-for-age (colinear with height-for-age), and CD4 count (colinear with viral load suppression).
Results
Participant clinical and socio-demographic characteristics
The study included 336 CLD+ and 74 CLD- participants, median (IQR) age 15 (13–18) years (Table 1). The median FEV1 z-score for CLD+ and CLD- children was − 1.96 (IQR -2.46, − 1.47) and 0.52 (IQR 0.23, 0.79), p < 0.001, respectively. The CLD+ group were more stunted (50% vs 30% [p < 0.001]) and underweight (52% vs 19% [p < 0.001]) compared to CLD- group, and a higher proportion of CLD+ had been previously treated for tuberculosis (29% vs 12% [p = 0.003]). Overall, 90% were taking cotrimoxazole prophylaxis. Both groups had been on ART for a median of 6.5 years but more CLD+ participants were on a protease inhibitor-based (second line) ART regimen than CLD- participants (26% vs 11% [p < 0.05]). Virologic suppression was similar between groups (CLD+: 56% vs CLD-: 66% [p = 0.12]). None of the participants reported smoking.
Prevalence of bacterial carriage
In the CLD+ group, 67% (226/336) of the NP swabs had at least one of the four bacterial species (SP, SA, HI and MC) compared to 39% (29/74) of swabs from the CLD- group (p < 0.001). Both SP (46% vs 26% [adjusted p = 0.008]), and MC (14% vs 3% [adjusted p = 0.012]) were more prevalent in the CLD+ group (Table 2).
In total, 400 sputa from 326 CLD+ and 74 CLD- participants were collected. At least one bacterial species was isolated from the sputa of half of the participants in each group, with no difference by CLD status (Table 2). There was no difference in semi-quantitative loads of any bacteria from either NP swabs or sputum between the two groups (Supplementary table: T1).
Thirteen different GNBs from NP and sputa were identified by MALDI-TOF MS platform. More GNBs were recovered from the CLD+ than in CLD- group (Supplementary table: T2). Among bacteria isolated from NP swabs and sputa, there were statistically significant co-carriage relationships between SP, HI, and MC carriage (Supplementary table: T3). These co-carriage relationships were independent of age or site.
Risk factors associated with carriage of bacteria
In multivariate analysis, participants on ART for two or more years were less likely to carry SP in both NP and sputum (Tables 3 and 4). Risk factors associated with SP carriage in the NP were having CLD (adjusted OR: 2.0 [1.06–3.89], p = 0.036), younger age (e.g., being 6 to 12 years old at the time of sampling compared to 17 to 19 years (adjusted OR: 3.2 [1.76–5.85], p > 0.001), and stunting (height-for-age-z score < − 2) (adjusted OR: 1.6 [1.05–2.58], p = 0.03). Participants with suppressed viral load (< 1000 copies/mL) (adjusted OR: 0.6 [0.38–0.95], p = 0.032), were less likely to carry SP in their NP (Table 3). Participants in Zimbabwe (adjusted OR: 3.1 [1.43–7.34], p = 0.006), sample collected in hot season (adjusted OR: 2.3 [1.22–4.4], p = 0.036) and previous tuberculosis treatment (adjusted OR: 1.8 [1.02–3.17], p = 0.043) were associated with sputum carriage of SP [Table 4].
Male participants had increased odds of carrying SA in their NP (adjusted OR: 1.7 [1.01–2.92], p = 0.048) whilst participants with suppressed viral loads (< 1000 copies/ml) were less likely to carry SA in their NP (adjusted OR: 0.5 [0.32–0.91], p = 0.021) (Table 3). For sputa, participants from Zimbabwe had higher odds of carrying SA (adjusted OR: 2.1 [1.05–4.2], p = 0.038) than those from Malawi (Table 4).
With regards to HI (Tables 5 and 6), participants from Zimbabwe (adjusted OR: 3.9 [1.47–11.74], p = 0.009), those aged 13 to 16 years at sampling (adjusted OR: 3.6 [1.46–10.22], p = 0.031), and those that had MRC dyspnoea score > 1 (adjusted OR: 2.6 [1.16–5.75], p = 0.02) were more likely to carry HI in their NP swabs (Table 5). No other variable tested was associated with sputum HI carriage (Table 6).
Sampling in the hot and cool seasons (May to November) compared to the rainy season (adjusted OR: 3.1 [1.25–8.08], p = 0.036), participants whose ages were less than 17 to 19 years (adjusted OR: 4 [1.39–13.22], p = 0.039), and participants who had MRC dyspnoea score > 1 (adjusted OR: 2.4 [1.06–5.43], p = 0.036), were more likely to carry MC in their NP. In contrast, participants who had used ART for two or more years (adjusted OR: 0.3 [0.07–0.93] p = 0.008) were less likely to carry MC in their NP (Table 5). No other variable tested was associated with sputum MC carriage (Table 6).
Antibiotic and multidrug resistance of isolates
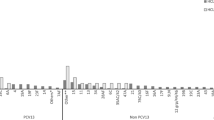
Some SP isolates failed to grow after initial isolation and therefore antibiotic susceptibility was conducted on 147/154 and 18/19 isolates from NP, and 75/83 and 15/17 isolates from sputa of CLD+ and CLD- participants, respectively. The proportion of NP isolates non-susceptible to penicillin was 32% (55/173) and that for sputa was 22% (20/90). A total of 29% (75/263) of all SP isolates regardless of sample type were penicillin non-susceptible. Penicillin non-susceptibility among SP was more common in the CLD+ participants (36% [53/147] vs 10% [2/19] p = 0.036). There were no other statistically significant differences in the antibiotic resistance profiles of SP, SA, HI and MC isolated from any sample type of the CLD+ vs CLD- participants. For all isolates recovered from both NP and sputa, there were generally low levels of resistance to azithromycin (SP = 16% [27/166]; SA = 8% [17/195]) and tetracycline (SP = 18% [45/253]; SA = 20% [39/195]; HI = 10% [6/58] and MC = 15% [12/81]) and moderate to very high cotrimoxazole resistance (SP = 95% [240/255]; SA = 67% [130/195], HI = 95% [55/58] and MC = 48% [38/81]) (Fig. 1). Methicillin-resistant SA was uncommon (4%, 7/195). β-lactamase production by MC was widespread (93%, 76/82) but not different between the two groups. Antibiotic susceptibility profiles did not differ between sputum or NP isolates for any bacterial species (Fig. 1).
Bar plot comparing the percentage of antibiotic resistance of S. aureus [n = 85 for nasopharyngeal swab isolates, n = 110 for sputum isolates] and S. pneumoniae [n = 165 nasopharyngeal swab isolates, n = 90 or sputum isolates] isolates recovered from the nasopharyngeal swabs and sputa. Only the antibiotics tested for each bacterium are shown
Discussion
The main finding of our study was that CLD+ CLWH were more likely to carry SP and MC in their NP than their CLD- counterparts whereas, participants with high MRC dyspnoea score (reflecting respiratory disability) were more likely to carry MC and HI. In addition, age, HIV viral load, duration of ART, the season of sample collection, site and nutritional status were associated with bacterial carriage among study participants. Longer period on ART or suppressed viral load were associated with reduced carriage for several bacterial species. The observed differences between the CLD+ and CLD- groups were more striking in NP compared to sputa. Antimicrobial susceptibility patterns were similar between the CLD+ and CLD- groups, apart from SP penicillin non-susceptibility, which was higher in the CLD+ group.
Studies of bacterial carriage among CLWH with CLD, but without acute infection, are limited. Masekela et al., [14] observed that among CLWH aged 6–14 years (mean 6.9 years), diagnosed with HIV-related bronchiectasis in South Africa, the most prevalent bacteria, from cumulative induced sputum samples collected over one year, were HI (30%) and H. parainfluenzae (21%) followed by Pseudomonas aeruginosa (2%) and SA (1%) [14]. Similarly, Verwey et al., [21] also found HI to be the most frequent bacterial species (38%) in respiratory samples (mainly sputum) of 52 CLWH with non-CF bronchiectasis [median age, 11.4 years (interquartile range 7.7–12.5)]. Samples were collected over a 2-year period and the prevalence of the other relevant bacterial species were SP (12%), MC (13%) and SA (11%) [21]. Ferrand et al., [6], also found HI (n = 6) to be the most frequent bacteria recovered from 18 expectorated sputa from Zimbabwean CLWH (mean age, 14 ± 2.6 years) with CLD and without acute respiratory infection [6].
Although, the most prevalent bacterial species that we identified were similar to those detected in previous studies using sputum samples, the prevalences differed substantially. SA (29%) followed by SP (25%) then MC (9%) were the most common bacteria isolated from our CLD+ subjects. HI was infrequently detected (4%). These differences in study results may be explained by a number of factors including sample type (lower respiratory tract samples vs NP swabs), clinical state of participants (acute exacerbation vs. stable) and age. The studies by Masekela [14] and Verwey [21] both included samples collected during acute exacerbations as well as intervening periods.
Differences in age between the studies may also explain the differences in bacterial carriage; participants in the studies by Masekela [14] and Verwey [21] were younger than those included in our study (Masekela: mean 6.9 years (range 6–14 years) [14], Verwey: median 9.1 years (IQR 7.2–12.1) [21], Ferrand: mean 14 (SD ± 2.6) years [6]) and 15(IQR: 13–18) in our study). Moreover, Masekela [14] investigated induced sputum while Verwey [21] included expectorated and induced sputum, bronchoalveolar lavage and tracheal samples. In Ferrand’s [6] study and ours, the samples collected were expectorated sputum for CLD+ subjects. Finally, both Masekela [14] and Verwey [21] studies also included multiple samples from the same participants – analysis did not adjust for multiple sampling from the same participant.
In our study, CLD+ participants were more stunted and underweight compared to their CLD- counterparts (Table 1). A meta-analysis showed that the prevalence of SP carriage is higher in malnourished children compared to their counterparts who were not malnourished [22]. This study also found that stunted and underweight children were also more likely to carry SP than children with normal weight and height, a finding which is consistent with our results. Malnutrition causes immune changes such as atrophy of the thymus, impairment of complement activity and immunoglobulin responses to encapsulated bacteria and a reduction in immunoglobulin A secretion [22].
The prevalence of SP in NP of CLD- CLWH (26%) is comparable to similar studies of CLWH in Ghana [27.1%, mean age was 5.8 ± 3.3 years with 59.3% falling within 9 to 15 years old range] [23] and India [27.8%, median age: 6 years, IQR:4,9 with 57% falling within 12 to 17 years old range] [24]. However, this prevalence is higher than that observed in CLWH from Cambodia [17.6%, median age: 7 years, IQR = 5–9 years] [25] and Ethiopia [10.3%, median age: 11 years (range was 6–16 years)] [26], and lower than CLWH from Zambia [51%, median age: 5.1 years (IQR = 2.8 to 8.7 years)] [27] and Tanzania [81%, mean age is 6.39, SD = 3.18] [28] . The participants in these other studies were not reported to have CLD. These differences in bacterial prevalence between studies may be related to sampling site (pharyngeal vs NP) [26], age of participants (younger children have higher carriage prevalence) [27,28,29], and method of detection; PCR is associated with increased detection of SP strains compared to culture [28].
While we did not record vaccination history, the majority of our study participants would not have been previously vaccinated against pneumococcal disease as these vaccines were introduced in both countries around 2012 [30, 31] when our study participants would have been too old to be eligible for immunisation. Pneumococcal vaccines have been shown to reduce both carriage and disease through individual protection of the vaccinated which in turn disrupts transmission [32]. Hence, the high SP carriage in our settings might also be attributed to the lack of pneumococcal vaccination.
The prevalence of SA in NP of CLD+ CLWH (23%) is comparable to similar studies conducted among CLWH in Ethiopia (29–31%) [26, 33] and India (24%) [34]. However, the prevalence in CLD- participants (12%) was lower in our study. These discrepancies in bacterial prevalence between studies may be explained by the differences in sampling sites (NP vs nasal) or age. The anterior nares are the natural niche for SA and may be expected to be more frequently colonised than the NP [35].
Zambian [27] and Indian [34] studies of CLWH reported a much higher prevalence of HI (29 and 26% respectively) than either of our groups [12%(CLD+) and 5% (CLD-)]. This higher prevalence may be due to the younger age of the study participants (median ages were 5.1 years and 6.5 years for the Zambian and Indian studies respectively, compared to median 15 years for our study. NP HI carriage declines with increasing age [29, 36].
The prevalence of MC in the NP of CLD- CLWH (3%) was much lower than similar studies conducted among CLWH in Ethiopia (12.3%, median age is 11 years) [26] and Ghana (39.8%, median age is 5.8 years) [37], but comparable to a Cambodian study (6%, median age is 7 years) [25]. Again, differences in age may explain these findings. NP MC carriage declines with increasing age from about 60% at 1–2 years to about 12% at 7–14 years [36]. MC is implicated in acute exacerbations of chronic bronchitis [38] and chronic obstructive pulmonary disease (COPD) [39], and therefore our finding of higher NP carriage in CLD+ participants (14%) vs CLD- participants (3%) is of interest. Sputum MC carriage was also higher in CLD+ (9%) vs CLD- CLWH (5%), but this difference was not statistically significant. Whether MC carriage plays a role in the pathogenesis of CLD or is a consequence of CLD requires further study.
The colonisation of the nasopharynx by multiple bacterial species may have important clinical consequences including biofilm formation, polymicrobial infections and antibiotic resistance [40, 41]. We found strong positive associations between NP carriage of SP, HI and MC. While some previous reports observed similar positive associations [34, 36, 42,43,44,45], others found the opposite [46,47,48]. Possible reasons for the varying observations include differences in age of participants enrolled, vaccination schedules, year of study, host-genetic background, antibiotic use and socio-economic status of the countries of study (low income compared to high-income status).
Interestingly, the differences we observed in NP bacterial carriage between CLD+ and CLD- groups was not mirrored in the sputum, despite the belief that the source of bacteria in the lower respiratory tract is largely from the upper airways through micro-aspiration [49]. Evidence from studies suggests that the oropharyngeal samples rather than the nasopharyngeal mirrors the lung microbiota (sputum) [50]. This may explain the discrepancies in NP and sputa bacterial carriage we observed.
We also found that ART for more than two years reduced the odds of pneumococcal carriage in both NP and sputa. This is consistent with a report by Nicoletti et al. [51] who found that consistent use of the same ART for a year or more was negatively associated with risk of NP pneumococcal colonisation in adults living with HIV. Incomplete recovery of B cell function was noted in children who were on ART for less than a year [52] and was associated with high NP pneumococcal carriage [52].
Increased odds of NP pneumococcal carriage in hot, dry seasons compared to rainy seasons is consistent with previous findings in Malawi [53] and other parts of Africa (Kenya [54] and Gambia [55]). Similar observations have also been made in Thailand [56] and the United States of America [57]. One reason for this observation is increased school attendance by children during the hot season, which may increase transmission. Furthermore, a study in Niger revealed that airborne dust and high temperatures were risk factors for invasive pneumococcal disease [56]. Dust exposure attenuates phagocyte-mediated bacterial killing whilst the high temperatures promoted SP autolysis, accompanied by the release of the toxin pneumolysin [58]. Our finding that pneumococcal carriage was higher in the hot, dry seasons may further explain the higher incidence of invasive pneumococcal disease (IPD) among both patients living with and without HIV irrespective of age in Malawi during these seasons [59].
The reason for the association between male sex and SA carriage, also reported previously [60], is incompletely understood. Potential reasons include poor hand hygiene and participation in contact sport by males [60]. Furthermore, physiological factors, including sex hormones, regulation of the immune system and bacterial virulence have also been suggested [60].
Our finding of higher prevalence of penicillin-non-susceptible SP in CLD+ participants is unsurprising since these participants were more likely to have been previously treated with antibiotics for acute exacerbations and were also more likely to have been treated for tuberculosis. Indeed, previous tuberculosis treatment was associated with increased odds of NP SP carriage in our multivariate model. Recent exposure to antibiotics is the strongest risk factor for NP carriage and spread of resistant SP [61]. Again, pneumococcal vaccination may be beneficial in this population. This is because vaccine serotypes are more likely to be resistant and therefore a reduction in carriage of vaccine serotypes resulting from prior immunisation can reduce antimicrobial resistance [62].
All four bacterial species tested exhibited moderate to high levels of resistance to cotrimoxazole. This was expected as 90% were receiving cotrimoxazole prophylaxis. Cotrimoxazole has been shown to reduce morbidity and mortality not only in patients living with HIV but also in their family members who are living without the infection [63], despite high background resistance in respiratory pathogens [27]. This positive effect may result from a reduction of systemic and intestinal inflammation via the modulation of the gut microbiome and immune and epithelial cell activation [64].
The strengths of our study include the enrolment of age-matched CLD- participants from the same area as the CLD+ group as a comparison group. We also included participants from two sites, which increased generalisability. Limitations of our study include the use of culture alone, without PCR, which may be more sensitive for detection of bacteria and would allow more precise quantitation, and the relatively small sample size for the comparator CLD- group which may have reduced statistical power. Also, the definition of CLD used may inadvertently include participants who may have normal lung function. However, our previous studies have shown that FEV1 z-score is an objective measure that correlates well with CLD based on radiological analysis (high-resolution computed tomography) [5,6,7]. Also, within the main trial under which this study was nested, we anticipated a reduced efficacy of azithromycin in participants with advanced disease, and therefore did not restrict enrolment to more severely ill, symptomatic children.
In conclusion, CLD+ CLWH were more likely to be colonised by MC and SP, including penicillin-resistant SP strains. The role of these bacteria in CLD pathogenesis, including the risk of acute exacerbations, should be further investigated.
Availability of data and materials
The datasets used and analysed during the current study are available from the corresponding author on reasonable request and ethical approval.
Abbreviations
- ART:
-
Antiretroviral therapy
- CLD:
-
HIV-associated chronic lung disease
- CLD:
-
Participants with HIV-associated chronic lung disease.
- CLD-:
-
Participants without HIV-associated chronic lung disease
- CLWH:
-
Children living with HIV
- GNB:
-
Other Gram negative bacilli other than HI
- HI:
-
Haemophilus influenzae
- IPD:
-
Invasive pneumococcal disease
- MC:
-
Moraxella catarrhalis
- NP:
-
Nasopharyngeal
- SA:
-
Staphylococcus aureus
- SP:
-
Streptococcus pneumoniae
References
HIV Statistics - Global and Regional Trends - UNICEF DATA. 2020. https://data.unicef.org/topic/hivaids/global-regional-trends/. Accessed 1 Apr 2020.
Peacock-Villada E, Richardson BA, John-Stewart GC. Post-HAART outcomes in pediatric populations: comparison of resource-limited and developed countries. Pediatrics. 2011;127:e423–41.
McHugh G, Rylance J, Mujuru H, Nathoo K, Chonzi P, Dauya E, et al. Chronic morbidity among older children and adolescents at diagnosis of HIV infection. J Acquir Immune Defic Syndr. 2016;73:275–81.
Cox JA, Lukande RL, Lucas S, Nelson AM, Van Marck E, Colebunders R. Autopsy causes of death in HIV-positive individuals in sub-Saharan Africa and correlation with clinical diagnoses. AIDS Rev. 2010;12:183–94.
Mwalukomo T, Rylance SJ, Webb EL, Anderson S, O’Hare B, Oosterhout V, et al. Clinical characteristics and lung function in older children vertically infected with human immunodeficiency virus in Malawi. J Pediatric Infect Dis Soc. 2016;5:161–9. https://doi.org/10.1093/jpids/piv045.
Ferrand RA, Desai SR, Hopkins C, Elston CM, Copley SJ, Nathoo K, et al. Chronic lung disease in adolescents with delayed diagnosis of vertically acquired HIV infection. Clin Infect Dis. 2012;55:145–52.
Desai SR, Nair A, Rylance J, Mujuru H, Nathoo K, McHugh G, et al. Human immunodeficiency virus-associated chronic lung disease in children and adolescents in Zimbabwe: chest radiographic and high-resolution computed tomographic findings. Clin Infect Dis. 2018;66:274–81.
Rylance J, McHugh G, Metcalfe J, Mujuru H, Nathoo K, Wilmore S, et al. Chronic lung disease in HIV-infected children established on antiretroviral therapy. Aids. 2016;30:2795–803.
du Plessis AM, Andronikou S, Machemedze T, Griffith-Richards S, Myer L, Mahtab S, et al. High-resolution computed tomography features of lung disease in perinatally HIV-infected adolescents on combined antiretroviral therapy. Pediatr Pulmonol. 2019;54:1765–73.
McHugh G, Rehman AM, Simms V, Gonzalez-Martinez C, Bandason T, Dauya E, et al. Chronic lung disease in children and adolescents with HIV: a case–control study. Trop Med Int Heal. 2020;25:590–9.
Githinji LN, Gray DM, Zar HJ. Lung function in HIV-infected children and adolescents. Pneumonia (Nathan). 2018;10:6.
Gonzalez-Martinez C, Kranzer K, McHugh G, Corbett EL, Mujuru H, Nicol MP, et al. Azithromycin versus placebo for the treatment of HIV-associated chronic lung disease in children and adolescents (BREATHE trial): study protocol for a randomised controlled trial. Trials. 2017;18:622.
Fitzpatrick ME, Nouraie M, Gingo MR, Camp D, Kessinger CJ, Sincebaugh JB, et al. Novel relationships of markers of monocyte activation and endothelial dysfunction with pulmonary dysfunction in HIV-infected persons. Aids. 2016;30:1327–39.
Masekela R, Anderson R, Moodley T, Kitchin OP, Risenga SM, Becker PJ, et al. HIV-related bronchiectasis in children: an emerging spectre in high tuberculosis burden areas. Int J Tuberc Lung Dis. 2012;16:114–9.
Zar HJ. Chronic lung disease in human immunodeficiency virus (HIV) infected children. Pediatr Pulmonol. 2008;43:1–10.
Rehman AM, Ferrand R, Allen E, Simms V, McHugh G, Weiss HA. Exclusion of enrolled participants in randomised controlled trials: what to do with ineligible participants? BMJ Open. 2020;10:e039546. https://doi.org/10.1136/bmjopen-2020-039546.
CLSI. Performance standards for antimicrobial susceptibility testing. 28th Editi. Wayne: Clinical Laboratory Standards Institute; 2018.
Cole TJ, Freeman JV, Preece MA. British 1990 growth reference centiles for weight, height, body mass index and head circumference fitted by maximum penalized likelihood. Stat Med. 1998;17:407–29.
Quanjer PH, Stanojevic S, Cole TJ, Baur X, Hall GL, Culver BH, et al. Multi-ethnic reference values for spirometry for the 3–95-yr age range: the global lung function 2012 equations. Eur Respir J. 2012;40:1324–43. https://doi.org/10.1183/09031936.00080312.
Madanhire T, Ferrand RA, Attia EF, Sibanda EN, Rusakaniko S, Rehman AM. Validation of the global lung initiative 2012 multi-ethnic spirometric reference equations in healthy urban Zimbabwean 7–13 year-old school children: a cross-sectional observational study. BMC Pulm Med. 2020;20:56. https://doi.org/10.1186/s12890-020-1091-4.
Verwey C, Velaphi S, Khan R. Bacteria isolated from the airways of paediatric patients with bronchiectasis according to HIV status. South Afr Med J. 2017;107:435. https://doi.org/10.7196/SAMJ.2017.v107i5.10692.
Smith HC, German E, Ferreira DM, Rylance J. Nasopharyngeal colonisation with Streptococcus pneumoniae in malnourished children: a systematic review and meta-analysis of prevalence. Trans R Soc Trop Med Hyg. 2019;113:227–33.
Donkor ES, Annan JA, Badoe EV, Dayie NT, Labi AK, Slotved HC. Pneumococcal carriage among HIV infected children in Accra, Ghana. BMC Infect Dis. 2017;17:133.
Bhattacharya SD, Niyogi SK, Bhattacharyya S, Fitzwater S, Chauhan N, Sudar A, et al. High rates of colonization with drug resistant hemophilus influenzae type B and Streptococccus Pneumoniae in unvaccinated HIV infected children from West Bengal. Indian J Pediatr. 2011;78:423–9.
Krcmery V, Sokolova J, Kulkova N, Liskova A, Shahum A, Benca G. Nasopharyngeal bacterial colonisation in HIV-positive children in Cambodia. Trop Med Int Heal. 2013;18:1267–8.
Mulu W, Yizengaw E, Alemu M, Mekonnen D, Hailu D, Ketemaw K, et al. Pharyngeal colonization and drug resistance profiles of Morraxella catarrrhalis, Streptococcus pneumoniae, Staphylococcus aureus, and Haemophilus influenzae among HIV infected children attending ART Clinic of Felegehiwot referral hospital, Ethiopia. PLoS One. 2018;13:e0196722.
Mwenya DM, Charalambous BM, Phillips PP, Mwansa JC, Batt SL, Nunn AJ, et al. Impact of cotrimoxazole on carriage and antibiotic resistance of Streptococcus pneumoniae and Haemophilus influenzae in HIV-infected children in Zambia. Antimicrob Agents Chemother. 2010;54:3756–62.
Anthony L, Meehan A, Amos B, Mtove G, Mjema J, Malahiyo R, et al. Nasopharyngeal carriage of Streptococcus pneumoniae: prevalence and risk factors in HIV-positive children in Tanzania. Int J Infect Dis. 2012;16:e753–7.
Abdullahi O, Nyiro J, Lewa P, Slack M, Scott JA. The descriptive epidemiology of Streptococcus pneumoniae and Haemophilus influenzae nasopharyngeal carriage in children and adults in Kilifi district, Kenya. Pediatr Infect Dis J. 2008;27:59–64.
Pneumococcal vaccine launched in Zimbabwe. Gavi, the Vaccine Alliance. 2020. https://www.gavi.org/news/media-room/pneumococcal-vaccine-launched-zimbabwe. Accessed 25 Apr 2020.
Pneumococcal vaccines introduced in Malawi. Gavi, the Vaccine Alliance. 2020. https://www.gavi.org/pneumococcal-vaccines-introduced-malawi. Accessed 25 Apr 2020.
Madhi SA, Nunes MC. The potential impact of pneumococcal conjugate vaccine in Africa: considerations and early lessons learned from the south African experience. Hum Vaccin Immunother. 2016;12:314–25.
Gebremedhn G, Gebremariam TT, Wasihun AG, Dejene TA, Saravanan M. Prevalence and risk factors of methicillin-resistant Staphylococcus aureus colonization among HIV patients in Mekelle, Northern Ethiopia. Springerplus. 2016;5:877.
Bhattacharya SD, Niyogi SK, Bhattacharyya S, Arya BK, Chauhan N, Mandal S. Associations between potential bacterial pathogens in the nasopharynx of HIV infected children. Indian J Pediatr. 2012;79:1447–53.
Krismer B, Weidenmaier C, Zipperer A, Peschel A. The commensal lifestyle of Staphylococcus aureus and its interactions with the nasal microbiota. Nat Rev Microbiol. 2017;15:675–87.
Kovács E, Sahin-Tóth J, Tóthpál A, van der Linden M, Tirczka T, Dobay O. Co-carriage of Staphylococcus aureus, Streptococcus pneumoniae, Haemophilus influenzae and Moraxella catarrhalis among three different age categories of children in Hungary. PLoS One. 2020;15:e0229021.
Sampane-Donkor E, Badoe EV, Annan JA, Nii-Trebi N. Colonisation of antibiotic resistant bacteria in a cohort of HIV infected children in Ghana. Pan Afr Med J. 2017;26:60.
Read RC. Infection in acute exacerbations of chronic bronchitis: a clinical perspective. Respir Med. 1999;93:845–50.
George LM, Haigh RD, Mistry V, Haldar K, Barer MR, Oggioni MR, et al. Sputum Moraxella catarrhalis strains exhibit diversity within and between COPD subjects. Int J Chron Obs Pulmon Dis. 2018;13:3663–7.
Krishnamurthy A, McGrath J, Cripps AW, Kyd JM. The incidence of Streptococcus pneumoniae otitis media is affected by the polymicrobial environment particularly Moraxella catarrhalis in a mouse nasal colonisation model. Microbes Infect. 2009;11:545–53.
Watson K, Carville K, Bowman J, Jacoby P, Riley TV, Leach AJ, et al. Upper respiratory tract bacterial carriage in Aboriginal and non-Aboriginal children in a semi-arid area of Western Australia. Pediatr Infect Dis J. 2006;25:782–90.
McNally LM, Jeena PM, Gajee K, Sturm AW, Tomkins AM, Coovadia HM, et al. Lack of association between the nasopharyngeal carriage of Streptococcus pneumoniae and Staphylococcus aureus in HIV-1-infected south African children. J Infect Dis. 2006;194:385–90.
Kwambana BA, Barer MR, Bottomley C, Adegbola RA, Antonio M. Early acquisition and high nasopharyngeal co-colonisation by Streptococcus pneumoniae and three respiratory pathogens amongst Gambian new-borns and infants. BMC Infect Dis. 2011;11:175.
Jacoby P, Watson K, Bowman J, Taylor A, Riley TV, Smith DW, et al. Modelling the co-occurrence of Streptococcus pneumoniae with other bacterial and viral pathogens in the upper respiratory tract. Vaccine. 2007;25:2458–64.
Bae S, Yu JY, Lee K, Lee S, Park B, Kang Y. Nasal colonization by four potential respiratory bacteria in healthy children attending kindergarten or elementary school in Seoul, Korea. J Med Microbiol. 2012;61(Pt 5):678–85.
Pettigrew MM, Gent JF, Revai K, Patel JA, Chonmaitree T. Microbial interactions during upper respiratory tract infections. Emerg Infect Dis. 2008;14:1584–91.
Dunne EM, Smith-Vaughan HC, Robins-Browne RM, Mulholland EK, Satzke C. Nasopharyngeal microbial interactions in the era of pneumococcal conjugate vaccination. Vaccine. 2013;31:2333–42.
Dahlblom V, Söderström M. Bacterial interactions in the nasopharynx - effects of host factors in children attending day-care centers. J Infect Public Heal. 2012;5:133–9.
Hare KM, Grimwood K, Leach AJ, Smith-Vaughan H, Torzillo PJ, Morris PS, et al. Respiratory bacterial pathogens in the nasopharynx and lower airways of Australian indigenous children with bronchiectasis. J Pediatr. 2010;157:1001–5.
Liu HY, Zhang SY, Yang WY, Su XF, He Y, Zhou HW, et al. Oropharyngeal and sputum microbiomes are similar following exacerbation of chronic obstructive pulmonary disease. Front Microbiol. 2017;8:1163.
Nicoletti C, Brandileone MC, Guerra ML, Levin AS. Prevalence, serotypes, and risk factors for pneumococcal carriage among HIV-infected adults. Diagn Microbiol Infect Dis. 2007;57:259–65.
Iwajomo OH, Moons P, Nkhata R, Mzinza D, Ogunniyi AD, Williams NA, et al. Delayed reconstitution of B cell immunity to pneumococcus in HIV-infected Malawian children on antiretroviral therapy. J Inf Secur. 2015;70:616–23.
Heinsbroek E, Tafatatha T, Phiri A, Swarthout TD, Alaerts M, Crampin AC, et al. Pneumococcal carriage in households in Karonga District, Malawi, before and after introduction of 13-valent pneumococcal conjugate vaccination. Vaccine. 2018;36:7369–76.
Abdullahi O, Karani A, Tigoi CC, Mugo D, Kungu S, Wanjiru E, et al. The prevalence and risk factors for pneumococcal colonization of the nasopharynx among children in Kilifi District, Kenya. PLoS One. 2012;7:e30787.
Bojang A, Jafali J, Egere UE, Hill PC, Antonio M, Jeffries D, et al. Seasonality of pneumococcal nasopharyngeal carriage in rural Gambia determined within the context of a cluster randomized pneumococcal vaccine trial. PLoS One. 2015;10:e0129649.
Numminen E, Chewapreecha C, Turner C, Goldblatt D, Nosten F, Bentley SD, et al. Climate induces seasonality in pneumococcal transmission. Sci Rep. 2015;5:11344.
Weinberger DM, Grant LR, Steiner CA, Weatherholtz R, Santosham M, Viboud C, et al. Seasonal drivers of pneumococcal disease incidence: impact of bacterial carriage and viral activity. Clin Infect Dis. 2014;58:188–94.
Jusot JF, Neill DR, Waters EM, Bangert M, Collins M, Bricio Moreno L, et al. Airborne dust and high temperatures are risk factors for invasive bacterial disease. J Allergy Clin Immunol. 2017;139:977–986.e2.
Everett DB, Mukaka M, Denis B, Gordon SB, Carrol ED, van Oosterhout JJ, et al. Ten years of surveillance for invasive Streptococcus pneumoniae during the era of antiretroviral scale-up and cotrimoxazole prophylaxis in Malawi. PLoS One. 2011;6:e17765.
Humphreys H, Fitzpatick F, Harvey BJ. Gender differences in rates of carriage and bloodstream infection caused by methicillin-resistant staphylococcus aureus: are they real, do they matter and why? Clin Infect Dis. 2015;61:1708–14.
Canet JJ, Garau J. Importance of dose and duration of beta-lactam therapy in nasopharyngeal colonization with resistant pneumococci. J Antimicrob Chemother. 2002;50 Suppl S:39–43.
Tin Tin Htar M, den Biggelaar AHJ v, Sings H, Ferreira G, Moffatt M, Hall-Murray C, et al. The impact of routine childhood immunization with higher-valent pneumococcal conjugate vaccines on antimicrobial-resistant pneumococcal diseases and carriage: a systematic literature review. Expert Rev Vaccines. 2019;18:1069–89.
Mermin J, Lule J, Ekwaru JP, Downing R, Hughes P, Bunnell R, et al. Cotrimoxazole prophylaxis by HIV-infected persons in Uganda reduces morbidity and mortality among HIV-uninfected family members. Aids. 2005;19:1035–42.
Bourke CD, Gough EK, Pimundu G, Shonhai A, Berejena C, Terry L, et al. Cotrimoxazole reduces systemic inflammation in HIV infection by altering the gut microbiome and immune activation. Sci Transl Med. 2019:11.
Acknowledgements
We would like to acknowledge the BREATHE trial participants and families and study team. We will also like to thank Division of Medical Microbiology staff, particularly Wendy Blose and members of Dube Lab, UCT and Dr. John Osei for editorial support on first draft.
Funding
This study was funded by the Global Health and Vaccination Research (GLOBVAC) Programme of the Medical Research Council of Norway. REA acknowledges the financial support of the Swedish International Development Cooperation Agency (SIDA) through Organisation of Women in Science for the developing world (OWSD) PhD Fellowship, Margaret McNamara Education Grants and L'Oréal UNESCO For Women in Science Fellowship. MNl is supported by an Australian National Health and Medical Research Council Investigator Grant [APP1174455]. AMR is additionally funded by the UK Medical Research Council (MRC) and the UK Department for International Development (DFID) under the MRC/DFID Concordat agreement which is also part of the EDCTP2 programme supported by the European Union, Grant Ref: MR/R010161/1. RAF is funded by the Wellcome Trust. FSD is supported by the National Research Foundation of South Africa (112160), Future Leaders – African Independent Research (FLAIR) Fellowship, the National Institute for Health Research (NIHR) Global Health Research Unit on Mucosal Pathogens using UK aid from the UK Government, the University of Cape Town and the Allergy Society of South Africa (ALLSA). The funders had no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Author information
Authors and Affiliations
Contributions
REA conducted the antimicrobial susceptibility testing, data analysis and drafted the first draft of the manuscript. MPN designed the study and co-supervised the study. GM coordinated sample collection in Zimbabwe. VS and AMR guided the direction of the statistical analysis, reporting of results and interpretation of the data. CB conducted microbiological cultures, bacteria recovery and identification. SM is data manager in South Africa. BWM is the data manager and LGN is the study coordinator in Malawi.HM provides clinical support for participants. BM and JM were responsible for sample processing in Zimbabwe. JOO and RAF conceived and led the parent BREATHe study. JOO secured funding from GLOBVAC on behalf of the consortium. FSD supervised this study. All authors contributed to the manuscript, and all authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The parent study (BREATHE), was approved by the Human Research and Ethics Committee of the University of Cape Town - UCT HREC (HREC/REF: 754/2015), London School of Hygiene and Tropical Medicine Ethics Committee (reference 8818), the Harare Central Hospital Ethics Committee and Medical Research Council of Zimbabwe (reference MRCZ/A/1946), the College of Medicine Research Ethics Committee Malawi (reference P.04/15/1719) and by the Regional Committee for Medical and Health Research Ethics, Northern Norway (reference 2015/1650). The University of Oxford waived approval. Additional ethical approval was received for this sub-study from the UCT HREC (HREC/REF: 092/2019). No additional data was collected other than that approved in the parent study. Written informed consent and assent were given by guardians and participants, respectively. Participants who were 18 years old, and above at the time of enrolment consented independently. All data obtained and generated during the study were kept confidential. This research was conducted in accordance with the Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Supplementary Table. T1
Semi-quantitative bacterial load distribution of isolates. This is a table comparing the distribution of the semi-quantitative bacterial loads of S. pneumoniae, S. aureus and H. influenzae, isolated from the respiratory samples of CLWH with or without chronic lung disease.
Additional file 2: Supplementary Table. T2
. Gram-negative bacilli other than Haemophilus influenzae isolated from respiratory samples. This is a table showing the identities of Gram negative bacilli other than H. influenzae, isolated from the respiratory samples of CLWH identified using Matrix-Assisted Laser Desorption/Ionization-Time-of-Flight mass spectrometry.
Additional file 3: Supplementary Table. T3
. Co-carriage of bacteria in nasopharyngeal swabs and sputa. This is a table showing the co-carriage relationship between S. pneumoniae, S. aureus and H. influenzae, isolated from respiratory samples of CLWH with or without chronic lung disease.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Abotsi, R.E., Nicol, M.P., McHugh, G. et al. Prevalence and antimicrobial resistance profiles of respiratory microbial flora in African children with HIV-associated chronic lung disease. BMC Infect Dis 21, 216 (2021). https://doi.org/10.1186/s12879-021-05904-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-021-05904-3