Abstract
Background
Acute Q fever usually presents as a nonspecific febrile illness, and its occurrence is rapidly increasing in South Korea. This study investigated the clinical characteristics of acute Q fever patients in South Korea and the time from symptom onset to serologic diagnosis. The clinical courses were examined according to antibiotic treatment.
Methods
Data of patients diagnosed with acute Q fever at Chungbuk National University Hospital between January 2015 and February 2018 were retrospectively collected. Demographic and epidemiologic data were reviewed. The time from symptom onset to serologic diagnosis by an immunofluorescence assay (IFA) was analyzed. Clinical courses and the percentage of patients with a high phase I immunoglobulin G titer (≥ 1:1024) were compared between patients administered antibiotics with anti-Coxiella burnetii activity and patients not administered such antibiotics.
Results
Forty-eight patients (median age: 51.5 years) were included. Most were male (95.8%) and had no history of animal contact (91.7%). The median time from illness onset to serologic diagnosis was 21 days. Thirty-nine patients received antibiotics with anti-C. burnetii activity. The length of hospital stay and fever duration did not significantly differ between patients who received antibiotics with anti-C. burnetii activity (7 and 15 days) and those who did not (5 and 8 days) (P = 0.110 and P = 0.137, respectively). The percentage of patients with a high phase I immunoglobulin G titer (≥ 1:1024) did not significantly differ between patients who received antibiotics with anti-C. burnetii activity and those who did not (P = 0.340).
Conclusions
Most acute Q fever patients had a nonspecific febrile illness with mild elevation of transaminases and no history of animal contact or occupational risk. The time from symptom onset to a positive IFA test was longer than the fever duration in most acute Q fever patients. Consequently, it may be difficult for clinicians to serologically diagnose acute Q fever. However, inappropriate antibiotic treatment was not associated with prolongation of symptoms or progression to chronic Q fever.
Similar content being viewed by others
Background
Human acute Q fever, a zoonosis caused by the obligate intracellular bacterium Coxiella burnetii, presents as various clinical manifestations such as self-limited febrile illness, pneumonia, endocarditis, vascular infections, hepatitis, osteomyelitis, and meningoencephalitis [1]. Although humans can be infected through direct contact (e.g., ingestion or skin inoculation of contaminated animal products), the primary mode of transmission is via inhalation of dust contaminated with C. burnetii [2].
In 2006, Q fever was designated a notifiable infectious disease in South Korea. Thereafter, around ten cases of Q fever were reported annually until 2015. However, the occurrence of Q fever has increased in recent years, with 81 cases in 2016 and 96 cases in 2017. This corresponds to a larger than 6-fold increase compared with the 12 cases reported in 2008 [3]. Although Q fever has been detected in all regions of South Korea, with the exception of Jeju island, its incidence is highest in the Chungcheong region, which is located in the center of the country. Approximately 45% of all cases were reported in this region [3]. Until now, it is not clear that which factors are associated with the high incidence of human Q fever in Chungcheong area of South Korea. It was suggested that increasing number of raised goats in this region may have a major effect on the high incidence of Q fever [4]. Previous serologic and bacteriologic studies suggest that C. burnetii is extensively distributed among host animals in South Korea [5, 6]. Seroprevalence of Q fever in Korean cattle is 9.5–11.6% and seroprevalence in goats are 15–19% [6,7,8,9]. The seroprevalence of C. burnetii is 1.5% in healthy people and 10.2% in slaughterhouse workers [10, 11].
Q fever is mainly diagnosed by a serologic test and therefore paired serum samples are required for confirmatory diagnosis. This disease is thought to be underrecognized and underdiagnosed, particularly in non-endemic and non-epidemic areas such as South Korea, due to its nonspecific symptoms and challenging diagnosis. It is important to understand the clinical courses and timing of seroconversion in acute Q fever patients in order to appropriately manage and diagnose patients with a nonspecific febrile illness. Chronic Q fever develops in < 5% of patients with acute disease and is associated with serious complications such as endocarditis and vasculitis. Therefore, it is important not to misdiagnose acute Q fever patients who present with a nonspecific febrile illness when antibodies against C. burnetii are not detected [12, 13].
This study investigated the clinical characteristics of acute Q fever patients in South Korea and the time from symptom onset to serologic diagnosis. Furthermore, we compared the clinical characteristics of patients administered antibiotics with anti-C. burnetii activity and those not administered such antibiotics.
Methods
Study design and definitions
The medical records of patients diagnosed with acute Q fever at Chungbuk National University Hospital, which is a tertiary teaching hospital located in the Chungcheong region, from January 2015 to February 2018 were retrospectively reviewed. This hospital diagnosed more acute Q fever cases than any other institution in South Korea during the study period. The following data were collected: demographic data, epidemiologic data (living area, occupation, and history of animal contact), time to defervescence (the interval between the onset of fever and the first day when the patient’s peak fever had been lower than 37.3 °C for at least two consecutive days without antipyretics), length of hospital stay, clinical findings, antibiotic treatment, and serologic and laboratory test results. Cases with pneumonia were defined as those with consolidation on a chest X-ray or chest computed tomography scan. Cases with elevated transaminases were defined as those whose aspartate aminotransferase (AST) or alanine aminotransferase (ALT) levels were more than 3-fold higher than the upper normal limits in laboratory tests. Cases with positive autoantibodies were defined as those with an anti-nuclear antibody (ANA) or anti-neutrophil cytoplasmic antibody (ANCA) titer ≥1:80.
Diagnosis of acute Q fever and analysis of clinical courses
Specimens of patients with suspected Q fever were sent to the Korea Centers for Disease Control and Prevention, where they were subjected to serologic testing for Q fever via an indirect immunofluorescence antibody (IFA) assay using a commercial kit (Focus Diagnostics, Cypress, CA, USA). Some specimens underwent PCR analysis as described in a previous study [14]. Acute Q fever was diagnosed based on the IFA or PCR results in patients with acute febrile illness. Cases with confirmed acute Q fever were defined as those with seroconversion to the phase II antigen, those in whom the phase II immunoglobulin G (IgG) titer differed by more than 4-fold between paired serum samples, or those with positive PCR or culture results with appropriate clinical findings. Cases with probable acute Q fever were defined as those with a phase II immunoglobulin M (IgM) titer of ≥1:16 or an IgG titer ≥1:256 in a single sample [2, 15, 16]. To investigate the serum antibody response to C. burnetii in detail, the time to serologic diagnosis was defined as the number of days from the onset of symptoms to the first positive result in the IFA test according to the aforementioned criteria.
Patients were categorized into two groups according to their treatment. Group 1 comprised patients who received antibiotics with activity against C. burnetii (tetracyclines, macrolides, quinolones, and rifampin) more than 3 days. Group 2 comprised patients who did not receive antibiotics with activity against C. burnetii more than 3 days. Time to defervescence, length of hospital stay, and the percentage of patients with a peak IgG titer ≥1:1024 were compared between the two groups.
Statistical analysis
Demographic and clinical data were statistically analyzed using the Statistical Package for the Social Sciences (SPSS) for Windows, version 24 (IBM Corp., Armonk, NY, USA). Categorical variables were analyzed using Pearson’s χ2 and Fisher’s exact tests. The Mann-Whitney U test was used to compare continuous variables between the two groups. P < 0.05 was considered statistically significant.
Ethics
This study was approved by the Institutional Review Board of Chungbuk National University Hospital (IRB No. 2012–03-024). The requirement for informed consent was waived because this was a retrospective study and there was no possibility of harming the enrolled subjects. All analyzed data were anonymized.
Results
From January 2015 to February 2018, 203 febrile patients (120 males, 59%) were tested for Q fever via an IFA at Chungbuk National University Hospital. Among them, 51 patients (25.1%) were diagnosed with Q fever. Of these, 48 patients with acute Q fever (38 confirmed cases and 10 probable cases) were included in this study. PCR analysis of C. burnetii was performed in three cases, all of whom tested positive.
Demographic and epidemiologic characteristics of acute Q fever patients
The median age of patients was 51.5 years [interquartile range (IQR): 46.3–58.8 years], and 46 (95.8%) patients were male (Table 1). In total, 27 (56.3%) patients had no underlying diseases, while 13 (27.0%) had hypertension and 11 (22.9%) had diabetes. Figure 1 shows the number of patients serologically diagnosed with acute Q fever in each month. Twenty-nine (60.4%) patients were diagnosed between June and September.
Number of patients who were diagnosed with acute Q fever in each month. Twenty-nine (60.4%) patients were diagnosed between June and September. In summer, animals are grazed in larger areas. People are more frequently exposed to the contaminated environments in farming season. It could be a reason of the slight increase of Q fever patients during summer
In relation to epidemiologic factors associated with Q fever, four (8.3%) patients had direct contact with animals due to their occupation (livestock raisers and veterinarians). These patients reported contact with goats (two cases), cattle (one case), and deer (one case). In total, 24 (50.0%) patients lived in rural areas and 6 (12.5%) patients were farmers; however, none of these patients reported any direct animal contact, except with companion dogs, or living near a barn. Thus, the majority of acute Q fever patients were previously healthy adults who lived in rural areas and lacked any known risk factors or underlying comorbidities.
The median time from illness onset to seeking of medical attention was 6.5 days (IQR: 4.0–14.0 days). The hospitalization rate was 89.6% and the median hospital stay was 6.5 days (IQR: 3.0–10.0 days). Most patients had a nonspecific acute febrile illness without localizing symptoms. Eleven (22.9%) patients had elevated transaminases and five (10.4%) patients had pneumonia. One patient had pericarditis. Table 2 summarizes the initial laboratory test results. Most patients exhibited mildly elevated C-reactive protein and transaminase levels. In total, 16 (66.4%) of 24 patients tested positive in autoantibody (ANA or ANCA) tests (Table 2).
Serologic diagnosis of acute Q fever
The median time from illness onset to serologic diagnosis was 21 days (IQR: 15–40 days). Among the 48 patients, 40 (83.3%) underwent more than one IFA test and eight were diagnosed based on the results of a single IFA test (probable cases). Seventeen (35.4%) patients tested negative in the first IFA test. However, 15 (88.2%) of these patients tested positive in the second follow-up IFA test, while two patients (11.8%) tested negative. Of these two patients, one tested positive in the third follow-up IFA test, while the other tested negative. The latter patient tested negative in the fourth IFA test. The median time from illness onset to the first, second, third, and fourth IFA tests was 14, 45, 144, and 248 days, respectively.
A total of 115 IFA tests were performed in the 48 patients as diagnostic work-up or follow-up of antibody titers. To analyze the serologic test results in more detail, we categorized the results according to the week in which testing was performed after illness onset. Twenty-six IFA tests were performed within 2 weeks of illness onset, 12 (46.2%) of which yielded positive results. Therefore, only 12 of the 48 patients were diagnosed with acute Q fever within 2 weeks of illness onset. Twenty-nine IFA tests were performed during the third and fourth weeks after illness onset, 25 (86.2%) of which yielded positive results. Twelve IFA tests were performed during the fifth, sixth, and seventh weeks after illness onset, all (100%) of which yielded positive results. A patient who was diagnosed with acute Q fever based on detection of C. burnetii by PCR analysis tested negative in serial IFA tests up to 124 days after illness onset. With the exception of one case, all IFA tests performed during the eighth week after illness onset yielded positive results.
Clinical courses of acute Q fever patients according to antibiotic treatment
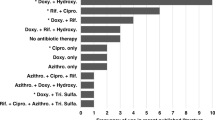
Among the 48 patients, 39 received antibiotics with activity against C. burnetii more than 3 days (group 1) and the other nine did not (group 2). We compared the clinical courses of patients between these two groups. The median time to defervescence did not significantly differ (P = 0.137) between group 1 (15 days, IQR: 7–24 days) and group 2 (8 days, IQR: 6.5–10.5 days). The median hospital stay did not significantly differ (P = 0.110) between group 1 (7 days, IQR: 3–10.5 days) and group 2 (5 days, IQR: 3.5–6 days). The percentage of patients with a phase I IgG titer ≥1:1024 in serial IFA tests did not significantly differ between the two groups (6/39, 15.4% vs 3/9, 33.3%, P = 0.340).
Discussion
We analyzed the clinical and epidemiological characteristics of patients diagnosed with acute Q fever between January 2015 and February 2018 in South Korea. The occurrence of Q fever increased rapidly during the study period [3]. The median age of patients was 51.5 years, and the majority of patients were previously healthy men who lived in rural areas and had no history of animal contact or occupational risk. Their clinical manifestations were nonspecific febrile illness. Due to these non-distinguishing clinical features and the lack of known risk factors such as animal contact, Q fever was underdiagnosed and underrecognized. The infection sources in this area are unclear. A further epidemiologic study including animals and environments might help to determine the origin of the infection.
In this study, 95.8% of the Q fever patients were male. In a survey on the 65 Korean Q fever patients reported in the national notifiable diseases surveillance system from 2006 to 2011, 57 patients were male (87.7%) [17]. Male predominance was also found in other study on Q fever of Australia [18] and in seroprevalence studies of the Netherlands and South Korea [19,20,21]. This gender imbalance is largely attributed to differential exposures to infected animals and contaminated environments thorough occupation. In addition to the different exposure risk between male and female, female sex hormone has some protective effect in Q fever [22,23,24]. This can potentiate gender disproportion of Q fever.
The time from illness onset to serologic diagnosis based on an IFA test (median: 21 days) was longer than the fever duration (median: 10 days). This is in contrast with patients with other rickettsial diseases, which usually show seroconversion in diagnostic tests within 7–10 days of symptom onset [25, 26]. The median time from illness onset to seeking of medical care was 6.5 days. Therefore, clinicians may fail to suspect and diagnose Q fever at an early stage in acute febrile patients. In this study, 35.4% of initial IFA tests yielded negative results, and these patients were diagnosed by follow-up tests at a late stage when they usually lacked clinical symptoms. To diagnose Q fever in non-endemic areas where this disease is underrecognized, such as South Korea, clinicians should suspect Q fever in patients with a nonspecific febrile illness who live in rural areas and should be aware of the delayed seroresponse.
After primary infection of C. burnetii, around 60% of patients are asymptomatic and the remainder display a fever and varying degrees of pneumonia or hepatitis [27, 28]. The major clinical manifestations of acute Q fever, such as hepatitis and pneumonia, differ between countries. Hepatitis is more frequently observed than pneumonia in France, southern Spain, and Taiwan [29,30,31], while pneumonia is the most prevalent manifestation in Nova Scotia in Canada, northern Spain, and the Netherlands [32,33,34]. This geographical variation might be due to differences in the route of infection, host factors, the infectious dose, and the strain of C. burnetii [27, 35,36,37]. In this study, 10.4% of patients had pneumonia and 22.9% of patients had elevated transaminases (more than 3-fold higher than the upper normal limits). Moreover, 85.4% of patients had an ALT concentration ≥ 40 U/L. Elevation of transaminases seems to be a more common clinical manifestation of acute Q fever than pneumonia in South Korea. However, a further study including more pneumonia patients is required to investigate the prevalence of C. burnetii in such patients because most patients included in the current study had a nonspecific febrile illness. Although hepatitis was the most prevalent feature of acute Q fever in this study, the AST and ALT concentrations were only modestly elevated (2–3-fold higher than the upper normal limits) in these patients. Autoantibody tests were performed as work-up in patients with a fever of unknown origin, 50% of whom tested positive. Immune reactions elicited by C. burnetii can produce various autoantibodies against cardiolipin, nuclear antigens, and smooth muscle antigens [38, 39]. In infective endocarditis, ANCA is associated with a longer duration of symptoms prior to diagnosis, and may result in multiple valve involvement and more frequent renal impairment [40]. Although it is unclear whether C. burnetii infection induces an autoimmune mechanism, circulating immune complexes might play a key role in the pathogenesis or severity of acute Q fever and lead to prolongation of fever, as observed in infective endocarditis.
Due to the considerable amount of time between illness onset and serologic diagnosis, diagnosis of Q fever and initiation of effective antibiotic treatment are often delayed. However, in this study, the time to defervescence and the hospital stay did not differ between patients who received antibiotics with anti-C. burnetii activity more than 3 days and those who did not. Other studies reported that doxycycline treatment significantly shortens the duration of fever in acute Q fever patients [41, 42]. It is likely that some acute Q fever patients have a self-remitting clinical course, while others have a protracted febrile illness that requires antibiotic treatment. Treatment of acute Q fever is not routinely recommended in asymptomatic cases or after resolution of symptoms [12]. A previous study reported that a phase I IgG antibody titer ≥1:800 at 3 and 6 months after illness onset is associated with chronic Q fever [43]. On the other hand, Wielders et al. demonstrated that early diagnosis and treatment of acute Q fever does not prohibit phase I IgG responses [44]. We analyzed whether inappropriate treatment of acute Q fever influences progression to chronic disease by assessing phase I IgG titers. The percentage of patients with a phase I IgG titer ≥1:1024 did not significantly differ between patients administered antibiotics with anti-C. burnetii activity and those not administered such antibiotics. Our results suggest that early initiation of appropriate antibiotic treatment does not affect the severity and duration of acute Q fever or progression to chronic Q fever.
Given the time delay and difficulties associated with serologic diagnosis and isolation of C. burnetii, PCR is an alternative option to diagnose acute Q fever within 2 weeks of illness onset [14]. In particular, real-time PCR analysis of IS1111 is a useful diagnostic tool in acute Q fever patients that are seronegative and only display phase II IgM [45]. In the current study, PCR analysis was performed in three patients, all of whom tested positive. One patient with positive PCR in this study showed negative IFA tests on day 22, 56, 75 and 128 from illness onset. The PCR result of this case could be a false positive or we could not detect the serologic change of the patient due to the relatively long IFA test intervals. A further study is required to compare the diagnostic accuracies of the IFA and PCR analysis in acute Q fever patients in South Korea.
Conclusions
The majority of patients diagnosed with acute Q fever were previously healthy males who lived in rural areas and presented with non-localizing febrile illness and mild elevation of transaminases. Serologic diagnosis of acute Q fever was usually achieved 3–4 weeks after illness onset. Late diagnosis and inappropriate antibiotic treatment were not associated with prolongation of acute Q fever or the development of chronic Q fever. These results provide baseline epidemiologic, clinical, and serologic data of acute Q fever patients in South Korea, a non-endemic area where this disease is underrecognized.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ALT:
-
Alanine aminotransferase
- ANA:
-
Anti-nuclear antibody
- ANCA:
-
Anti-neutrophil cytoplasmic antibody
- AST:
-
Aspartate aminotransferase
- C. burnetii :
-
Coxiella burnetii
- IFA assay:
-
Indirect immunofluorescence antibody assay
- IgG:
-
Immunoglobulin G
- IgM:
-
Immunoglobulin M
- IQR:
-
Interquartile range
- PCR:
-
Polymerase chain reaction
References
Bennett JE, Dolin R, Blaser MJ. Mandell, Douglas, and Bennett's principles and practice of infectious diseases: 2-volume set, vol. 1. Philadelphia: Elsevier Health Sciences; 2014. p. 220–16
Maurin M, Raoult D. Q fever. Clin Microbiol Rev. 1999;12(4):518–53.
prevention KCfdca: Infectious disease statistics system. 2018 Available at: https://www.iscdcgokr/dstat/indexjsp Accessed 1 Sept 2018.
Korean Ministry of Agriculture, Food and Rural Affairs: Number of raised goats in each region of South Korea. Annual statistics for the number of farmed and domestic animals in South Korea 2012, 2013, 2014, 2015, 2016, 2017.
Jung BY, Seo M-G, Lee S-H, Byun J-W, Oem J-K, Kwak D. Molecular and serologic detection of Coxiella burnetii in native Korean goats (Capra hircus coreanae). Vet Microbiol. 2014;173(1–2):152–5.
Lyoo K-S, Kim D, Jang HG, Lee S-J, Park MY, Hahn T-W. Prevalence of antibodies against Coxiella burnetii in Korean native cattle, dairy cattle, and dogs in South Korea. Vector-Borne Zoonotic Dis. 2017;17(3):213–6.
Gang S-J, Jeong J-M, Kim H-K, Lee J-W, Shon K-R, Park T-W. Prevalence of Coxiella burnetii in native Korean goat in Jeonbuk province. Kor J Vet Serv. 2016;39(4):239–46.
Ouh I-O, Seo M-G, Jang Y-S, Kim S-Y, Kwak D-M. Seroprevalence of Coxiella burnetii in cattle with reproductive disorders in eastern Gyeongbuk province, Korea. Kor J Vet Serv. 2013;36(4):249–54.
Kim N-H, Kim H-R, Park H-S, Kim Y-s, Lee J-H. Seroprevalence of Coxiella burnetii and Toxoplasam gondii in cattle in Seoul, Korea. Kor J Vet Serv. 2015;38(4):233–9.
Kim WJ, Hahn T-W, Kim D-Y, Lee M-G, Jung K-S, Ogawa M, et al. Seroprevalence of Coxiella burnetii infection in dairy cattle and non-symptomatic people for routine health screening in Korea. J Korean Med Sci. 2006;21(5):823–6.
Chu H, Yoo S-J, Hwang K-J, Lim H-S, Lee K, Park M-Y. Seroreactivity to Q fever among slaughterhouse workers in South Korea. J Prev Med Public Health. 2017;50(3):195.
Anderson A, Bijlmer H, Fournier P-E, Graves S, Hartzell J, Kersh GJ, et al. Diagnosis and management of Q fever—United States, 2013: recommendations from CDC and the Q fever working group. Morb Mortal Wkly Rep Recomm Rep. 2013;62(3):1–29.
van der Hoek W, Versteeg B, Meekelenkamp JC, Renders NH, Leenders AC, Weers-Pothoff I, et al. Follow-up of 686 patients with acute Q fever and detection of chronic infection. Clin Infect Dis. 2011;52(12):1431–6.
Fournier P-E, Raoult D. Comparison of PCR and serology assays for early diagnosis of acute Q fever. J Clin Microbiol. 2003;41(11):5094–8.
prevention KCfdca: Integrated guidelines for the diagnostic tests of legal communicable disease. Available at: http://www.cdcgokr/CDC/together/CdcKrTogether0302jsp?menuIds=HOME006-MNU2804-MNU3027-MNU2979&fid=10713&q_type=title&q_value=%EA%B0%90EC%97%BCEBB3%91&cid=138031&pageNum=1 Accessed 1 Sept 2018(Korean).
Dupont HT, Thirion X, Raoult D. Q fever serology: cutoff determination for microimmunofluorescence. Clin Diagn Lab Immunol. 1994;1(2):189–96.
Kwak W, Chu H, Hwang S, Park J-H, Hwang KJ, Gwack J, et al. Epidemiological characteristics of serologically confirmed q fever cases in South Korea, 2006–2011. Osong Publ Health ResPerspect. 2013;4(1):34–8.
Sloan-Gardner T, Massey P, Hutchinson P, Knope K, Fearnley E. Trends and risk factors for human Q fever in Australia, 1991–2014. Epidemiol Infect. 2017;145(4):787–95.
Richardus J, Donkers A, Dumas A, Schaap G, Akkermans J, Huisman J, et al. Q fever in the Netherlands: a sero-epidemiological survey among human population groups from 1968 to 1983. Epidemiol Infect. 1987;98(2):211–9.
Schimmer B, Schotten N, Van Engelen E, Hautvast J, Schneeberger P, Van Duijnhoven Y. Coxiella burnetii seroprevalence and risk for humans on dairy cattle farms, the Netherlands, 2010–2011. Emerg Infect Dis. 2014;20(3):417.
Park J-H, Chu H, Yoo S-J, Hwang K-J, Lim H-S. Serologic survey and risk factors for Coxiella burnetii infection among dairy cattle farmers in Korea. J Korean Med Sci. 2018;33(39):e245.
Leone M, Honstettre A, Lepidi H, Capo C, Bayard F, Raoult D, et al. Effect of sex on Coxiella burnetii infection: protective role of 17β-estradiol. J Infect Dis. 2004;189(2):339–45.
Tissot-Dupont H, Vaillant V, Rey S, Raoult D. Role of sex, age, previous valve lesion, and pregnancy in the clinical expression and outcome of Q fever after a large outbreak. Clin Infect Dis. 2007;44(2):232–7.
Leone M, Textoris J, Capo C, Mege J-L. Sex hormones and bacterial infections. In: Sex hormones. Croatia: Intech; 2012. p. 237–54.
Premaratna R, Weerasinghe S, Ranaweera A, Chandrasena TN, Bandara NW, Dasch GA, et al. Clinically helpful rickettsial disease diagnostic IgG titers in relation to duration of illness in an endemic setting in Sri Lanka. BMC Res Notes. 2012;5(1):662.
Jeong HW, Choi YK, Baek YH, Seong MH. Phylogenetic analysis of the 56-kDa type-specific protein genes of Orientia tsutsugamushi in Central Korea. J Korean Med Sci. 2012;27(11):1315–9.
Raoult D, Marrie T, Mege J. Natural history and pathophysiology of Q fever. Lancet Infect Dis. 2005;5(4):219–26.
Dupuis G, Petite J, Péter O, Vouilloz M. An important outbreak of human Q fever in a Swiss Alpine valley. Int J Epidemiol. 1987;16(2):282–7.
Domingo P, Muñoz C, Franquet T, Gurguí M, Sancho F, Vazquez G. Acute Q fever in adult patients: report on 63 sporadic cases in an urban area. Clin Infect Dis. 1999;29(4):874–9.
Lai C-H, Chang L-L, Lin J-N, Chen W-F, Wei Y-F, Chiu C-T, et al. Clinical characteristics of Q fever and etiology of community-acquired pneumonia in a tropical region of southern Taiwan: a prospective observational study. PLoS One. 2014;9(7):e102808.
Raoult D, Tissot-Dupont H, Foucault C, Gouvernet J, Fournier PE, Bernit E, et al. Q fever 1985-1998. Clinical and epidemiologic features of 1,383 infections. Medicine. 2000;79(2):109–23.
Alende-Castro V, Macía-Rodríguez C, Novo-Veleiro I, García-Fernández X, Treviño-Castellano M, Rodríguez-Fernández S, et al. Q fever in Spain: description of a new series, and systematic review. PLoS Negl Trop Dis. 2018;12(3):e0006338.
Marrie TJ, Campbell N, McNeil SA, Webster D, Hatchette TF. Q fever update, maritime Canada. Emerg Infect Dis. 2008;14(1):67.
Wielders CC, Wuister AM, de Visser VL, de Jager-Leclercq MG, Groot CA, Dijkstra F, et al. Characteristics of hospitalized acute Q fever patients during a large epidemic, the Netherlands. PLoS One. 2014;9(3):e91764.
Eldin C, Melenotte C, Mediannikov O, Ghigo E, Million M, Edouard S, et al. From Q fever to Coxiella burnetii infection: a paradigm change. Clin Microbiol Rev. 2017;30(1):115–90.
Zhang G, To H, Russell KE, Hendrix LR, Yamaguchi T, Fukushi H, et al. Identification and characterization of an immunodominant 28-kilodalton Coxiella burnetii outer membrane protein specific to isolates associated with acute disease. Infect Immun. 2005;73(3):1561–7.
Marrie TJ, Stein A, Janigan D, Raoult D. Route of infection determines the clinical manifestations of acute Q fever. J Infect Dis. 1996;173(2):484–7.
Camacho M, Outschoorn I, Tellez A, Sequí J. Autoantibody profiles in the sera of patients with Q fever: characterization of antigens by immunofluorescence, immunoblot and sequence analysis. J Autoimmune Dis. 2005;2(1):10.
Vardi M, Petersil N, Keysary A, Rzotkiewicz S, Laor A, Bitterman H. Immunological arousal during acute Q fever infection. Eur J Clin Microbiol Infect Dis. 2011;30(12):1527–30.
Langlois V, Lesourd A, Girszyn N, Ménard J-F, Levesque H, Caron F, et al. Antineutrophil cytoplasmic antibodies associated with infective endocarditis. Medicine. 2016;95(3):e2564.
Fenollar F, Fournier P-E, Carrieri MP, Habib G, Messana T, Raoult D. Risks factors and prevention of Q fever endocarditis. Clin Infect Dis. 2001;33(3):312–6.
Sobradillo V, Zalacain R, Capelastegui A, Uresandi F, Corral J. Antibiotic treatment in pneumonia due to Q fever. Thorax. 1992;47(4):276–8.
Landais C, Fenollar F, Thuny F, Raoult D. From acute Q fever to endocarditis: serological follow-up strategy. Clin Infect Dis. 2007;44(10):1337–40.
Wielders C, Kampschreur L, Schneeberger P, Jager M, Hoepelman A, Leenders A, et al. Early diagnosis and treatment of patients with symptomatic acute Q fever do not prohibit IgG antibody responses to Coxiella burnetii. Clin Vaccine Immunol. 2012;19(10):1661–6.
Schneeberger PM, Hermans MH, van Hannen EJ, Schellekens JJ, Leenders AC, Wever PC. Real-time PCR with serum samples is indispensable for early diagnosis of acute Q fever. Clin Vaccine Immunol. 2010;17(2):286–90.
Acknowledgments
We thank the Division of Bacterial Diseases, Center for Laboratory Control of Infectious Diseases, Korea Centers for Disease Control and Prevention for assistance with IFA tests of Q fever patients.
Funding
None.
Author information
Authors and Affiliations
Contributions
JYH, YWC and HWJ conceived and designed this study. EJK, SKL and JYL reviewed and analyzed data. SHL and SDH performed IFA and analyzed the results. Drafting of the manuscript was done by JYH, YWC, EJK and JYL. Revision of the manuscript and acquisition of data was done by HWJ. Approval of the final version of the manuscript was received from the co-authors before submission to the journal. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Institutional Review Board of Chungbuk National University Hospital, Cheongju, Republic of Korea has approved this study (IRB No.2012–03-024). The requirement of informed consent was waived since it was a retrospective study and there was no possibility to cause harm to the enrolled subjects. Medical information center of Chungbuk National University Hospital granted permission to access the raw data of patients’ records. All data analyzed were anonymized.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Heo, J., Choi, Y., Kim, E. et al. Clinical characteristics of acute Q fever patients in South Korea and time from symptom onset to serologic diagnosis. BMC Infect Dis 19, 903 (2019). https://doi.org/10.1186/s12879-019-4479-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-019-4479-0