Abstract
Background
Non-structural 5A protein (NS5A) resistance-associated substitutions (RASs) have been identified in patients infected with hepatitis C virus (HCV), even prior to exposure to direct-acting antiviral agents (DAAs). Selection for these variants occurs rapidly during treatment and, in some cases, leads to antiviral treatment failure. DAAs are currently the standard of care for hepatitis C treatment in many parts of the world. Nevertheless, in Brazil, the prevalence of pre-existing NS5A RASs is largely unknown. In this study, we evaluated the frequency of naturally occurring NS5A RASs in Brazilian patients infected with HCV as either a monoinfection or coinfection with human immunodeficiency virus (HIV).
Methods
Direct Sanger sequencing of the NS5A region was performed in 257 DAA-naïve patients chronically infected with HCV (156 monoinfected with HCV and 101 coinfected with HIV/HCV).
Results
The frequencies of specific RASs in monoinfected patients were 14.6% for HCV GT-1a (M28 V and Q30H/R), 6.0% for GT-1b (L31F/V and Y93H), and 22.6% for GT-3a (A30K and Y93H). For HIV/HCV-coinfected patients, the frequencies of RAS were 3.9% for GT-1a (M28 T and Q30H/R), and 11.1% for GT-1b (Y93H); no RASs were found in GT-3a sequences.
Conclusions
Substitutions that may confer resistance to NS5A inhibitors exist at baseline in Brazilian DAA-naïve patients infected with HCV GT-1a, −1b, and -3a. Standardization of RAS definitions is needed to improve resistance analyses and to facilitate comparisons of substitutions reported across studies worldwide. Therapeutic strategies should be optimized to efficiently prevent DAA treatment failure due to selection for RASs, especially in difficult-to-cure patients.
Similar content being viewed by others
Background
Due to their shared routes of transmission, co-infection with hepatitis C virus (HCV) and human immunodeficiency virus (HIV) is common worldwide, it is estimated that 2.3 million HIV-infected people also produce antibodies against HCV [1]. This population has long been considered a special at-risk population in terms of disease progression with subsequent mortality and a lower response to HCV therapies using interferon and ribavirin [2].
Currently, HCV-infected patients are treated with direct-acting antiviral agents (DAAs) using effective and well-tolerated completely oral regimens. DAAs target HCV non-structural proteins essential for genome replication and virion assembly, i.e. NS3/4A, NS5B, and NS5A. In addition, NS5A has also been implicated in host immune evasion [3]. To improve patient response to therapeutics and avoid viral resistance, new regimens consist of combinations of inhibitors of non-structural proteins NS3/4A, NS5B, and/or NS5A [4, 5].
In the era of these DAA therapies, the treatment response rates have not differed between HIV/HCV-coinfected and HCV-monoinfected patients [6]. HIV coinfection is no longer considered a risk factor for poor response to HCV treatment. Nevertheless, the presence of resistance-associated substitutions (RASs) prior to treatment may contribute to treatment failure, regardless of the presence of HIV infection [7]. A sustained virological response is a multifactorial event reliant on the therapeutic regimen and viral and host factors. Predictors of treatment failure include infection with HCV GT-1a and 3a, the presence of cirrhosis, and prior treatment with interferon-containing regimens [8].
NS5A substitutions have been identified in DAA treatment-naïve patients and may reduce the antiviral activity of NS5A inhibitors currently used in the clinic, such as daclatasvir, elbasvir, ledipasvir, ombitasvir and velpatasvir [9,10,11,12]. In Brazil, the interferon-free regimen for HCV treatment consists of a combination of sofosbuvir and daclatasvir, or sofosbuvir and simeprevir, with or without ribavirin [13].
Considering that there is little data on the prevalence of naturally occurring NS5A RASs in Brazil, the aim of the present study was to determine the frequency of natural NS5A RASs patients infected with HCV or HCV/HIV.
Methods
Study population
In this transversal study, a total of 257 DAA-naïve patients with chronic HCV-infections were enrolled. Of these patients, 156 were monoinfected (GT-1a: n = 41, GT-1b: n = 84, and GT-3a: n = 31) and 101 were coinfected with HIV/HCV (GT-1a: n = 77, GT-1b: n = 9, and GT-3a: n = 15). Patients were followed up at the Clinics Hospital of the School of Medicine of University of São Paulo.
HCV viral load of monoinfected patients was 6.17 Log UI/mL (IQR 5.78–6.51) and 6.31 Log UI/mL (IQR 6.02–6.71) for coinfected patients. At the time of this study, none of the enrolled patients had been treated with DAAs. However, 50.6% (79/156) of HCV monoinfected and 41.6% (42/101) of coinfected patients had previously been treated with PEGylated interferon-alpha (PEG-IFNa) and ribavirin (RBV), and, thus, were treatment-experienced without virological response. In addition, the majority of HCV/HIV co-infected patients was on antiretroviral therapy and thus has a good immune profile (data not shown).
RNA extraction and NS5A amplification
Viral RNA was extracted from 200 μL of each plasma sample using QIAamp MinElute Virus Spin (Qiagen, Hilden, Germany) according to the manufacturer’s recommendations. cDNA synthesis and the first round of polymerase chain reaction (PCR) were performed using the SuperScript III One-Step RT-PCR system with Platinum Taq High Fidelity DNA Polymerase (Invitrogen™, Thermo Fisher Brand, Carlsbad, CA, USA). Next, a nested-PCR was performed using Platinum Taq DNA Polymerase (Invitrogen™, Thermo Fisher Brand, Carlsbad, CA, USA). NS5A genotype-specific primers and the amplification conditions for HCV GT-1a and GT-1b are described in Paolucci et al. [14] and, for HCV GT-3a, in previous work [15].
Sequencing by Sanger method
Nested PCR products were purified using 4 μl of ExoSAP-IT (Affymetrix, Santa Clara, CA, USA) according to the manufacturer’s instructions. A cycle-sequencing reaction was performed using the BigDye® Terminator v3.1 Kit (Applied Biosystems, Thermo Fisher Brand, Carlsbad, CA, USA) with the same primers used in the nested PCR. Direct sequencing by the Sanger method was performed using the automatic sequencer ABI 3500 genetic analyzer (Applied Biosystems, Thermo Fisher Brand, Carlsbad, CA, USA).
Sequence analysis
Electropherogram quality analyses were conducted and consensus sequences were obtained using the online software (http://asparagin.cenargen.embrapa.br/phph/). Nucleotide sequences were aligned against the reference genomes NC_004102 for GT-1a, AJ238799 for GT-1b, and D28917 for GT-3a using ClustalW integrated into Bioedit software (v. 7.0.8) [16]. In addition, all sequences were analyzed by geno2pheno[hcv] [17] to confirm amino acid substitutions and drug sensitivity. Substitutions at amino acid positions 28, 30, 31, and 93 in NS5A were considered RASs and have been previously reported as clinically relevant [18,19,20,21,22,23].
Statistical analysis
The Fisher exact test was applied to assess an association between identified RASs and each patient group (HCV mono and HCV / HIV co-infected patients) according to HCV subtype, p values were calculated and considered statistically significant if P < 0.05. Analyses were conducted using SPSS software, version 15.0 (SPSS Inc., Chicago. IL, USA).
Results
Sequencing of the HCV NS5A region
The NS5A region was successfully sequenced from all 257 samples tested in this study and RASs were found in both treatment-naïve patient groups: HCV monoinfected and HIV/HCV coinfected.
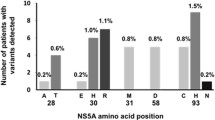
Using Sanger-based sequencing, RASs were observed in 10% (22/257) of the amplified NS5A region sequences. Specifically, RASs were detected in sequences from 7.6% (9/118) of GT-1a-, 6.5% (6/93) of GT-1b-, and 15.2% (7/46) of GT-3a-infected patients. Population sequencing generated the sequence of the most common virus present in a sample (sensitivity ~20%).
Table 1 summarizes all detected amino acid substitutions (RASs and non-RASs) observed at NS5A amino acid residues 28, 30, 31, and 93. HCV GT-1a-monoinfected patients more frequently had NS5A RASs than those who were coinfected with HIV/HCV GT-1a (14.6% and 3.9%, respectively; Table 2), highlighting the increased prevalence of Q30H in HCV GT-1a-monoinfected patients.
RASs in HCV-monoinfected patients
Among the HCV-monoinfected patients, RASs were observed in 11.5% (18/156). Among the HCV subtypes, 14.6% of RASs in GT-1a were M28 V and Q30H/R, 6.0% in GT-1b were L31F/V and Y93H, and 22.6% in GT-3a were A30K and Y93H. None of the NS5A sequences from HCV GT-1a harbored the Y93H variant (Table 1). Furthermore, in HCV subtype 1b sequences, P58S (2/84), E62H/E/P (3/84 were E62H, 2/84 were E62E, and 1/84 were E62P), and A92V/T (1/84 were A92V and 4/84 were A92T) polymorphisms were also detected.
Multiple amino acid substitutions were observed at a low frequency (<10%) in NS5A sequences. From monoinfected patients, GT-1a sequences had combinations of M28I + Q30H (1/41; 2.4%) and M28 L + Q30R (1/41; 2.4%), GT-1b had R30K + L31F (1/84; 1.2%), and GT-3a had S62 L + Y93H (1/31; 3.2%) and P58S + Y93H (1/31; 3.2%).
RASs in HCV/HIV-coinfected patients
The RAS frequency in HCV/HIV-coinfected patients was 4% (4/101). Grouping by HCV subtypes, RASs were detected in 3.9% of GT-1a sequences (M28 T and Q30H/R) and 11.1% of GT-1b sequences (Y93H) (Table 1). Other polymorphisms also observed in GT-1a sequences were H58S/R/P (H58S was found in 1/77, H58R in 7/77, and H58P in 13/77) and E62D (5/77). GT-1b sequences contained P58S (3/9) and E62D/V (E62D was found in 1/9 and E62V in 1/9). Meanwhile, GT-3a sequences contained S62 T/Q (S62 T was found in 6/15 and S62Q in1/15).
In HCV/HIV-coinfected patients, multiple amino acid substitutions were detected only in GT-1a sequences. The combinations observed were M28 T + Q30L + H58S (1/77; 1.3%), M28I + H58R (2/77; 2.6%), and Q30R + H58P (1/77; 1.3%).
Discussion
In this present study, the NS5A region was analyzed in HCV GT-1a, −1b, and -3a isolated from DAA treatment-naïve patients that were either monoinfected with HCV or coinfected with HCV/HIV from a major public hospital in São Paulo city.
Currently available HCV treatments are highly efficacious in and well-tolerated by most patients. Nevertheless, HCV resistance to DAAs has an important role in the failure of interferon-free treatment regimens and is a major challenge faced by future treatment strategies [18, 19]. Previous work has described the rapid selection of NS5A RASs, particularly at residues 28, 30, 31, and 93 [5].
The presence of NS5A RASs at baseline is associated with the virologic failure of DAAs. However, RASs have different effects based on the DAA regimen, viral genotype/subtype, and population characteristics (e.g. treatment-experience and cirrhosis status) [8].
NS5A RASs appear to have an impact on patient response to treatment, especially in those infected with HCV-1a and HCV-3a. Testing for baseline RASs is recommended for determining treatment duration in HCV GT-1a-infected patients who are being considered for therapy with elbasvir/grazoprevir. This testing is also recommended for patients infected with HCV GT-1a and GT-3a with cirrhosis (American Association for the Study of Liver Diseases guidelines) and in all patients before retreatment following a failed therapeutic regimen with DAAs [24,25,26].
In this present study, NS5A HCV sequences from HCV-monoinfected and HCV/HIV-coinfected patients revealed RASs were more frequent in GT-1a (7.6%; 9/118) than GT-1b sequences (6.5%; 6/93). This observation is contrary to previous studies [14, 27,28,29], and is likely a result of study differences in classification of RASs (e.g. inclusion of substitutions not previously shown to be relevant in clinical trials), as well as sample size. In addition, it is plausible that the different geographic origins of the samples may be involved in the resistance profiles described. The proportion of detectable pre-existing RASs reported in previous studies is 13% of cases in North America and 14% in Europe [30].
In this study, the amino acid substitutions M28 V and Q30H/R were observed within the GT-1a sequences from monoinfected patients. Previously published work has shown that M28 V confers low-level resistance to ombitasvir [19], while Q30H/R confers high-level resistance to daclatasvir, ledipasvir, and ombitasvir (fold change >100) [18, 23].
In sequences from GT-1b-monoinfected patients, the RASs L31F/V and Y93H were observed. Y93H confers different levels of resistance to approved NS5A inhibitors, including elbasvir and velpatasvir [30,31,32]. Finally, in GT-3a sequences from monoinfections, the Y93H and A30K substitutions were present at low to moderate frequencies. Hernandez et al. [33] observed that A30K and Y93H variants in NS5A reduce viral sensitivity to daclatasvir and ledipasvir, respectively. The clinical interpretation of these findings remains challenging since the impact of NS5A RAS to any DAA is multifactorial (e.g. drug regimen specific, cirrhosis or treatment-experienced patients without cirrhosis).
In regard to HCV/HIV-coinfected patients enrolled in this study, M28 T and Q30H/R variants were found in GT-1a isolates and may confer variable levels of resistance depending on the approved NS5A inhibitors used. Moreover, GT-1b isolates were found with the Y93H substitution, which is associated with resistance to daclatasvir, ombitasvir, and elbasvir (24–93-fold) [34], as well as ledipasvir (>1000-fold) [18, 19]. No RASs were identified in GT-3a isolates. Importantly, the presence of HIV appears to have had no influence on RAS selection.
Additionally, in this present work, the amino acid substitution P58S was observed in HCV GT-1b sequences from HCV-monoinfected and HCV/HIV-coinfected patients. While this substitution alone has been found to confer no to minimal resistance to NS5A inhibitors, it can increase viral resistance to NS5A inhibitors when in combination with certain NS5A RASs (e.g. L31 + Y93H) [19, 24, 35]. Moreover, multiple combinations of variants, consisting of two or three substitutions, were observed, but the clinical importance of these is currently unknown.
Special attention should be given to NS5A RASs that only minimally impair viral fitness and persist for long periods after treatment failure [36,37,38]. Another important point to emphasize is that NS5A inhibitors are currently used in the majority of treatment regimens for hepatitis C in different parts of the world, including Brazil.
Therefore, information on the frequency of NS5A RAS is very important as it can guide therapeutic strategies and diagnostic decisions by assessing resistance in candidates when treating hepatitis C in Brazil. Standardization of RAS definitions is needed to improve resistance analyses and to facilitate comparisons of substitutions between studies worldwide. To our knowledge, this is the first study on the presence of NS5A RASs in GT-3a strains from Brazil.
Conclusion
In conclusion, this study found NS5A RASs were a natural occurrence in both HCV-monoinfected and HIV/HCV-coinfected patients that were DAA naive. A limitation of this study was the small sample size and the lack of follow-up on the subsequent clinical courses with DAAs. It is important to perform this analysis on patients from other regions to more thoroughly characterize the pattern of resistance across both the country of Brazil and continent of South America.
Abbreviations
- DAA:
-
Direct-acting antiviral
- GT:
-
Genotype
- HCV:
-
Hepatitis C virus
- HIV:
-
Human immunodeficiency virus
- RAS:
-
Resistance-associated substitution
- SVR:
-
Sustained viral response
References
Platt L, Easterbrook P, Gower E, McDonald B, Sabin K, McGowan C, Yanny I, Razavi H, Vickerman P. Prevalence and burden of HCV co-infection in people living with HIV: a global systematic review and meta-analysis. Lancet Infect Dis. 2016;16(7):797–08.
Karageorgopoulos DE, Allen J, Bhagani S. Hepatitis C in human immunodeficiency virus co-infected individuals: is this still a “special population”? World J Hepatol. 2015;7(15):1936–52.
Ross-Thriepland D, Harris M. Hepatitis C virus NS5A: enigmatic but still promiscuous 10 years on! J Gen Virol. 2015;96(Pt 4):727–38.
Asselah T, Boyer N, Saadoun D, Martinot-Peignoux M, Marcellin P. Direct-acting antivirals for the treatment of hepatitis C virus infection: optimizing current IFN-free treatment and future perspectives. Liver Int. 2016;36(Suppl 1):47–57.
Sarrazin C. The importance of resistance to direct antiviral drugs in HCV infection in clinical practice. J Hepatol. 2016;64(2):486–04.
Wyles DL, Ruane PJ, Sulkowski MS, Dieterich D, Luetkemeyer A, Morgan TR, Sherman KE, Dretler R, Fishbein D, Gathe JC Jr, et al. Daclatasvir plus Sofosbuvir for HCV in patients Coinfected with HIV-1. N Engl J Med. 2015;373(8):714–25.
Boesecke C, Ingiliz P, Reiberger T, Stellbrink HJ, Bhagani S, Page E, Mauss S, Lutz T, Voigt E, Guiguet M, et al. Dual treatment of acute HCV infection in HIV co-infection: influence of HCV genotype upon treatment outcome. Infection. 2015;44(1):93–101.
Feld JJ. Resistance testing: interpretation and incorporation into HCV treatment algorithms. Clini Liver Dis. 2017;9(5):115–20.
Feld JJ, Jacobson IM, Hezode C, Asselah T, Ruane PJ, Gruener N, Abergel A, Mangia A, Lai CL, Chan HL, et al. Sofosbuvir and Velpatasvir for HCV genotype 1, 2, 4, 5, and 6 infection. N Engl J Med. 2015;373(27):2599–07.
Foster GR, Afdhal N, Roberts SK, Brau N, Gane EJ, Pianko S, Lawitz E, Thompson A, Shiffman ML, Cooper C, et al. Sofosbuvir and Velpatasvir for HCV genotype 2 and 3 infection. N Engl J Med. 2015;373(27):2608–17.
Rockstroh JK, Nelson M, Katlama C, Lalezari J, Mallolas J, Bloch M, Matthews GV, Saag MS, Zamor PJ, Orkin C, et al. Efficacy and safety of grazoprevir (MK-5172) and elbasvir (MK-8742) in patients with hepatitis C virus and HIV co-infection (C-EDGE CO-INFECTION): a non-randomised, open-label trial. Lancet HIV. 2015;2(8):e319–27.
Zeuzem S, Ghalib R, Reddy KR, Pockros PJ, Ben Ari Z, Zhao Y, Brown DD, Wan S, DiNubile MJ, Nguyen BY, et al. Grazoprevir-Elbasvir combination therapy for treatment-naive cirrhotic and noncirrhotic patients with chronic hepatitis C virus genotype 1, 4, or 6 infection: a randomized trial. Ann Intern Med. 2015;163(1):1–13.
Mesquita F, Santos ME, Benzaken A, Correa RG, Cattapan E, Sereno LS, Naveira MC. The Brazilian comprehensive response to hepatitis C: from strategic thinking to access to interferon-free therapy. BMC Public Health. 2016;16(1):1132.
Paolucci S, Fiorina L, Mariani B, Gulminetti R, Novati S, Barbarini G, Bruno R, Baldanti F. Naturally occurring resistance mutations to inhibitors of HCV NS5A region and NS5B polymerase in DAA treatment-naive patients. Virol J. 2013;10:355.
Malta FM, Medeiros-Filho JE, Azevedo RS, Goncalves L, Silva LC, Carrilho FJ, Pinho JR. Sequencing of E2 and NS5A regions of HCV genotype 3a in Brazilian patients with chronic hepatitis. Mem Inst Oswaldo Cruz. 2010;105(1):92–8.
Hall T. BioEdit: a user-friendly biological sequence alignment editor and analysis program for windows 95/98/NT. Nucl Acids Symp Ser. 1999;41:95–8.
Kalaghatgi P, Sikorski AM, Knops E, Rupp D, Sierra S, Heger E, Neumann-Fraune M, Beggel B, Walker A, Timm J, et al. Geno2pheno[HCV] - a web-based interpretation system to support hepatitis C treatment decisions in the era of direct-acting antiviral agents. PLoS One. 2016;11(5):e0155869.
Fridell RA, Wang C, Sun JH, O’Boyle DR 2nd, Nower P, Valera L, Qiu D, Roberts S, Huang X, Kienzle B, et al. Genotypic and phenotypic analysis of variants resistant to hepatitis C virus nonstructural protein 5A replication complex inhibitor BMS-790052 in humans: in vitro and in vivo correlations. Hepatology. 2011;54(6):1924–35.
Krishnan P, Beyer J, Mistry N, Koev G, Reisch T, DeGoey D, Kati W, Campbell A, Williams L, Xie W, et al. In vitro and in vivo antiviral activity and resistance profile of ombitasvir, an inhibitor of hepatitis C virus NS5A. Antimicrob Agents Chemother. 2015;59(2):979–87.
Lawitz EJ, Gruener D, Hill JM, Marbury T, Moorehead L, Mathias A, Cheng G, Link JO, Wong KA, Mo H, et al. A phase 1, randomized, placebo-controlled, 3-day, dose-ranging study of GS-5885, an NS5A inhibitor, in patients with genotype 1 hepatitis C. J Hepatol. 2012;57(1):24–31.
Nettles RE, Gao M, Bifano M, Chung E, Persson A, Marbury TC, Goldwater R, DeMicco MP, Rodriguez-Torres M, Vutikullird A, et al. Multiple ascending dose study of BMS-790052, a nonstructural protein 5A replication complex inhibitor, in patients infected with hepatitis C virus genotype 1. Hepatology. 2011;54(6):1956–65.
Bagaglio S, Andolina A, Merli M, Uberti-Foppa C, Morsica G. Frequency of natural resistance within NS5a replication complex domain in hepatitis C genotypes 1a, 1b: possible implication of subtype-specific resistance selection in multiple direct acting Antivirals drugs combination treatment. Viruses. 2016;8(4):91.
Lontok E, Harrington P, Howe A, Kieffer T, Lennerstrand J, Lenz O, McPhee F, Mo H, Parkin N, Pilot-Matias T, et al. Hepatitis C virus drug resistance-associated substitutions: state of the art summary. Hepatology. 2015;62(5):1623–32.
Pawlotsky JM, Hepatitis C. Virus resistance to direct-acting antiviral drugs in interferon-free regimens. Gastroenterology. 2016;151(1):70–86.
Lawitz E, Gane E, Pearlman B, Tam E, Ghesquiere W, Guyader D, Alric L, Bronowicki JP, Lester L, Sievert W, et al. Efficacy and safety of 12 weeks versus 18 weeks of treatment with grazoprevir (MK-5172) and elbasvir (MK-8742) with or without ribavirin for hepatitis C virus genotype 1 infection in previously untreated patients with cirrhosis and patients with previous null response with or without cirrhosis (C-WORTHY): a randomised, open-label phase 2 trial. Lancet. 2015;385(9973):1075–86.
Hepatitis C. Guidance: AASLD-IDSA recommendations for testing, managing, and treating adults infected with hepatitis C virus. Hepatology. 2015;62(3):932–54.
Dietz J, Susser S, Berkowski C, Perner D, Zeuzem S, Sarrazin C. Consideration of viral resistance for optimization of direct antiviral therapy of hepatitis C virus genotype 1-infected patients. PLoS One. 2015;10(8):e0134395.
Peres-da-Silva A, de Almeida AJ, Lampe E. NS5A inhibitor resistance-associated polymorphisms in Brazilian treatment-naive patients infected with genotype 1 hepatitis C virus. J Antimicrob Chemother. 2015;70(3):726–30.
Nguyen LT, Hall N, Sheerin D, Carr M, De Gascun CF. Naturally occurring HCV NS5A/B inhibitor resistance-associated mutations to direct-acting antivirals. Antivir Ther. 2016;21(5):447–53.
Sarrazin C, Dvory-Sobol H, Svarovskaia ES, Doehle BP, Pang PS, Chuang SM, Ma J, Ding X, Afdhal NH, Kowdley KV, et al. Prevalence of resistance-associated substitutions in HCV NS5A, NS5B, or NS3 and outcomes of treatment with Ledipasvir and Sofosbuvir. Gastroenterology. 2016;151(3):501–12.
Welzel TM, Bhardwaj N, Hedskog C, Chodavarapu K, Camus G, McNally J, Brainard D, Miller MD, Mo H, Svarovskaia E, et al. Global epidemiology of HCV subtypes and resistance-associated substitutions evaluated by sequencing-based subtype analyses. J Hepatol. 2017;67(2):224–36.
Krishnan P, Schnell G, Tripathi R, Beyer J, Reisch T, Zhang X, Setze C, Rodrigues L Jr, Burroughs M, Redman R, et al. Analysis of hepatitis C virus genotype 1b resistance variants in Japanese patients treated with Paritaprevir-Ritonavir and Ombitasvir. Antimicrob Agents Chemother. 2016;60(2):1106–13.
Hernandez D, Zhou N, Ueland J, Monikowski A, McPhee F. Natural prevalence of NS5A polymorphisms in subjects infected with hepatitis C virus genotype 3 and their effects on the antiviral activity of NS5A inhibitors. J Clin Virol. 2013;57(1):13–8.
Liu R, Curry S, McMonagle P, Yeh WW, Ludmerer SW, Jumes PA, Marshall WL, Kong S, Ingravallo P, Black S, et al. Susceptibilities of genotype 1a, 1b, and 3 hepatitis C virus variants to the NS5A inhibitor elbasvir. Antimicrob Agents Chemother. 2015;59(11):6922–9.
Nakamoto S, Kanda T, Wu S, Shirasawa H, Yokosuka O. Hepatitis C virus NS5A inhibitors and drug resistance mutations. World J Gastroenterol. 2014;20(11):2902–12.
Wang C, Sun JH, O'Boyle DR 2nd, Nower P, Valera L, Roberts S, Fridell RA, Gao M. Persistence of resistant variants in hepatitis C virus-infected patients treated with the NS5A replication complex inhibitor daclatasvir. Antimicrob Agents Chemother. 2013;57(5):2054–65.
Yoshimi S, Imamura M, Murakami E, Hiraga N, Tsuge M, Kawakami Y, Aikata H, Abe H, Hayes CN, Sasaki T, et al. Long term persistence of NS5A inhibitor-resistant hepatitis C virus in patients who failed daclatasvir and asunaprevir therapy. J Med Virol. 2015;87(11):1913–20.
Wyles D, Mangia A, Cheng W, Shafran S, Schwabe C, Ouyang W, Hedskog C, McNally J, Brainard DM, Doehle BP, et al. Long-term persistence of HCV NS5A resistance associated substitutions after treatment with the HCV NS5A inhibitor, ledipasvir, without sofosbuvir. Antivir Ther. 2017; doi: 10.3851/IMP3181.
Bartels DJ, Sullivan JC, Zhang EZ, Tigges AM, Dorrian JL, De Meyer S, Takemoto D, Dondero E, Kwong AD, Picchio G, et al. Hepatitis C virus variants with decreased sensitivity to direct-acting antivirals (DAAs) were rarely observed in DAA-naive patients prior to treatment. J Virol. 2013;87(3):1544–53.
Plaza Z, Soriano V, Vispo E, del Mar Gonzalez M, Barreiro P, Seclen E, Poveda E. Prevalence of natural polymorphisms at the HCV NS5A gene associated with resistance to daclatasvir, an NS5A inhibitor. Antivir Ther. 2012;17(5):921–6.
Aissa Larousse J, Trimoulet P, Recordon Pinson P, Tauzin B, Azzouz MM, Ben Mami N, Cheikh I, Triki H, Fleury H. Prevalence of hepatitis C virus (HCV) variants resistant to NS5A inhibitors in naive patients infected with HCV genotype 1 in Tunisia. Virol J. 2015;12:84.
Uchida Y, Kouyama JI, Naiki K, Sugawarav K, Inao M, Imai Y, Nakayama N, Mochida S. Development of rare RAVs that are extremely tolerant against NS5A inhibitors during daclatasvir/asunaprevir therapy via a two-hit mechanism. Hepatol Res. 2016;46(12):1234–46.
Acknowledgments
We thank each patient included in this study.
Funding
This study was supported by grant 443,152/2014–4 from CNPq, 2011/50633–4 from the São Paulo Research Foundation (FAPESP) and the Alves de Queiroz Family Fund for Research. João Renato Rebello Pinho and Maria Cassia Mendes Correa received a fellowship from CNPq (Bolsista de Produtividade).
Availability of data and materials
The data supporting the study conclusions are included within this article. The datasets used for this present study are available from the corresponding author upon request.
Author information
Authors and Affiliations
Contributions
FMM, MCMC, and JRRP designed the study. FMM performed the experiments. FMM, KVG, and GLN analyzed the data. FMM and MCMC wrote the paper. JRRP and FJC revised the manuscript for publication. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Ethics Committee of the School of Medicine of University of São Paulo (process number 0850/11). In accordance with the Declaration of Helsinki, plasma samples were collected from each patient after obtaining written informed consent.
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Malta, F., Gaspareto, K.V., Lisboa-Neto, G. et al. Prevalence of naturally occurring NS5A resistance-associated substitutions in patients infected with hepatitis C virus subtype 1a, 1b, and 3a, co-infected or not with HIV in Brazil. BMC Infect Dis 17, 716 (2017). https://doi.org/10.1186/s12879-017-2817-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-017-2817-7