Abstract
Background
There have been various reports concerning Helicobacter cinaedi infections. However, few reports have examined central nervous system infections.
Case presentation
A 52-year-old man was transferred from the local hospital because of a persistent headache and suspected intracranial subdural empyema. Neurosurgical drainage was performed via burr holes. Gram staining and results from abscess cultures were negative. The blood culture yielded H. cinaedi. He was given an antibiotic regimen consisting of 2 g of ceftriaxone twice a day, but the size of the abscess was not reduced in size at all after 3 weeks of treatment. Neurosurgical drainage was performed again, and the antimicrobial regimen was switched to 2 g of meropenem 3 times a day. The size of the abscess was reduced after 2 weeks of the second drainage and antimicrobial drug change to meropenem. After 4 weeks treatment with meropenem, the patient was discharged, and his symptoms had completely resolved.
Conclusions
H. cinaedi infection should be considered in the differential diagnosis of subdural empyema cases for which Gram staining and abscess culture results are negative. Meropenem can be a first-line drug of choice or an effective alternative treatment for H. cinaedi central nervous system infections.
Similar content being viewed by others
Background
The first report of a Helicobacter cinaedi infection involved a man with proctitis in 1984 [1]. Since then, various foci of H. cinaedi infection have been reported. However, few reports have examined central nervous system (CNS) infections, and the optimal therapy for CNS infection is unknown. Here, we report a case of intracranial subdural empyema and bacteremia due to H. cinaedi, in which the patient experienced treatment failure after a maximum dose of ceftriaxone. Finally, he was treated successfully with adequate drainage and meropenem.
Case presentation
A 52-year-old man with a history of epilepsy and drug eruption due to an amoxicillin/clavulanate was transferred from the local hospital because of a persistent headache and a suspicion of chronic subdural hematoma. There were no other symptoms before the persistent headache occurred, and he could perform his work normally. He had no history of head trauma and no meningeal irritation symptoms, such as neck stiffness. He kept an outdoor dog for years, but he had no contact with other animals, such as rats, hamsters, dogs, cats, birds, or monkeys, during the past year.
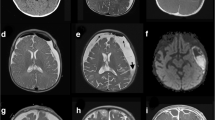
Computed tomography and nuclear magnetic resonance imaging of the head showed a right subdural mass with high and mixed density/intensity (Fig. 1a). Because of these findings, we suspected intracranial subdural empyema (SDE).
Nuclear magnetic resonance imaging of the head and brain. a Gadolinium-enhanced T1-weighted imaging (Gd T1WI) at the time of admission showed right subdural empyema (white arrowhead, 106 × 33 × 53 mm) with mixed low intensity. b Gd T1WI after 3 weeks of treatment. The size of the abscess was not reduced (white arrowhead, 109 × 35 × 60 mm). c Gadolinium-enhanced T1-weighted imaging 2 weeks after the second drainage and antimicrobial drug change to meropenem. The abscess was decreased in size. d T1-weighted imaging without contrast, 1 year after the treatment. There was no recurrence of the abscess
Neurosurgical drainage was performed via burr holes. Gram staining of the purulent material showed no bacteria, and he had no predisposition for sinusitis or periodontal disease. Therefore, we thought the possibility of anaerobic and aerobic gram-negative bacteria involvement was low. Two sets of blood cultures were drawn, and empirical antibiotic therapy was started with intravenous vancomycin targeting only aerobic streptococci and staphylococci. However, the results from abscess cultures were negative. After 7 days of incubation, the blood culture (BACTEC FX system, Nippon BD, Tokyo, Japan) yielded Gram-negative, long spiral-shaped bacillus. Microaerobic subculture on blood agar plates with hydrogen gas showed thin transparent colonies. Because of these findings, we suspected Helicobacter cinaedi intracranial subdural empyema. The human immunodeficiency virus antibody screening test administered just after surgery showed negative results. An additional two sets of blood culture specimens were drawn, and he was given an antibiotic regimen consisting of 2 g of ceftriaxone twice a day. The blood culture results were negative, but the size of the abscess was not reduced in size at all after 3 weeks of treatment (Fig. 1b).
Neurosurgical drainage was performed again, and the antimicrobial regimen was switched to 2 g of meropenem 3 times a day. Although the microaerobic culture of the abscess was negative, H. cinaedi was identified from the abscess and blood culture by cdtB virulence factor gene-based PCR. The 16S rRNA sequence analysis revealed with 100% similarity between the abscess and blood culture (Fig. 2). Antimicrobial susceptibility testing for H. cinaedi using the broth microdilution method revealed minimum inhibitory concentrations (MICs) of 4 μg/mL for ceftriaxone and 0.06 μg/mL for meropenem (Table 1). The size of the abscess was reduced after 2 weeks of the second drainage and antimicrobial drug change to meropenem (Fig. 1c). After 4 weeks treatment with meropenem, the patient was discharged, and his symptoms had completely resolved. The patient has continued visiting the hospital for more than 1 year after discharge, and there has been no recurrence to date (Fig. 1d).
Phylogenetic tree of helicobacter cinaedi. Neighbor-joining tree showing the position within the species of the genus Helicobacter, based on 16S rRNA gene sequence. The numbers at the branching points are bootstrap values. Campylobacter fetus subsp. fetus was used as the out group. The numbers in parentheses are the accession numbers of the gene sequences. Arrows indicate the position of strains from the abscess or blood source in this case
Conclusion
This case revealed two important clinical issues. First, H. cinaedi can cause an intracranial SDE. Secondly, treatment failure with ceftriaxone can occur when the MIC value for ceftriaxone is 4 μg/mL or higher in an H. cinaedi intracranial SDE case.
To our knowledge, this is the first published case of H. cinaedi intracranial SDE. There have been four reported cases of H. cinaedi central nervous system infections. Three cases concerned meningitis in adults [2–4], and the final case was a case of meningitis and bacteremia in a neonate [5]. None of those reported cases were associated with abscess formation. We must consider H. cinaedi as a causative organism of culture-negative intracranial SDE.
Treatment failure with ceftriaxone in cases of H. cinaedi intracranial SDE can occur if the MIC value for ceftriaxone is 4 μg/mL or higher. We initially chose ceftriaxone as definitive therapy because antibiotic regimens including ceftriaxone effectively treated H. cinaedi meningitis in the previous four case reports. Antimicrobial susceptibility testing (AST) for H. cinaedi isolates was not performed in these cases, probably because AST for H. cinaedi is too cumbersome to perform routinely in hospital laboratories [6].
However, in the present case, AST yielded useful information for changing the therapeutic strategy. With the doses normally used to treat bacterial meningitis, the concentrations of ceftriaxone in cerebrospinal fluid range from 2 to 8 μg/mL, and levels are nearly constant in children and adults [7, 8]. These concentrations are close to the MIC observed in this case (4 μg/mL), which may negate the culture results, but there is a possibility of treatment failure especially in the presence of an abscess. On the other hand, when 2 g of meropenem was administered every 8 h, the concentration in the cerebrospinal fluid was reported to be 1.29 μg/mL, even in the trough value [9], which is considerably higher than the MIC in this case (0.06 μg/mL). Therefore, meropenem can be a first-line drug of choice or an effective alternative treatment for CNS infection, especially when the MIC value is 0.06 μg/mL or lower.
In conclusion, H. cinaedi infection should be considered in the differential diagnosis of SDE cases for which Gram staining and abscess culture results are negative. AST for H. cinaedi isolates must be performed for cases of CNS infections. When the MIC value to ceftriaxone is 4 μg/mL or higher, treatment failure can occur. Meropenem can be a first-line drug of choice or an effective alternative treatment for H. cinaedi CNS infections.
Abbreviations
- AST:
-
Antimicrobial susceptibility testing
- CNS:
-
Central nervous system
- MIC:
-
Minimum inhibitory concentration
- SDE:
-
Subdural empyema
References
Pasternak J, Bolivar R, Hopfer RL, Fainstein V, Mills K, Rios A, Bodey GP, Fennell CL, Totten PA, Stamm WE. Bacteremia caused by campylobacter-like organisms in two male homosexuals. Ann Intern Med. 1984;101:339–41.
Sugiyama A, Mori M, Ishiwada N, Himuro K, Kuwabara S. First adult case of helicobacter cinaedi meningitis. J Neurol Sci. 2014;336:263–4.
Okubo H, Goto M, Sato M, Sugiyama T, Kawano M, Matsunaga T, Akaike T. Helicobacter cinaedi meningitis: a case report and review of previous cases. J Neurol Sci. 2014;347:396–7.
Uwamino Y, Muranaka K, Hase R, Otsuka Y, Hosokawa N. Clinical features of community-acquired helicobacter cinaedi bacteremia. Helicobacter. 2016;21:24–8.
Orlicek SL, Welch DF, Kuhls TL. Septicemia and meningitis caused by helicobacter cinaedi in a neonate. J Clin Microbiol. 1993;31:569–71.
Oyama K, Khan S, Okamoto T, Fujii S, Ono K, Matsunaga T, Yoshitake J, Sawa T, Tomida J, Kawamura Y, et al. Identification of and screening for human helicobacter cinaedi infections and carriers via nested PCR. J Clin Microbiol. 2012;50:3893–900.
Del Rio M, McCracken Jr GH, Nelson JD, Chrane D, Shelton S. Pharmacokinetics and cerebrospinal fluid bactericidal activity of ceftriaxone in the treatment of pediatric patients with bacterial meningitis. Antimicrob Agents Chemother. 1982;22:622–7.
Cabellos C, Viladrich PF, Verdaguer R, Pallares R, Linares J, Gudiol F. A single daily dose of ceftriaxone for bacterial meningitis in adults: experience with 84 patients and review of the literature. Clin Infect Dis. 1995;20:1164–8.
Blassmann U, Roehr AC, Frey OR, Vetter-Kerkhoff C, Thon N, Hope W, Briegel J, Huge V. Cerebrospinal fluid penetration of meropenem in neurocritical care patients with proven or suspected ventriculitis: a prospective observational study. Crit Care. 2016;20:343.
Acknowledgements
We would like to extend our sincere appreciation to Dr. Rei Yamaguchi for treating the patient together.
Funding
None.
Availability of data and materials
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
Authors’ contributions
MY, IY, and SK performed the isolation and identification of the organism. JT and YK performed 16S rRNA sequence analysis and antimicrobial susceptibility testing. TH treated the patient, and was a major contributor in writing the manuscript. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
We have had a signed consent for publication from the patient.
Ethics approval and consent to participate
Not applicable.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Hayashi, T., Tomida, J., Kawamura, Y. et al. Unusual manifestation of Helicobacter cinaedi infection: a case report of intracranial subdural empyema and bacteremia. BMC Infect Dis 17, 40 (2017). https://doi.org/10.1186/s12879-016-2129-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-016-2129-3