Abstract
Background
Genotypic molecular testing may be very helpful for tuberculosis (TB) drug-resistance surveillance and for treatment guidance in low resource settings.
Methods
Descriptive analysis of M. tuberculosis isolates from Beira Central Hospital, Mozambique, during 2014–2015. Genotype MTBDRplus and MTBDRsl were used and patient medical records reviewed.
To explore genotypic susceptibility profile of Mycobacterium tuberculosis, to first and second line drugs (SLD) in Beira Mozambique.
Results
Of 155 isolates, 16.1 % (25) were multidrug resistant (MDR), 8.4 % (13) isoniazid-monoresistant and 1.3 % (2) rifampicin-monoresistant. Among MDR-TB, 22.2 % showed primary and 77.8 % represented acquired resistance. The majority of patients with drug resistance had a history of previous TB treatment. Among 125 isolates tested for ethambutol and SLD, 7.2 % (9) were resistant to ethambutol, 4.8 % (6) to fluoroquinolones and 0.8 % (1) to ethambutol and fluoroquinolones. Resistance to injectable SLD was not detected.
Conclusions
As far as we know this is the first report of a genotypic testing used to provide information about SLD resistance in Mozambique, where phenotypic susceptibility testing is usually unavailable. Extensively drug resistant TB was not detected in this isolates from Beira Mozambique.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Drug resistant tuberculosis (DR-TB) is a major threat worldwide. It is estimated that 3.5 % of new cases and 20.5 % of previously treated cases have multidrug resistant TB (MDR-TB) and almost 9 % of MDR-TB patients are carrying extensively drug resistant TB (XDR-TB) worldwide [1–4].
Sub-Saharan Africa is a region with the highest TB burden, where the numbers of DR-TB and HIV coinfection are increasing continuously [1, 5]. In Mozambique, drug resistance is a great challenge for the National Program of TB Control (NPTBC). From 2010 to 2014, NPTBC reported 482 DR-TB cases but there is scarce information about the occurrence of XDR-TB in the country. Data from a national drug resistance survey indicated that 3.5 % of DR-TB in the country is primary resistance while 11.6 % is secondarily acquired [6, 7]. These numbers may be an underestimate because of the lack of laboratory facilities for phenotypic drug susceptibility test (DST) in the country, especially for second line TB drugs (SLDs).
The diagnosis of DR-TB requires susceptibility testing to anti-TB drugs, either by phenotypic DST or rapid molecular tests such as Line Probe Assay (LPA). Genotype MTBDRplus is an LPA recommended by WHO for DR-TB surveillance and diagnostic worldwide [8]. Genotype MTBDRplus evaluates resistance to both isoniazid (H) and rifampicin (R), which is the definition of MDR-TB. The test also classifies resistance to H according to the mutation detected: high level resistance (mutation in katG gene) and low level resistance (mutation in inhA gene regulatory region) [9]. In Mozambique this test is available only at the National Laboratory of TB in the capital city, Maputo.
Genotype MTBDRsl is the only commercial rapid molecular test available for detection of resistance to the SLDs. The WHO Expert Group recommended that the Genotype MTBDRsl assay cannot be used as a replacement test for conventional phenotypic DST, but it may be used as a rule-in test for XDR-TB while waiting for confirmatory results [10–13]. This test has low sensitivity especially for detection of resistance to injectable SLD. When used, Genotype MTBDRsl can reduce dramatically the delay to the initiation of adequate therapy, as it detects 2 in 3 or even 3 in 4 of XDR-TB cases [10].
Treatment of MDR-TB in Mozambique is standardized with 6 classes of anti-TB drugs (kanamycin/capreomycin, levofloxacin/ofloxacin, ethionamide/prothionamide, cycloserine, ethambutol and pyrazinamide) [14].
Timely information provided by LPAs regarding to the first- and second-line TB drugs would be helpful for individual decision-making and for review and improvement of the DR-TB management protocols in Mozambique. The aim of this study was to evaluate the role of the genotypic susceptibility testing to the first- and second-line drugs in surveillance and treatment of MDR- and XDR-TB.
Methods
Study population
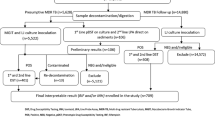
This is a descriptive study, where patients diagnosed with TB, confirmed with liquid medium (MGIT) culture at the Tuberculosis Reference Laboratory in Beira Central Hospital, Beira, Mozambique, from January 2014 to March 2015 were analyzed. Only one isolate of Mycobacterium tuberculosis per patient was tested. If a patient had more than one isolate, the first one was included in the study.
Data collection
Specimens were collect during routine diagnostic investigation of TB buy the clinical team that was taking care of the patient, according to the routine of the laboratory: direct exam after auramine O staining was done, and then the sample was incubated in liquid medium culture in an MGIT 960 automated system. Identification of M. tuberculosis complex was done using rapid test SD BIOLINE TB Ag MPT64 Rapid, an immunochromatographic test that identifies M. tuberculosis complex via monoclonal anti-MPT64 mouse antibody. MPT64 is a specific protein of the M. tuberculosis complex.
Patients information
Patient medical records were reviewed to obtain demographic data, HIV serology results, and a history of past or previous treatment of tuberculosis.
Genotypic susceptibility testing
All isolates were freshly subcultured on liquid medium incubated in MGIT 960, before being tested. The Genotype MTBDRplus and MTBDRsl (Hain Lifescience, GmbH, Germany) were performed according to the manufacturer’s instructions. These are Line Probe Assays (LPAs) that are based on DNA strip technology. Genotype MTBDRsl was performed by an experienced operator, who received standardized training on the method and it has been done, routinely, in the past 4 years in our Lab. The test itself has internal quality control and the final results were double checked by two blinded experienced operators.
Genotype® MTBDRplus
MTBDRplus is a rapid molecular test that detects M. tuberculosis complex and mutations in: rpoB gene, this encodes RNA polymerase β-subunit, mutation in this gene causes resistance to rifampicin; katG gene (encodes catalase peroxidase enzyme) and inhA gene (encodes enoyl-acyl carrier protein redutase) mutation in these two genes causes resistance to isoniazid. We screened for mutations D516V, H526Y, H526D, and S531L in the rpoB gene, mutations S315T1, S315T2 in the katG gene, and mutations C15T, A16G, T8C and T8A in the inhA gene regulatory region. Any sample that had these mutations or absence of wild-type was categorized as resistant to the evaluated drug [15].
Genotype® MTBDRsl
The MTBDRsl assay identifies M. tuberculosis complex and important mutations that confer resistance to the second line Injectable anti-TB drugs (SLIDs), fluoroquinolones (FQ) and etambutol (EMB). To detect resistance for the SLIDs (kanamycin, amikacin and or capreomycin), the rrs gene was screened. For FQs (levofloxacin and ofloxacin), the gyrA gene was screened and for EMB the embB gene was screened. The following mutations A90V, S91P, D94A, D94N/Y, D94G, and D94H in the gyrA gene are associated with resistance to FQ. In the rrs gene, A1401G and G1484T mutations cause resistance to the SLIDs, while M306I and M306V mutations in the embB gene confer resistance to EMB. The strip contained conjugated control (CC) and amplification control band (AC). The MTBDRsl strip is also composed by six wild-type (WT) bands: three gyrA WT, two rrs WT and one embB WT. For interpretation of the strip, the presence of any mutation described above or absence of wild-type band was considered as indication of resistance to the evaluated drug [16, 17].
Statistical analysis
Statistical test were performed using STATA 13.1 software (StataCorp LP College Station, Texas 77845 USA). Chi-square and Fisher exact test was used to determine differences between susceptible and resistant group. The results was considered significant for those having p < 0.05.
Results
A total of 155 TB patients confirmed by culture and Genotype MTBDRplus were included. There were 77 (50 %) males, 62 (40 %) females and 16 (10 %) for whom gender was unknown. Pulmonary disease was diagnosed in 153 (98.7 %) patients. There were two cases of extra-pulmonary TB. TB/HIV coinfection was present in 61.1 % (44/72) of the 72/155 (46.5 %) patients who had HIV serology performed. MTBDRplus results: From the 155 isolates tested, 115 (74.2 %) where susceptible to isoniazid (H) and rifampicin (R) while any resistance to H or R was detected in 40 (25.8 %) isolates. The prevalence of MDR-TB meaning resistance to both R and H was 16.1 % (25/155), H-monoresistance (H-monoR) detected in 13 (8.4 %) isolates while R-monoresistance (R-monoR) occurred in 2 (1.3 %). Regarding to the previous use of anti-TB drugs, 4 (16 %) patients with MDR-TB had no previous treatment of TB, while 20 (80 %) patients reported previous TB treatment for more than 30 days. There was 1 (4 %) patient with unknown information about previous use of anti-TB drugs.
Among the population with any resistance to the evaluated drugs (40 isolates), the frequency of MDR-TB was 62.5 %, H-monoR 32.5 % and R-monoR 5 %, the frequency of primary DR-TB was 22.2 % (8) while in 77.8 % (28) the drug resistance was acquired (Table 1).
Genotypic profile
Of the 27 isolates resistant to R with mutations detected in the rpoB gene, 37 % (10 isolates) presented S531L mutations; this was the most frequent profile (Table 2).
Genotype MTBDRsl
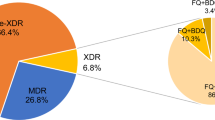
There were no discrepancies between the operators in the interpretation of the final result with the strip tests. A total of 125 isolates tested with MTBDRplus including the 25 isolates with resistance to R or H, were screened for resistance to the SLIDs, FQ and EMB using MTBDRsl test in other to determine the frequency of XDR-TB in this studied region of Mozambique. Of these, 109 (88 %) were susceptible while 16 (12 %) had any resistance detected. Within the resistant isolates, 37.5 % (6/16) were monoresistant to FQ, 56.25 % (9/16) monoresistant to EMB and 6.25 % (1/16) resistant to both FQ and EMB. There were no resistant detected to SLIDs as a consequence of this, XDR-TB did not occur in this sample. All isolates detected with resistance to EMB had previous use of anti-Tb drugs.
Genotypic profile
The most frequent mutation detected in the isolates with resistance to FQ was A90V, this occurred in 100 % (7/7) while in those resistant to EMB loss of embB WT1 was more prevalent (90 %) (Table 3).
Discussion
In Mozambique, surveillance for tuberculosis is poor [2, 18] and the true magnitude of drug resistance is unknown [2, 19]. Operations researches are needed to provide estimates of drug resistance and HIV coinfection burdens to improve treatment and prevention.
Our study is the first from Mozambique that provides an estimate the frequency DR-TB, coinfection DR-TB/HIV and the magnitude of resistance to SLIDs. Compared to national data, [7] our finding shows high frequency of resistance to the first line anti-TB drugs (R or H) (25.8 %) and high frequency of MDR-TB (16.1 %). Based on the history of previous therapy in the majority of our patients, drug resistant TB was mainly acquired as shown previously [6, 20]. However, the frequency of primary drug resistant TB (22.2 %) at Beira Hospital, in Central Mozambique may be an indication of failure to prevent transmission during management of DR-TB cases.
Because of the high prevalence of HIV in Mozambique, HIV infection was common among patients with DR-TB infection (62.5 %) although there is no association between HIV and drug resistant TB [21–23]. However, it was shown by the epidemics of DR-TB in Kwazulu Natal, South Africa (SA) that there is high case fatality among DR-TB/HIV co-infected patients [24, 25] so rapid diagnosis of drug resistance and guided treatment is important both for improved outcomes and to avoid progression to XDR-TB or “TDR-TB”. Genotypic molecular tests such as LPA (MTBDRplus, MTBDRsl), which are endorsed by WHO for surveillance of drug resistance have been revolutionary in shortening the time between DR-TB diagnostic and initiation of treatment [23]. In addition, these tests are improving the understanding of drug resistant TB worldwide and may also improve local protocols for treatment of DR-TB in settings with lack of specialized physicians for DR-TB management such as Mozambique.
It is expected that almost 95 % of strains resistant to R are also resistant to H, this supports the recommendation of WHO that all R resistant cases in regions with high prevalence of TB should receive treatment for MDR-TB [2], our findings support this recommendation because 93 % of R resistant isolates in our study were concomitantly resistant to H.
The first generation of Genotype MTBDRsl has low sensitivity especially for detection of resistance to SLIDs [10–13] but this test may be used for purpose of infection control and rule-in XDR-TB in settings such us Mozambique, where LAP technology is available and conventional DST capacity is limited. However, the test should not replace phenotypic DST as the gold standard for diagnosis of XDR-TB and clinicians must not rule out the investigation of XDR-TB when resistance is not detected on MTBDRsl [13]. This test has already a new version (MTBDRsl version 2.0) which shows better performance characteristics in detection of XDR-TB with high sensitivity and specificity, especially for kanamycin detection. This new test may shorten the time between diagnostic of DR-TB and initiation of treatment as well as estimates the prevalence of SLD resistance accurately [26].
In Mozambique there is a lack of information about resistance to the second line drugs. Samo Gudo et al., in their study using phenotypic DST within 41 strains obtained from national drug resistance surveillance, found no XDR-TB case in 2008 [6]. Pires et al., with 280 MDR-TB isolates using phenotypic DST, found only 2 (0.71 %) XDR-TB cases [20]. Using MTBDRsl we found low frequency of resistance to the SLD (12 %), almost 37.5 % (6/16) resistant to FQ, 56.3 % (9/16) to EMB and 6.3 % (1/16) resistant to both FQ and EMB, no resistance detected for SLIDs, and no XDR-TB case confirmed in this study. Based on these limited data, we suspect that XDR-TB is still infrequent in Beira Mozambique.
The major limitations of this study were the incomplete medical records, lack of information about phenotypic susceptibility tests and Genotype MTBDRsl low sensitivity to detect resistance to SLIDs.
This study is also part of an initiative to evaluate the cost/effectiveness of LPA test introduction in the drug resistant tuberculosis routine investigation at Beira, central Mozambique.
Conclusions
Despite a high frequency of MDR, XDR-TB was not found in our patients. This study emphasizes the rational use of molecular tests for improving local protocols for treatment and prevention of drug resistant TB. Genotypic assays improve local information about SLD resistance in settings in which phenotypic testing is unavailable.
Abbreviations
AC, amplification control; CC, conjugated control; DR-TB, drug resistant tuberculosis; DST, drug susceptibility test; EMB, etambutol; FQ, fluoroquinolones; H, isoniazid; HIV, human immunodeficiency virus; LPA, line probe assay; MDR, multidrug resistant; NPTBC, national program of TB control; R, rifampicin; SLD, second line drugs; SLIDs, second line Injectable anti-TB drugs; TB, tuberculosis; WHO, world health organization; WT, wild-type; XDR-TB, extensively drug resistant tuberculosis.
References
Zumla A, Chakaya J, Centis R, D'Ambrosio L, Mwaba P, Bates M, et al. Tuberculosis treatment and management-an update on treatment regimens, trials, new drugs, and adjunct therapies. Lancet Respir Med. 2015;3:220–34.
World Health Organization. Global Tuberculosis report 2014. Geneva. World Health Organization Document 2014; WHO/HTM/TB/2014.8: 1-171.
Parida SK, Axelsson-Robertson R, Rao MV, Singh N, Master I, Lutckii A, et al. Totally drug-resistant tuberculosis and adjunct therapies. J Intern Med. 2015;277:388–405.
Migliori GB, De Iaco G, Besozzi G, Centis R, Cirillo DM. First tuberculosis cases in Italy resistant to all tested drugs. Euro Surveill. 2007;12:E070517.1.
Zumla A, Petersen E, Nyirenda T, Chakaya J. Tackling the Tuberculosis Epidemic in sub-Saharan Africa - unique opportunities arising from the second European Developing Countries Clinical Trials Partnership (EDCTP) programme 2015–2024. Int J Infect Dis. 2015;32:46–9.
Samo Gudo P, Cuna Z, Coelho E, Maungate S, Borroni E, Miotto P, et al. Is multidrug-resistant tuberculosis on the rise in Mozambique? Results of a national drug resistance survey. Eur Respir J. 2011;38:222–4.
Moçambique. Ministério da Saúde. Direção nacional de Saúde Publica. Relatório de Actividade desenvolvidas durante o ano 2014. Maputo; 2015.
World Health Organization (WHO). Molecular line probe assays for rapid screening of patients at risk of multi-drug resistant tuberculosis (MDR-TB). Policy statement. Geneva: World Health Organization; 2008. http://www.who.int/tb/features_archive/policy_statement.pdf.
Torres JN, Paul LV, Rodwell TC, Victor TC, Amallraja AM, Elghraoui A, et al. Novel katG mutations causing isoniazid resistance in clinical M. tuberculosis isolates. Emerg Microbes Infect. 2015;4:e42.
Theron G, Peter J, Richardson M, Barnard M, Donegan S, Warren R, et al. The diagnostic accuracy of the GenoType(®) MTBDRsl assay for the detection of resistance to second-line anti-tuberculosis drugs. Cochrane Database Syst Rev. 2014;10:CD010705.
Feng Y, Liu S, Wang Q, Wang L, Tang S, Wang J, et al. Rapid diagnosis of drug resistance to fluoroquinolones, amikacin, capreomycin, kanamycin and ethambutol using genotype MTBDRsl assay: a meta-analysis. PLoS One. 2013;8:e55292.
Jin J, Shen Y, Fan X, Diao N, Wang F, Wang S, et al. Underestimation of the resistance of Mycobacterium tuberculosis to second-line drugs by the new GenoType MTBDRsl test. J Mol Diagn. 2013;15:44–50.
World Health Organization. The use of molecular line probe assay for the detection of resistance to second-line anti-tuberculosis drugs. Expert group meeting report – Geneva: February 2013. Geneva: World Health Organization; 2013. http://apps.who.int/iris/bitstream/10665/78099/1/WHO_HTM_TB_2013.01.eng.pdf.
Moçambique. Ministerio da Saude. Manual de tratamento da Tuberculose Multiresistente em Mocambique, 2012. Maputo; 2012.
Vijdea R, Stegger M, Sosnovskaja A, Andersen AB, Thomsen VO, Bang D. Multidrug-resistant tuberculosis: rapid detection of resistance to rifampin and high or low levels of isoniazid in clinical specimens and isolates. Eur J Clin Microbiol Infect Dis. 2008;27:1079–86.
Singh AK, Maurya AK, Kant S, Umrao J, Kushwaha RA, Nag VL, et al. Rapid detection of drug resistance and mutational patterns of extensively drug-resistant strains by a novel GenoType® MTBDRsl assay. J Postgrad Med. 2013;59:179–85.
Brossier F, Veziris N, Aubry A, Jarlier V, Sougakoff W. Detection by GenoType MTBDRsl test of complex mechanisms of resistance to second-line drugs and ethambutol in multidrug-resistant Mycobacterium tuberculosis complex isolates. J Clin Microbiol. 2010;48:1683–9.
García-Basteiro AL, López-Varela E, Manhiça I, Macete E, Alonso PL. Mozambique faces challenges in the fight against tuberculosis. Lancet. 2014;383:215–6.
Falzon D, Jaramillo E, Wares F, Zignol M, Floyd K, Raviglione MC. Universal access to care for multidrug-resistant tuberculosis: an analysis of surveillance data. Lancet Infect Dis. 2013;13:690–7.
Pires GM, Folgosa E, Nquobile N, Gitta S, Cadir N. Mycobacterium tuberculosis resistance to antituberculosis drugs in Mozambique. J Bras Pneumol. 2014;40:142–7.
Vadwai V, Shetty A, Soman R, Rodrigues C. Determination of risk factors for isoniazid monoresistance and multidrug-resistant tuberculosis in treatment failure patients. Scand J Infect Dis. 2012;44:48–50.
Lukoye D, Ssengooba W, Musisi K, Kasule GW, Cobelens FG, Joloba M, et al. Variation and risk factors of drug resistant tuberculosis in sub-Saharan Africa: a systematic review and meta-analysis. BMC Public Health. 2015;15:291.
Feliciano CS, Nascimento MM, Anselmo LM, Pocente RH, Bellissimo-Rodrigues F, Bollela VR. Role of a GenoType MTBDRplus line probe assay in early detection of multidrug-resistant tuberculosis at a Brazilian reference center. Braz J Med Biol Res. 2015;48:759–64.
Cohen T, Murray M, Wallengren K, et al. The prevalence and drug sensitivity of tuberculosis among patients dying in hospital in KwaZulu-Natal, South Africa: a postmortem study. PLoS Med. 2010;22:e1000296.
Gandhi NR, Moll A, Sturm AW, et al. Extensively drug-resistant tuberculosis as a cause of death in patients co-infected with tuberculosis and HIV in a rural area of South Africa. Lancet. 2006;368:1575–80.
Tagliani E, Cabibbe AM, Miotto P, Borroni E, Toro JC, Mansjö M, et al. Diagnostic Performance of the New Version (v2.0) of GenoType MTBDRsl Assay for Detection of Resistance to Fluoroquinolones and Second-Line Injectable Drugs: a Multicenter Study. J Clin Microbiol. 2015;53:2961–9.
Acknowledgements
The authors thanks the Referral Laboratory of Beira, National Laboratory in Maputo and Laboratory of Mycobacterium of HCRP-USP for assistance in performing the study assays, the National TB Control Program (NPTBC- MISAU), CIOB, Dr. Manuel Napua, Dra. Sofia Viegas, José Carlos Beirão, Domitila Ferrao and Mara Ribeiro for assistance in data collection; and Kathleen Stewart, Joyce Snyder, Sarifa Mulina, Diana Pakstis, and Linda DeLuco for administrative assistance.
Funding
This work was supported in part by a Fogarty International Center HIV Research Training Program grant, National Institutes of Health, to the University of Pittsburgh (D43TW009753). Support was also provided by FAEPA and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) - Brazil.
Availability of data and materials
Data supporting the findings (patient records) cannot be shared because the study was taken as part of routine at the Beira central Hospital in Mozambique, and data contain patients information but if requested can be found in the data base of the hospital. Line probe assays results and spreadsheets raw data can be accessed by contacting the corresponding author. They are available at the laboratory records.
Authors’ contributions
Conceived and designed the study (EIN, VRB, JF and LH). Data Collection (EIN, IT, ML). Tests performing and analysis (IT, MP, RP). Data analysis and writing the manuscript (EN, VB and LH). Review the final draft all authors. All authors read and approved the final manuscript.
Competing interest
The authors declare that they have no competing interest.
Consent to publish
Not applicable.
Ethics and consent to participate
Clinical samples were taken anyway as part of routine, so informed consent was not required. The study protocol was approved by the National Bioethics Committee for Health of the Ministry of Health in Mozambique reference number CNBS/82/2014.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Namburete, E.I., Tivane, I., Lisboa, M. et al. Drug-resistant tuberculosis in Central Mozambique: the role of a rapid genotypic susceptibility testing. BMC Infect Dis 16, 423 (2016). https://doi.org/10.1186/s12879-016-1766-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-016-1766-x