Abstract
Background
Bacterial meningitis remains an important infection globally, with the greatest burden in children in low-income settings, including Papua New Guinea (PNG). We present serotype, antimicrobial susceptibility and outcome data from paediatric meningitis patients prior to introduction of Haemophilus influenzae type b (Hib) and pneumococcal conjugate vaccines (PCVs) in PNG, providing a baseline for evaluation of immunisation programs.
Methods
Cerebrospinal fluid (CSF) was collected from children admitted to Goroka General Hospital with suspected meningitis between 1996 and 2005. Culture and sensitivity was conducted, and pneumococci and H. influenzae were serotyped. Laboratory findings were linked to clinical outcomes.
Results
We enrolled 1884 children. A recognised pathogen was identified in 375 children (19.9 %). Streptococcus pneumoniae (n = 180) and Hib (n = 153) accounted for 88.8 % of pathogens isolated. 24 different pneumococcal serogroups were identified; non-PCV types 2, 24 and 46 accounted for 31.6 % of pneumococcal meningitis. 10- and 13-valent PCVs would cover 44.1 % and 45.4 % of pneumococcal meningitis respectively. Pneumococcal isolates were commonly resistant to penicillin (21.5 %) and 23 % of Hib isolates were simultaneously resistant to ampicillin, co-trimoxazole and chloramphenicol. The case fatality rate in patients with a recognised bacterial pathogen was 13.4 % compared to 8.5 % in culture-negative patients.
Conclusions
If implemented in routine expanded programme of immunisation (EPI) with high coverage, current PCVs could prevent almost half of pneumococcal meningitis cases. Given the diversity of circulating serotypes in PNG serotype replacement is of concern. Ongoing surveillance is imperative to monitor the impact of vaccines. In the longer term vaccines providing broader protection against pneumococcal meningitis will be needed.
Similar content being viewed by others
Background
Bacterial meningitis is an important cause of morbidity and mortality in children in low-income countries [1]. The two most important etiological agents of bacterial meningitis are Streptococcus pneumoniae and Haemophilus influenzae type b (Hib). In total, S. pneumoniae kills over 800,000 children (<5 years old) each year, with meningitis being the most common severe form of invasive pneumococcal disease (IPD) [2]. Prior to introduction of Hib conjugate vaccine there were ~2.2 million cases of serious Hib disease annually, the vast majority in low-income settings [3]. In low-income countries the case fatality rate (CFR) for acute bacterial meningitis is commonly above 30 % and usually higher for pneumococcal than Hib meningitis [2, 4–7]. In children who survive acute bacterial meningitis, neurological complications are common with approximately one in four children in developing countries suffering long-term neurological sequelae following pneumococcal meningitis [8].
Vaccines have been developed to reduce mortality and morbidity due to IPD and Hib disease. Hib vaccine is effective in high and low socio-economic settings. In The Gambia the incidence of Hib meningitis remains below 5 cases/100,000 14 years after the introduction of a three dose course of Hib vaccine [9, 10]. In Indigenous children in Western Australia the introduction of Hib vaccine in 1993 resulted in a significant decline in hospital admission for meningitis [11]. Despite the availability of conjugate Hib vaccines since the 1980s, there has been a delay in their inclusion in national immunisation programs in many low-income countries [12].
The 23-valent pneumococcal polysaccharide vaccine (PPV) (serotypes 1, 2, 3, 4, 5, 6B, 7 F, 8, 9 N, 9 V, 10, 11, 12 F, 14, 15B, 17 F, 18C, 19 F, 19A, 20, 22 F, 23 and 33 F) is generally considered to be poorly immunogenic in children under 2 years old. The pneumococcal conjugate vaccines (PCVs) consist of serotype-specific polysaccharides conjugated to a protein to improve immunogenicity in children <2 years old. Introduction of the heptavalent conjugate vaccine, PCV7 (serotypes 4, 6B, 9 V, 14, 18C, 19 F and 23 F) into national immunisation programs has reduced the incidence of IPD in a number of industrialised countries [13, 14]. Studies have demonstrated efficacy of a 9-valent PCV (PCV7 serotypes plus serotypes 1 and 5) in two African settings [15, 16]. PCV7 has been superseded in recent years by PCV10 (PCV7 serotypes plus 1, 5 and 7 F) and PCV13 (PCV10 serotypes plus 3, 6A, and 19A). Rollout of these higher valency vaccines is now occurring in low-income countries.
Previous studies in PNG have shown S. pneumoniae and Hib to be the most common causes of bacterial meningitis and have provided data on serotype distribution and antimicrobial susceptibility of isolates [6, 17]. With the introduction of Hib vaccine into the PNG national program in 2008 and rollout of PCV13 commencing in 2014, ongoing surveillance of serotype distribution and antimicrobial susceptibility is essential to ensure optimal prevention and treatment strategies.
Acute flaccid paralysis surveillance has been conducted in Goroka in the PNG highlands since 1996 as part of the global polio eradication campaign. As such, suspected cases of meningitis have been investigated, providing data of Hib and pneumococcal meningitis, serotype distribution and antimicrobial susceptibility in the pre-vaccine era. We report here on data collected from children admitted to Goroka General Hospital (GGH; now called Eastern Highlands Provincial Hospital) between August 21st, 1996 and June 17th, 2005.
Methods
Setting and study population
GGH is the referral hospital for Eastern Highlands Province (population ~433,000 in 2000) of PNG. The provincial capital Goroka (altitude 1546 m asl) has a population of ~20,000 (70,000 in the surrounding district). The majority of people are subsistence farmers, with the major cash crop in the province being coffee.
We have conducted surveillance of suspected meningitis in children aged <15 years admitted to GGH since 1996. Case identification was based on any of the following clinical signs or symptoms: history of convulsion, altered level of consciousness, neck stiffness, bulging or tense fontanelle at rest, focal neurological signs associated with history of recent febrile illness, refusal or inability to feed associated with a febrile illness, or paediatrician’s suspicion of meningitis in the absence of above signs and symptoms. We documented whether patients were discharged, absconded or died from the hospital records.
Laboratory methods
Cerebrospinal fluid (CSF) was collected via lumbar puncture using aseptic technique. Where possible CSF was collected prior to administration of antibiotics in the hospital. Samples were processed at the PNG Institute of Medical Research (PNGIMR) as soon as possible after collection. Standard methods, namely microscopy and bacterial culture were used to diagnose meningitis and determine etiological agents: these methods are well established in this setting [17]. Microscopy included cell counts (polymorphonuclear neutrophils, lymphocytes and erythrocytes) and Gram stain of pelleted CSF. Evidence of prior antimicrobial treatment was garnered through an assay in which a disk impregnated with the patient’s CSF was placed on an agar plate seeded with S. aureus ATCC 25923. H. influenzae was serotyped at PNGIMR using H. influenzae antisera a-f (Remel, Thermo Fisher Scientific, Australia). Pneumococci were serogrouped at PNGIMR by the Quellung reaction (Statens Serum Institut, Copenhagen, Denmark) and a subset sent to Queensland Health Pathology Service (Brisbane, Australia) for confirmation and factor typing.
Antibiotic susceptibility testing was conducted by disk diffusion (Oxoid, Thermo Fisher Scientific, Australia) following CLSI guidelines [18]. Isolates were tested for susceptibility to chloramphenicol, tetracycline, co-trimoxazole, ceftriaxone, ampicillin (H. influenzae only), oxacillin (S. pneumoniae only) and erythromycin (S. pneumoniae only). Minimum inhibitory concentration (MIC) testing was conducted using E-test (AB Biodisk, Sweden) following CLSI guidelines [18]. MIC tests were conducted on pneumococcal isolates to determine susceptibility to penicillin, chloramphenicol and cotrimoxazole, with a subset tested for susceptibility to tetracycline, ceftriaxone and erythromycin. H. influenzae isolates had MICs determined for ampicillin, chloramphenicol and cotrimoxazole. At the time this study was conducted (1996–2005) the MIC for resistance to penicillin in S. pneumoniae was ≥2 μg/ml (as it remains currently for non-meningitis cases). CSF pneumococcal isolates now are considered resistant to penicillin at MIC ≥0.12 μg/ml [18]. We applied the current guidelines when determining resistance to penicillin in pneumococcal isolates. Serotyping and sensitivity testing was conducted at the time of isolation.
Data were double-entered using FoxPro 8 (Microsoft Corp, USA) and analysed using Excel (Microsoft Corp, USA). The Yates chi-square test was used to compare proportions between groups of interest. Ethics approval was granted by the PNG Medical Research Advisory Committee to conduct CSF bacterial culture and biochemistry as part of the acute flaccid paralysis surveillance. The need for written informed consent was waivered by the ethics committee as this work was conducted as part of good clinical care of the patients.
Results
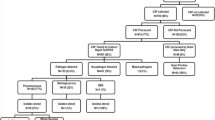
Between August 1996 and June 2005 CSFs were collected from 1884 patients: 1126 males and 758 females. The median age of patients was 6 months (lower quartile 3 months, upper quartile 12 months). Bacteria were isolated from 480 (25.5 %) samples; two organisms were isolated from five CSF samples, resulting in 485 bacterial isolates. S. pneumoniae (180) and H. influenzae (165, of which 153 were Hib) were the most commonly isolated pathogens, accounting for 91.5 % of recognised pathogens isolated. Other bacterial pathogens included Enterobacteriaceae (14), β-haemolytic streptococci (8) and Acinetobacter spp (6) (Table 1). Thirty-two isolates were considered probable contaminants: 12 Staphylococcus epidermidis, 10 Bacillus spp., 4 non-aeruginosa pseudomonads, 1 Micrococcus sp. and 5 non-specified. A further 76 isolates were considered possible pathogens (68 Staphylococcus aureus, 5 viridans streptococci, 2 Enterococcus faecalis and 1 Klebsiella oxytoca). The temporal distribution of S. aureus isolates was indicative of contamination for a large proportion of these isolations: 45 of 68 isolations occurred in 2004 and 2005 and 94.4 % (34 of 36 that had cell count conducted within this period) occurred in the absence of polymorphonucleocytes (PMN) in the CSF. In total, 375 CSF (19.9 %) samples were positive for a recognised bacterial pathogen (excluding probable contaminants and possible pathogens). In two of these samples multiple bacteria were isolated, resulting in a total of 377 recognised pathogens, as listed in Table 1.
Of the 1404 culture negative CSF samples, in 263 samples PMN were >10 × 106/l (10–100 × 106/l in 147 samples; >100 × 106/l in 116 samples). We also detected antimicrobial activity in the CSF of 126 of 1775 samples tested (including 10 samples with high PMN counts). A summary of microscopy results is provided in Additional file 1: Table S1.
Outcomes (died, discharged or absconded) were documented for 1351 patients (71.7 % of participants). 71 (5 %) children were taken home while still sick. The CFR during hospitalisation among remaining children was 9.5 %. The CFR for patients in whom a recognised bacterial pathogen was isolated (i.e. excluding possible pathogens and probable contaminants) was 13.4 % compared to a CFR of 8.5 % in patients with no bacterial pathogen isolated (probable contaminant or no bacteria isolated) (χ 2 = 4.94, degrees of freedom (df) = 1, p = 0.026). The CFR for pneumococcal meningitis was 15.4 %, and 8.9 % for patients with Hib meningitis (χ 2 = 1.82, df = 1, p = 0.177).
Significant differences in age distribution of pneumococcal and Hib meningitis were noticed (Table 2). Pneumococcal meningitis most frequently occurred in infants aged < 6 months (52.8 % of all pneumococcal meningitis compared with 36.2 % of Hib meningitis, χ 2 = 8.50, 1df, p = 0.004) whereas Hib meningitis was most frequent in children aged 6–11 months (50.0 % of Hib cases compared with 20.0 % of pneumococcal meningitis cases, χ 2 = 31.85, 1df, p = 0.000).
Serotype distribution of S. pneumoniae and H. influenzae
Of the 180 pneumococcal isolates, 171 were serogrouped; yielding 24 different serogroups (29 serotypes) and one non-typable isolate (Additional file 2: Table S2). The most common serogroups were 2 (17.5 % of all serogrouped isolates), 5 (10.5 %), 46 (8.8 %) and 7 (7.0 %).
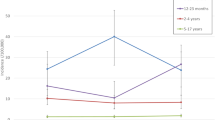
The proportion of cases of culture-confirmed pneumococcal meningitis that would be covered by 10- and 13-valent PCVs and PPV is shown in Fig. 1. Where factor type was relevant, non-factor-typed isolates were excluded from analysis, resulting in 152 cases. The PCV10 would cover 44.1 % of all cases in our setting in PNG; an additional 1.3 % of cases (45.4 %) would be covered by PCV13.
The proportion of cases with culture-confirmed pneumococcal meningitis that would be covered by 10vPCV, by the additional coverage offered by PCV13 and by PPV by age group. The number in parenthesis (n) represents the number of cases in that age group for which serogroup/type data are available. In three age groups (<2, 6–11, and 12–23 months) PCV13 would offer no additional protection above that of PCV10. The serotype 6A was not tested with 6C antisera, thus reported as 6A in this analysis
Hib accounted for 153 (93 %) of the 165 of H. influenzae isolated from CSF; nine isolates (5.5 %) were serotype a, two (1.2 %) serotype c and one (0.6 %) non-typable.
Antimicrobial susceptibility
Susceptibility testing was conducted by disk diffusion on 177 S. pneumoniae isolates. Reduced susceptibility to oxacillin was observed in 34 (17.2 %) S. pneumoniae isolates; resistance to other antibiotics was uncommon. Pneumococcal resistance to penicillin (MIC determined by E-test) was observed in 21.5 % of isolates (Table 3). Some serotypes commonly exhibited reduced susceptibility to penicillin: serogroups/types 19 (5/6 isolates), 6 (9/11), 10 (3/4), 14 (5/8) 24 (4/9) and 9 (2/5). The one pneumococcal isolate with reduced susceptibility to ceftriaxone (serotype 14) was also resistant to penicillin and cotrimoxazole; three other isolates were resistant to both penicillin and cotrimoxazole. All four tetracycline-resistant S. pneumoniae were also resistant to cotrimoxazole; two isolates were resistant to additional antibiotics (one to penicillin and one to chloramphenicol).
All 165 H. influenzae isolates had β-lactamase test conducted, and susceptibility testing was conducted by disk diffusion on 163 isolates. Fifty-three (32.5 %) isolates were β-lactamase positive. All resistant H. influenzae isolates were Hib. All β-lactamase positive isolates were resistant to ampicillin and cotrimoxazole by disk diffusion, 52/53 were also resistant to chloramphenicol and 51/52 resistant to tetracycline. One-hundred and sixty-two isolates had MICs determined for ampicillin, chloramphenicol and cotrimoxazole. One-third of isolates demonstrated reduced susceptibility to ampicillin, with a similar proportion of isolates having reduced susceptibility to cotrimoxazole and chloramphenicol (Table 3). Multiple resistance was common in H. influenzae isolates, with 38 (23 %) isolates resistant to ampicillin, chloramphenicol and cotrimoxazole. An additional 15 isolates showed multiple resistance or intermediate resistance to two or three of those antibiotics.
Only disk diffusion testing was conducted for tetracycline (n = 162) and ceftriaxone (n = 74). Fifty-seven H. influenzae isolates were resistant to tetracycline (35 %) with one additional isolate demonstrating intermediate resistance. Fifty-two of the tetracycline-resistant isolates were also resistant to ampicillin, chloramphenicol and cotrimoxazole. Four isolates (5 %) were non-susceptible to ceftriaxone.
Analysis was conducted to determine if the rate of antibiotic resistance increased during the study period, using the end of 2000 as the approximate mid-point of the surveillance (Table 4). There was no statistical difference in the proportion of pneumococci that were resistant pre-2001 compared to the later period (2001–June 2005). However, the proportion of H. influenzae that were resistant to ampicillin increased significantly between the two periods (Table 4).
Discussion
Our data demonstrate the importance of pneumococcus and Hib in the aetiology of meningitis in PNG; with the serotype distribution of pathogenic pneumococci and age of infection having implications for vaccine efficacy. Potential changes in serotype distribution (post-vaccine) and antimicrobial susceptibility dictate the need for ongoing surveillance.
Hib accounts for the vast majority of pathogenic H. influenzae isolated in this setting (and other settings), enabling the Hib vaccine to have a significant impact on disease. In contrast, non-PCV13 serogroups/types 2, 8, 12, 18A, 19B, 24 and 46 were amongst the most commonly isolated strains of S. pneumoniae, accounting for >40 % of all pneumococcal isolates in this study. The broad range of serotypes observed in this study is in keeping with our earlier study in which Lehmann et al found so-called ‘adult’ serogroups of pneumococci were more commonly isolated from CSF of children than ‘paediatric’ serotypes (i.e. 6, 14) [17].
Serotype 2, the most commonly isolated serotype in this study, has recently been described as a “newly recognised pneumococcal infection threat” [19]; however, it has been noted as a major cause of pneumococcal meningitis in PNG for the past 30 years with no obvious temporal clustering [6, 17]. Saha and colleagues noted that serotype 2 affected younger children relative to other serotypes [19]. In contrast, in our study the age distribution of serotype 2 reflected the pneumococcal isolation rate over the age groups (Table 2 and Additional file 2: Table S2). Our study provides evidence that serotype 2 presents a threat to this region. PCV13 does not include serotype 2, and upper respiratory tract carriage of other non-vaccine serotypes of pneumococcus is common in infants in this setting [20]. Thus, there may be the potential for an increase in disease due to non-vaccine serotypes post pneumococcal vaccine introduction in PNG, as was seen after the introduction of PCV7 in other settings [21].
Despite the potential for serotype replacement, the overall impact of PCVs has been positive, particularly in high income settings and with broader spectrum (PCV10 and PCV13) vaccines. It is difficult to make direct comparisons due to lack of meningitis-specific data, but in high-income settings PCVs have reduced overall IPD rates by up to 80 % as a result of the better match between serotypes in PCVs and serotypes causing disease [13]. Even with lower serotype coverage (relative to high income settings) and the potential for serotype replacement, a vaccine that offers 40–50 % coverage in a high burden setting will save lives. When consideration is given to the roll of pneumococcus in pneumonia, the case for immediate PCV rollout in high-burden settings becomes even stronger.
Our data demonstrate differences in age distribution and CFR between Hib meningitis and pneumococcal meningitis (Table 2). The CFRs for laboratory-confirmed bacterial meningitis was higher than the CFR for patients in whom no bacterial pathogen was isolated, and there was a trend towards a higher CFR in patients with pneumococcal meningitis than in those with H. influenzae meningitis (though not statistically significant). The CFRs observed in the current surveillance are a considerable improvement on those observed previously in the same setting, when approximately one-third of children with probable or confirmed bacterial meningitis died [17]. It is difficult to ascertain the reasons for this decrease in CFR over the two study periods; though better overall health of the population resulting in less severe disease, and/or improved management, may be contributing factors.
We observed high and increasing rates of antimicrobial resistance in Hib isolates. An increase in resistance relative to our previous study was observed, in which all Hib were susceptible to ampicillin and chloramphenicol [17]. A recent study in the lowlands of PNG found all H. influenzae CSF isolates tested (n = 14) were chloramphenicol-resistant [22]. Until recently chloramphenicol was the first-line treatment for meningitis in children in PNG: due to increasing resistance of Hib to chloramphenicol, ceftriaxone has now replaced it as the treatment of choice [23]. The observation that four Hib isolates were non-susceptible to ceftriaxone using the disk-diffusion method is of concern; however MICs were not conducted to confirm non-susceptibility (due to loss of viability of the isolates). Resistance to ceftriaxone in Hib remains uncommon in other settings [24, 25]; nonetheless, ongoing monitoring of ceftriaxone susceptibility of Hib is imperative given its current use for treatment of meningitis in PNG.
Penicillin-resistant pneumococci have long been recognised in PNG. In this study 21.5 % of isolates were penicillin resistant: a similar proportion of isolates (7/31; 22.6 %) had an MIC ≥0.125 μg/ml in the previous study conducted in this setting [17]. Thus, on the basis of current and previous findings [17, 22], there is no evidence of increasing prevalence of antimicrobial resistant pneumococci in PNG.
Tetracycline and erythromycin are not well suited for the treatment of meningitis; however, monitoring resistance to these antibiotics in pneumococcal isoaltes is of value. With limited routine diagnostic culture and sensitivity conducted in PNG, it is important to gain an insight into resistance patterns for a wide range of antimicrobial agents from relatively few clinical isolates. Moreover, baseline data on macrolide resistance in malaria endemic settings is of value as trials are conducted on malaria prophylaxis [26].
Our study provides important data leading up to the introduction of Hib and PCV vaccines. The Hib vaccine (introduced in 2008) and the PCV13 (rollout commenced in 2014) should reduce the number of cases of bacterial meningitis. The predominance of pneumococcal meningitis in the first 6 months of life highlights the need for early protection. In PNG both an accelerated 1-2-3-month PCV schedule (which ties in with PNG’s standard EPI schedule) and a schedule including a neonatal dose (0,1 and 2 months) have been shown to be safe and immunogenic [27] and should assist in protecting young children from disease caused by vaccine serotypes. Recent data from GGH show the benefit of the introduction of Hib vaccination into the national EPI program. Analysis conducted by our research team [28] reveal that the isolation rate of Hib from CSF fell significantly from 6.0 % pre-introduction (2004–7) to 0.94 % following introduction (2009–13) (χ2, P < 0.001). There was no change in the isolation rate of S. pneumoniae over the same period [28].
We acknowledge that there are some limitations of the study and resulting data. We isolated higher numbers of S. aureus than expected. Further investigation indicated that CSF collection methods were inadequate and likely to have contributed to high isolation rate of S. aureus in 2004–2005. Some of these isolates, and some or all of those in previous years (in which no more than 6 were isolated in any given year between 1997 and 2003) may have been the causative agent of meningitis. Of the 68 S. aureus isolates, 11 corresponding CSF specimens were observed to have elevated PMN counts. However, even in samples with high PMN counts we cannot discount the possibility of another undetected bacterial pathogen being the causative agent of meningitis. This cautious supposition is supported by the fact that elevated PMN counts were detected in some specimens from which no bacteria were isolated (Additional file 2: Tables S2 and Additional file 3: Table S3). Given that S. aureus is rarely a cause of paediatric meningitis, and is generally associated with pre-existing abnormalities of the central nervous system or recent surgery (which were not present in our patients) [29, 30], we concluded that S. aureus were most likely contaminants.
Our isolation rate of other contaminants (aside from S. aureus) was <2 %, which is consistent with other CSF culture studies (e.g. Dunbar et al. [31]). One additional limitation of our study is that we obtained data from only one site within PNG, which may not be representative of the whole country.
The benefits of ongoing multi-site surveillance of bacterial diseases in high-burden settings are well recognised; however, conducting such surveillance is costly and the level of expertise required is in short supply. At regional sites non-culture based methods could be applied. However, antigen detection assays have short-comings, and currently available culture-independent nucleic acid detection methods appear to lack the robustness and user-friendliness required for resource-poor regional settings [32]. Moreover, neither method enables antimicrobial susceptibility testing to be conducting (though resistance can be inferred through the detection of genes). Concerted efforts are required to develop expertise and methods to enable more widespread and sustainable surveillance of S. pneumoniae and H. influenzae disease and upper respiratory tract carriage, as vaccines that reduce the impact of these pathogens are introduced globally.
Conclusions
Meningitis remains an important cause of severe childhood illness and death globally. However, vaccines are now available for two of the bacterial pathogens commonly associated with meningitis, namely H. influenzae type B and up to 13 serotypes of S. pneumoniae. Pre-vaccine surveillance data is imperative to gain an insight into the impact of the vaccines when they are introduced. Our data demonstrate the important role that non-PCV serotypes play in childhood disease in PNG. While the introduction of PCV is welcome in PNG, ongoing surveillance is imperative to monitor the role of non-vaccine serotypes in disease.
Abbreviations
- CFR:
-
Case fatality rate
- CLSI:
-
Clinical and Laboratory Standards Institute
- CSF:
-
Cerebral spinal fluid
- Hib:
-
Haemophilus influenzae type b
- GGH:
-
Goroka General Hospital
- IPD:
-
Invasive pneumococcal disease
- MIC:
-
Minimum inhibitory concentration
- PCV:
-
Pneumococcal conjugate vaccine
- PNG:
-
Papua New Guinea
- PNGIMR:
-
Papua New Guinea Institute of Medical Research
- PPV:
-
Pneumococcal polysaccharide vaccine
References
Greenwood BM. Selective primary health care: strategies for control of disease in the developing world. XIII. Acute bacterial meningitis. Rev Infect Dis. 1984;6:374–89.
O’Brien KL, Wolfson LJ, Watt JP, Henkle E, Deloria-Knoll M, McCall N, et al. Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet. 2009;374:893–902.
Peltola H. Worldwide Haemophilus influenzae type b disease at the beginning of the 21st century: global analysis of the disease burden 25 years after the use of the polysaccharide vaccine and a decade after the advent of conjugates. Clin Microbiol Rev. 2000;13:302–17.
Al Khorasani A, Banajeh S. Bacterial profile and clinical outcome of childhood meningitis in rural Yemen: a 2-year hospital-based study. J Infect. 2006;53:228–34.
Boisier P, Mainassara HB, Sidikou F, Djibo S, Kairo KK, Chanteau S. Case-fatality ratio of bacterial meningitis in the African meningitis belt: we can do better. Vaccine. 2007;25 Suppl 1:A24–9.
Gratten M, Barker J, Shann F, Gerega G, Montgomery J, Kajoi M, et al. The aetiology of purulent meningitis in highland children: a bacteriological study. P N G Med J. 1985;28:233–40.
Mackie EJ, Shears P, Frimpong E, Mustafa-Kutana SN. A study of bacterial meningitis in Kumasi, Ghana. Ann Trop Paediatr. 1992;12:143–8.
Edmond K, Clark A, Korczak VS, Sanderson C, Griffiths UK, Rudan I. Global and regional risk of disabling sequelae from bacterial meningitis: a systematic review and meta-analysis. Lancet Infect Dis. 2010;10:317–28.
Howie SR, Oluwalana C, Secka O, Scott S, Ideh RC, Ebruke BE, et al. The effectiveness of conjugate Haemophilus influenzae type B vaccine in The Gambia 14 years after introduction. Clin Infect Dis. 2013;57:1527–34.
Oluwalana C, Howie SR, Secka O, Ideh RC, Ebruke B, Sambou S, et al. Incidence of Haemophilus influenzae type b disease in The Gambia 14 years after introduction of routine Haemophilus influenzae type b conjugate vaccine immunization. J Pediatr. 2013;163:S4–7.
Moore HC, Lehmann D. Decline in meningitis admissions in young children: vaccines make a difference. Med J Aust. 2006;185:404.
Rossi IA, Zuber PL, Dumolard L, Walker DG, Watt J. Introduction of Hib vaccine into national immunization programmes: a descriptive analysis of global trends. Vaccine. 2007;25:7075–80.
Fitzwater SP, Chandran A, Santosham M, Johnson HL. The worldwide impact of the seven-valent pneumococcal conjugate vaccine. Pediatr Infect Dis J. 2012;31:501–8.
Whitney CG, Farley MM, Hadler J, Harrison LH, Bennett NM, Lynfield R, et al. Decline in invasive pneumococcal disease after the introduction of protein-polysaccharide conjugate vaccine. N Engl J Med. 2003;348:1737–46.
Cutts FT, Zaman SM, Enwere G, Jaffar S, Levine OS, Okoko JB, et al. Efficacy of nine-valent pneumococcal conjugate vaccine against pneumonia and invasive pneumococcal disease in The Gambia: randomised, double-blind, placebo-controlled trial. Lancet. 2005;365:1139–46.
Klugman KP, Madhi SA, Huebner RE, Kohberger R, Mbelle N, Pierce N. A trial of a 9-valent pneumococcal conjugate vaccine in children with and those without HIV infection. N Engl J Med. 2003;349:1341–8.
Lehmann D, Yeka W, Rongap T, Javati A, Saleu G, Clegg A, et al. Aetiology and clinical signs of bacterial meningitis in children admitted to Goroka Base Hospital, Papua New Guinea, 1989-1992. Ann Trop Paediatr. 1999;19:21–32.
CLSI. Performance standards for antimicrobial susceptibility testing; twenty-first informational supplement. CLSI document M100-S21. Wayne: Clinical and Laboratory Standards Institute; 2011.
Saha SK, Al Emran HM, Hossain B, Darmstadt GL, Saha S, Islam M, et al. Streptococcus pneumoniae serotype-2 childhood meningitis in Bangladesh: a newly recognized pneumococcal infection threat. PLoS One. 2012;7:e32134.
Aho C, Dangy J, Lamelas A, Greenhill AR, Pomat WS, Lehmann D, et al. Diversity of pneumococcal isolates in carriage and causing meningitis in Papua New Guinea [Abstract ISPPD - 0200]. Pneumonia. 2014;3:16.
Feikin DR, Kagucia EW, Loo JD, Link-Gelles R, Puhan MA, Cherian T, et al. Serotype-specific changes in invasive pneumococcal disease after pneumococcal conjugate vaccine introduction: a pooled analysis of multiple surveillance sites. PLoS Med. 2013;10:e1001517.
Manning L, Laman M, Greenhill AR, Michael A, Siba P, Mueller I, et al. Increasing chloramphenicol resistance in Streptococcus pneumoniae isolates from Papua New Guinean children with acute bacterial meningitis. Antimicrob Agents Chemother. 2011;55:4454–6.
Paediatrics Society of Papua New Guinea. Standard treatment for common illnesses of children in Papua New Guinea. A manual for nurses, community health workers, health extension officers and doctors. Port Moresby: National Department of Health; 2011.
Pfaller MA, Farrell DJ, Sader HS, Jones RN. AWARE Ceftaroline Surveillance Program (2008-2010): trends in resistance patterns among Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis in the United States. Clin Infect Dis. 2012;55 Suppl 3:S187–93.
Tristram S, Jacobs MR, Appelbaum PC. Antimicrobial resistance in Haemophilus influenzae. Clin Microbiol Rev. 2007;20:368–9.
Unger HW, Aho C, Ome-Kaius M, Wangnapi RA, Umbers AJ, Jack W, et al. Impact of intermittent preventive treatment in pregnancy with azithromycin-containing regimens on maternal nasopharyngeal carriage and antibiotic sensitivity of Streptococcus pneumoniae, Haemophilus influenzae, and Staphylococcus aureus: a cross-sectional survey at delivery. J Clin Microbiol. 2015;53:1317–23.
Pomat WS, van den Biggelaar AH, Phuanukoonnon S, Francis J, Jacoby P, Siba PM, et al. Safety and immunogenicity of neonatal pneumococcal conjugate vaccination in Papua New Guinean children: a randomised controlled trial. PLoS One. 2013;8:e56698.
Yoannes M, Michael A, Aho C, Siba P, Pui L, Waure C, et al. Haemophilus influenzae type b (Hib) meningitis pre- and post-Hib vaccine introduction in Papua New Guinea [abstract 29]. In: Program and abstracts of the 49th Annual Medical Symposium (Lae). Port Moresby: Medical Society of Papua New Guinea and National Department of Health; 2013.
Givner LB, Kaplan SL. Meningitis due to Staphylococcus aureus in children. Clin Infect Dis. 1993;16:766–71.
Rodrigues MM, Patrocinio SJ, Rodrigues MG. Staphylococcus aureus meningitis in children: a review of 30 community-acquired cases. Arq Neuropsiquiatr. 2000;58:843–51.
Dunbar SA, Eason RA, Musher DM, Clarridge JE. Microscopic examination and broth culture of cerebrospinal fluid in diagnosis of meningitis. J Clin Microbiol. 1998;36:1617–20.
Kirkham LA, Smith-Vaughan HC, Greenhill AR. Improving the aetiological diagnosis of bacterial pneumonia and meningitis in Papua New Guinea. P N G Med J. 2010;53:139–46.
Acknowledgements
This work was supported by the core operating costs of the Papua New Guinea Institute of Medical Research. Rebecca Sehuko from IMR conducted patient recruitment and sample collection. Gimana Nale assisted with laboratory analysis. We thank Dr Dale Frank, Dr Naomi Pomat, Dr Wendy Pameh and other clinical staff at GGH for assisting with patient recruitment and sample collection.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Competing interests
AG, CB, DL, WP and PS have received research support through a Pfizer Investigator Initiated Grant. DL has been a member of the GlaxoSmithKline Australia Pneumococcal-Haemophilus influenzae-Protein D conjugate vaccine Advisory Panel, has received support from Pfizer Australia and GSK Australia to attend conferences, and has received an honorarium from Merck Vaccines to give a seminar at their offices in Pennsylvania and to attend a conference. WP received a travel grant from Pfizer Australia to attend ISPPD in 2010.
Authors’ contributions
JR, WP, AM and PS managed the meningitis surveillance. AM, MY, TO, DM and HS conducted laboratory analysis and data management. AG, SP, JR, CB and DL conducted data analysis. AG and SP wrote the manuscript. DL, JR and CB contributed to the manuscript. All authors reviewed the manuscript. AM passed away prior to completion of the final manuscript. She reviewed earlier drafts, and consented to be an author of this work prior to her passing. All authors read and approved the final manuscript.
Author’s information
Denise Murphy Retired and does not have a work-related email address.
Andrew R. Greenhill and Suparat Phuanukoonnon contributed equally to this work.
Audrey Michael deceased.
Additional files
Additional file 1: Table S1.
Overview of microscopy results for cases of suspected meningitis in children in Goroka, Papua New Guinea. In this study, unusually high rates of S. aureus positive CSF was detected. Microscopy results support the notion that S. aureus is likely a contaminant in the majority of samples from which it was isolated. (DOCX 15 kb)
Additional file 2: Table S2.
Prevalence of serogroups and serotypes of S. pneumoniae isolated from children with meningitis admitted to Goroka General Hospital. (DOCX 22 kb)
Additional file 3: Table S3.
Comparison of number of polymorphonucleocytes in CSF samples positive for S. aureus isolation versus recognised pathogens (S. pneumoniae and H. influenzae), other pathogens (non-pneumococcus, non-Hi), probable contaminants and samples from which no bacteria were isolated. There is no evidence of significance difference between PMN numbers in CSF with S. aureus compared to CSF with no pathogens isolated. Analysis conducted using a non-parametric independent samples median test. (DOCX 16 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Greenhill, A.R., Phuanukoonnon, S., Michael, A. et al. Streptococcus pneumoniae and Haemophilus influenzae in paediatric meningitis patients at Goroka General Hospital, Papua New Guinea: serotype distribution and antimicrobial susceptibility in the pre-vaccine era. BMC Infect Dis 15, 485 (2015). https://doi.org/10.1186/s12879-015-1197-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-015-1197-0