Abstract
Background
Among methods for preventing pneumonia and possibly also bacteremia in intensive care unit (ICU) patients, Selective Digestive Decontamination (SDD) appears most effective within randomized concurrent controlled trials (RCCT’s) although more recent trials have been cluster randomized. However, of the SDD components, whether protocolized parenteral antibiotic prophylaxis (PPAP) is required, and whether the topical antibiotic actually presents a contextual hazard, remain unresolved. The objective here is to compare the bacteremia rates and patterns of isolates in SDD-RCCT’s versus the broader evidence base.
Methods
Bacteremia incidence proportion data were extracted from component (control and intervention) groups decanted from studies investigating antibiotic (SDD) or non-antibiotic methods of VAP prevention and summarized using random effects meta-analysis of study and group level data. A reference category of groups derived from purely observational studies without any prevention method under study provided a benchmark incidence.
Results
Within SDD RCCTs, the mean bacteremia incidence among concurrent component groups not exposed to PPAP (27 control; 17.1%; 13.1-22.1% and 12 intervention groups; 16.2%; 9.1-27.3%) is double that of the benchmark bacteremia incidence derived from 39 benchmark groups (8.3; 6.8-10.2%) and also 20 control groups from studies of non-antibiotic methods (7.1%; 4.8 – 10.5). There is a selective increase in coagulase negative staphylococci (CNS) but not in Pseudomonas aeruginosa among bacteremia isolates within control groups of SDD-RCCT’s versus benchmark groups with data available.
Conclusions
The topical antibiotic component of SDD presents a major contextual hazard toward bacteremia against which the PPAP component partially mitigates.
Similar content being viewed by others
Background
Infections acquired by patients requiring a prolonged stay in the intensive care unit (ICU) have been studied extensively [1]-[112]. These are a leading cause of potentially preventable illness and death [113],[114]. In this patient group, the acquisition of colonizing bacteria is a key intermediary step toward the development of both bacteremia and ventilator associated pneumonia (VAP) [115],[116].
Among an extensive range of methods for the prevention of VAP in this patient group, Selective Digestive Decontamination (SDD) and Selective Oro-pharyngeal Decontamination (SOD) are of great interest for several reasons [117]-[121]. Firstly, as a counterfactual, the reduction in VAP incidence observed in 36 randomized concurrent controlled trials (RCCTs) of SDD is 65% [119] versus less than 40% for prevention methods that are non-antibiotic based. Second, SDD is postulated to have multi-site actions mediated against colonizing bacteria both at the oro-pharynx and gastro-intestinal tracts resulting in reductions in bacteremia as great as 27% [118],[120].
Thirdly, SDD is postulated to impart contextual effects mediated through cross colonization within the ICU. That SDD could influence the infection incidences beyond the intervention groups of concurrent design studies was postulated in the original 1984 SDD study [71] and others [60],[63] which as a consequence were either intentionally non-concurrent in design or more recently used cluster randomized design. Testing for the postulated SDD contextual effects on infection incidences is difficult due to the methodological and analytical challenges which cannot be adequately addressed within the confines of the typical single center RCCT.
Finally, SDD has been evaluated with at least five major variations in study design [121]; using either concurrent versus non-concurrent designs, with either or both of bacteremia and VAP as study end points, with different compositions of multi-component antibiotics in the SDD regimens, with SDD administered factorized as topical antibiotic prophylaxis alone or together with protocolized parenteral antibiotic prophylaxis (PPAP), with the PPAP component of SDD administered sometimes to the control groups in addition to the intervention groups (duplex studies), and with at risk ICU populations including varying proportions of trauma, medical and surgical patients under evaluation. This multiplicity of study designs creates a natural experiment in which the contextual effect of any group wide intervention, such as the factorized components of SDD might be inferred. This can be achieved through a calibration of bacteremia, or any other end point of interest, across component groups decanted from the various design types of these SDD studies versus component groups decanted from studies within the broader evidence base using methods analogous to those used in cluster randomized trials. In such a calibration, studies of non-antibiotic methods for the prevention of VAP are included to provide additional reference.
Such a calibration of the VAP end point among the component groups of 36 SDD studies with concurrent design reveals an SDD contextual hazard as follows; the VAP incidence is 14 percentage points higher among control groups [122] together with a selective increase in the proportion of Staphylococci [123] but not Pseudomonas aeruginosa [124] among the VAP isolates in both intervention and also control groups [123] of SDD-RCCTs versus observational study groups. Moreover these hazards are not seen among studies of non-antibiotic methods for the prevention of VAP.
Methods
Overview
The purpose of this analysis is to compare the bacteremia incidence and patterns of isolates in groups of ICU patients exposed directly or indirectly (contextually) to the topical and or parenteral components of SDD within RCCT’s versus component groups from studies within the broader evidence base that relates to the ICU patient group at risk of bacteremia and VAP. Of interest are comparisons of bacteremia not only versus other study designs of SDD but also versus studies of other interventions used to prevent infection in ICU patients in which the bacteremia incidence has been measured. Of secondary interest are comparisons of the VAP end point among these studies and the effect sizes of the various interventions that were under study against the two end points.
Study selection
The seven steps in the selection of studies and subsequent decanting of component groups and the plan of analysis is as depicted in Figure 1. These steps are detailed as follows;
-
1.
An electronic search of PubMed, The Cochrane database and Google Scholar for systematic reviews containing potentially eligible studies was undertaken using the following search terms; “ventilator associated pneumonia”, “mechanical ventilation”, “intensive care unit”, “blood stream infection”, “bacteremia”, “meta-analysis” and “systematic review” up to December 2013.
-
2.
Systematic reviews of studies of patient populations requiring prolonged (>48 hours) ICU admission were then streamed into one of three categories; systematic reviews containing studies in which there was no intervention, a non-antibiotic based intervention, or SDD as an antibiotic based intervention for the prevention of VAP. For the purpose of this study, SDD was factorized into protocolized topical and protocolized parenteral antibiotic prophylaxis (PPAP) components. An SDD study is defined here as the use of protocolized topical antibiotic prophylaxis applied by the gastric or oro-pharyngeal route in the intervention group with or without the additional use of PPAP.
-
3.
The studies were screened against the following eligibility criteria. Inclusion criteria; Bacteremia incidence data for all bacteremias totalled and extractable as an incidence proportion per patient. Exclusion criteria; studies limited to patients with the acute respiratory distress syndrome. Studies in a language other than English were included when the required data had been abstracted in an English language systematic review.
-
4.
A hand search was undertaken for additional studies meeting the eligibility criteria.
-
5.
All eligible studies were then collated and any duplicate studies were removed. Ineligible studies that were not evaluable for the bacteremia end point but evaluable within a sensitivity analysis or for bacteremia isolate data were identified.
-
6.
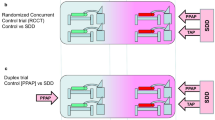
Groups of patients receiving mechanical ventilation from studies without a VAP prevention method under study were labelled as observational groups (Figure 2). The studies of intervention studies were classified as follows. Among the non-antibiotic based methods of VAP prevention are studies with interventions delivered at either the gastric site or the airway or oral sites. The SDD studies were further sub-classified (Figure 3); firstly as to whether the control group was concurrent and co-located within the same ICU as the intervention group (RCCT; Figure 3b) or not (non-concurrent; Figure 3a); and secondly on the basis of the additional use or not of PPAP in either the intervention group or the control group. Studies that used PPAP in the control group are referred to as duplex studies (Figure 3c).
Schematics of SDD study designs with intervention and control groups being either non-concurrent (a) or concurrent (b & c) and with intervention groups receiving prophylaxis with either or both protocolized parenteral and topical antibiotics (dual colour stripes; a, b & c) and with control group patients receiving the protocolized parenteral antibiotic prophylaxis component alone (RCCT-duplex studies; dual colour stripes; c); or not (monochrome; a & b). Note the non-concurrent control and intervention patient groups were separated by a physical or temporal barrier (a).
-
7.
The component groups were decanted from each study as follows;
The control and intervention groups from non-antibiotic based methods were classified as indicated in the original study
All groups that received topical antibiotic prophylaxis with or without PPAP were designated as an SDD intervention group and all other groups from SDD studies are classified as a control group regardless of whether or not they may have received PPAP (duplex studies).
Search method (numbered arrow 1) and streaming (arrow 2) of systematic reviews, screening (arrow 3, 4 & 5) and classification (arrow 6) of eligible studies, and decant and analytic plan (arrow 7) of component groups being control (rectangles) and intervention (ovals) groups from studies of VAP prevention methods and a reference category of observation (diamond) groups from cohorts of ICU patients without a pneumonia prevention method under study. Dotted rectangles and ovals represent component groups within studies of antibiotic based methods of VAP prevention (SDD) which received protocolized parenteral antibiotic prophylaxis. Analytic plan; the vertical dotted lines connecting the component groups represent the group contrasts used towards the calculation of the counterfactual effects and the horizontal dotted rectangles represent the calibrations used toward the estimation of contextual effects among the component groups referent to the observation groups. Note; the total numbers do not tally as some systematic reviews provided studies in more than one category and some studies provided groups in more than one category.
Data extraction
The primary outcome here is the bacteremia incidence proportion per 100 patients (B-IP) for each identified component group. Any studies with bacteremia incidences expressed only as a number of episodes or on a per-day basis were non evaluable for the primary outcome. Studies that reported bacteremia incidence proportion using a composite total of specific bacteremia sub-types were analysed here within a sensitivity analysis only. Coagulase negative Staphylococci (CNS) are common among bacteremia isolates of patients receiving SDD. Hence the defining criteria for bacteremia for each study was determined in relation to whether or not the CDC criteria for a Coagulase negative Staphylococcal isolate being detected in two or more separate blood cultures was specified [125].
For many SDD studies, but not all, the primary end point was VAP occurrence whereas bacteremia was a secondary end point. Hence for this analysis, the VAP incidence proportion per 100 patients (VAP-IP) was also extracted and analysed in parallel to enable an assessment of comparability between the end points of SDD studies selected here which had B-IP data available versus the broader evidence base for which B-IP data was not available.
The following bacteremia isolate data was extracted; numbers of coagulase negative Staphylococci (CNS); numbers of Pseudomonas aeruginosa isolates and total numbers of bacteraemia isolates.
Statistical analysis
The bacteremia data were logit transformed for analysis as previously [122]; with the total number of patients as the denominator (D), the number of patients with bacteremia as the numerator (N), and R being the B-IP proportion (N/D), the logit(bacteremia-IP) is log(N/(D-N)) and its variance is 1/(D*R*(1-R)) [126]. Using these pre-calculated logits and logit variances, group specific 95% confidence intervals, summary logits and the associated summary 95% CIs were generated using the ‘metan’ command in STATA (release 12.0, STATA Corp., College Station, TX, USA) [127],[128].
For each category of component group the summary mean logit B–IP and associated 95% confidence interval were calculated using random effects methods. These were then back-transformed to the percentage scale. On the logit scale the 95% confidence intervals for a proportion are symmetrical and remain within the interval of 0 to 100%. The summary mean B–IP derived from the observational studies is the benchmark. The caterpillar plots have the studies ranked in order of increasing B-IP in relation to the benchmark.
VAP-IP data per total number of patients and the bacteremia isolate data per total number of isolates were likewise logit transformed to enable the analysis of these data.
A conventional meta-analysis of effect of study intervention was undertaken to derive study specific and summary measures using random effects meta-analysis methods and expressed as an odds ratio and displayed in forest plots. These summary measures derived from the category of non-antibiotic methods include a broad category of heterogenous interventions and the summary measures derived here are merely indicative. Caterpillar plots are forest plots which have been transformed by the ranking of studies in order of increasing study or group specific effect size.
Contextual effects of control and intervention group membership were each estimated in separate models versus the benchmark groups as the reference category using random effects meta-regression of group level logit transformed data. The use of CDC defining criteria for bacteremia and use of SDD factorized as topical and or parenteral antibiotic use and were entered as group level variables.
Sensitivity analyses
Three sensitivity tests were undertaken to test the robustness of the findings here as follows; to the inclusion of additional data from studies that might be unpublished or missing; to the exclusion of groups from studies not cited in systematic reviews; and also to the inclusion of study results from two recent large cluster randomized studies of SDD and SOD [60],[63]. In sensitivity test one, the control groups from studies of non-antibiotic methods were used as a source of simulated ‘missing’ data in the recalculation of mean B-IP. In sensitivity test two, the meta-regression model for observational and control groups was repeated with restriction to only those component group obtained from studies sourced exclusively from systematic reviews. In sensitivity test three, the meta-regression model for observational and intervention groups was repeated including the bacteremia incidence data for intervention groups obtained from the two recent large cluster randomized studies of SDD and SOD. For the purpose of sensitivity test three the non-standard bacteremia definition as reported in these two studies was included in the meta-regression model after transformation to the logit scale but otherwise without adjustment.
Results
Description of studies
Of the 112 studies (Figure 1; Additional file 1: Tables S1-S5), 71 were sourced from at least one of 20 systematic reviews and 41 were sourced from elsewhere. Six were not evaluable for bacteremia incidence; two reported incidence for only specific bacteremia sub-types [64],[74], two reported an incidence of several specific bacteremia sub-types as a composite [60],[63] and two reported numbers of bacteremia episodes [69],[86]. The data from these six studies was analysed either only for bacteremia isolate data or only within sensitivity test three.
Of the 106 remaining studies, the CDC defining criteria for bacteremia were used in 49 studies. Following the decant of groups from the studies, there were 39 observational groups, 69 control and 77 intervention groups (Table 1). 13 studies either had a second control group or a second intervention group. Most studies were published in the 1990s. The SDD studies tended to be smaller in size and all but three were of European origin. There were 25 different antibiotic regimens under study in the 55 SDD intervention groups. The study groups were classified according to the type of study design in which they were located (Figures 2 & 3). All of the studies of non-antibiotic methods were concurrent in design. There were 13 control groups (duplex studies) and 40 intervention groups in which all patients received protocolized parenteral antibiotic prophylaxis (PPAP).
VAP-IP data was available for 158 groups (Additional files 1 and 2: Figures S1-4) and bacteremia-IP data was available for 186 groups (Additional file 1; Figures 4, 5, 6 and 7).
Among all SDD studies with a concurrent design, the effect size expressed as an odds ratio for the difference for VAP incidences between control and intervention groups was 0.36 (0.31 – 0.42; n = 35) and for bacteremia incidences was 0.69 (0.59 – 0.81; n = 41). Among all ten SDD studies with a non-concurrent design, the effect size expressed as an odds ratio for the difference for VAP incidences between control and intervention groups was 0.50 (0.41 – 0.61) for bacteremia incidences was 0.75 (0.51– 0.99), respectively (Forest plots showing summary and study specific effect sizes are displayed in Additional file 3).
The bacteremia incidence benchmark was 8.3 (6.8-10.2) (Table 1; Figure 4). The mean bacteremia-IP for each of the four categories of component group from studies of non-antibiotic methods were within 4 percentage points of the bacteremia incidence benchmark (Table 1; Figure 5). Among the 8 categories of component group from the SDD studies, the mean bacteremia incidence among the four that received PPAP (three intervention and one control) were all also within 4 percentage points of the bacteremia incidence benchmark whereas three categories that did not receive PPAP (two intervention and one control groups) were > 4 percentage points greater than the benchmark (Table 1; Figures 6 and 7).
Caterpillar plots of the group specific (small diamonds) and summary (large open diamond, vertical line) bacteremia incidence proportion (B-IP) and 95% CI of observational groups of observational studies (Benchmark groups). Studies are listed in Additional file 1: Table S1. Note that the x axis is a logit scale.
Caterpillar plots of the group specific (small diamonds) and summary (large open diamond) B-IP and 95% CI of control and intervention groups from studies of VAP prevention using non-antibiotic methods. For comparison, the summary B-IP (vertical line) derived from the benchmark groups from Figure 4 is shown. Studies are listed in Additional file 1: Table S2. Note that the x axis is a logit scale.
Caterpillar plots of the group specific (small diamonds) and summary (large open diamond) B-IP and 95% CI of control groups of studies of VAP prevention using SDD. Duplex study control groups received protocolized parenteral antibiotic prophylaxis. For comparison, the summary B-IP (vertical line) derived from the benchmark groups from Figure 4 are shown. Studies are listed in Additional file 1: Tables S3, S4 and S5. Note that the x axis is a logit scale.
Caterpillar plots of the group specific (small diamonds) and summary (large open diamond) B-IP and 95% CI of intervention groups of studies of VAP prevention using SDD. For comparison, the summary B-IP (vertical line) derived from the benchmark groups from Figure 4 are shown. Studies are listed in Additional file 1: Tables S3, S4 and S5. Note that the x axis is a logit scale.
As a sensitivity test to groups from potentially unpublished or missing studies, the mean bacteremia incidence was re-calculated including all 46 concurrent control groups not receiving PPAP (i.e. the 20 control groups from studies of non-antibiotic based methods together with the 27 control groups from SDD studies not using PPAP) and this remains >1.5 percentage points greater than the upper 95% confidence limit of the mean B-IP of the benchmark (sensitivity test 1; Table 1, footnote n).
Meta-regression models of logit B-IP
The effect of membership of the various categories of component group together with the effect of exposure to PPAP were examined in meta-regression models of logit B-IP separately for control and for intervention groups (Table 2). The effects of membership of either a control or an intervention group of an SDD RCCT were each significant, positive and similar in magnitude to the negative effect of exposure to PPAP on bacteremia incidence. The influences of all other factors in each model were non-significant.
Repeating the meta-regression model for observational and control groups with restriction to only those component group obtained from studies sourced exclusively from systematic reviews gave a coefficient that remained significant and positive (sensitivity test two; Table 2, footnote c).
The two large cluster randomized studies of SDD and SOD that had used a non-standard bacteremia end point, being the composite total of specific bacteremia sub-types, contained 1990 control group and 14940 intervention group patients [60],[63] versus the 4575 control group and 5238 intervention group patients from the 54 studies of SDD that had used a standard bacteremia end point, being incidence proportion totalled for all bacteremias. The meta-regression model for observational and intervention groups including unadjusted non-standard bacteremia end point data from the intervention groups from these two recent large cluster randomized studies of SDD and SOD was repeated. With the bacteremia data for these groups included in the model, the coefficient remained non-significant and positive (sensitivity test three; Table 2, footnote e)
Bacteremia isolates
The proportion of CNS (Figure 8) and Pseudomonas aeruginosa (Figure 9) among bacteremia isolates was examined among groups from 15 benchmark and seven SDD-RCCT studies reporting bacteremia using the CDC criteria here (Table 1, Additional file 1: Tables S6). The proportion of CNS isolates among the control (p = 0.027) groups of the SDD-RCCT studies is double that versus the benchmark groups (Figure 8). By contrast, the proportion of Pseudomonas aeruginosa among the bacteremia isolates among the control groups of SDD-RCCT studies is similar to that of the benchmark groups (Figure 9; p = 0.64).
Caterpillar plots of the group specific (small diamonds) and summary (large open diamond) coagulase negative Staphylococcus (CNS) as an isolate proportion (CNS-IP) and 95% CI of component groups of studies of VAP prevention using SDD and non-antibiotic methods. The summary CNS-IP derived from the benchmark groups at the top of the figure is shown (vertical line). Note that the x axis is a logit scale. Studies are listed in Additional file 1: Tables S6.
Caterpillar plots of the group specific (small diamonds) and summary (large open diamond) Pseudomonas aeruginosa as an isolate proportion (Ps-IP) and 95% CI of component groups of studies of VAP prevention using SDD and non-antibiotic methods. The summary Ps-IP derived from the benchmark groups at the top of the figure is shown (vertical line). Note that the x axis is a logit scale. Studies are listed in Additional file 1: Tables S6.
Discussion
The effect of SDD on bacteremia is of great interest for five reasons. Bacteremia acquired by patients in the intensive care unit is associated with a high attributable mortality, especially so in patients either receiving mechanical ventilation [129] or who have pneumonia as the source of the bacteremia [129],[130].
Secondly, the defining criteria for bacteremia are less diverse than is the case for VAP. Hence bacteremia serves as a more stable study end point than is VAP toward estimating contextual effects [131]. Thirdly, SDD has complex ecological effects on colonization within the ICU [132] and clarifying the nature, direction and extent of these effects are crucial in defining the role of SDD going forward. Fourth, the numbers of patients assessed for a bacteremia end point in the SDD and SOD evidence has recently nearly doubled with the publication of two large cluster randomized studies [60],[63].
Finally, the effect of SDD on bacteremia within the ICU patient population is unclear and the evidence is conflicting. On the one hand, the evidence for protection against bacteremia [118],[120], as with protection against VAP, appears compelling [119],[120]. Among the 35 SDD RCCTs studies here, SDD appears to reduce bacteremia incidence by up to 31% and VAP by up to 64% [see Additional file 3: Figures S9 and S10]. Indeed the summary ORs derived elsewhere for SDD on both bacteremia among 31 studies [118] and pneumonia among 36 studies [119], are respectively similar to the counterfactual effect for each derived here among the SDD-RCCTs (see Additional file 3).
On the other hand, protection against bacteremia is unequal among the different types of SDD-RCCT’s. It is most apparent among the 18 SDD-RCCT’s for which the intervention groups received both topical and PPAP components of SDD. However, the protection appears marginal and non-significant among the remaining SDD-RCCT’s for which either the intervention groups received only the topical component of SDD and not the PPAP component or among the SDD-RCCT’s for which the control groups received the PPAP component (duplex studies) (Additional file 3: Figure S10).
Moreover, protection against bacteremia is not apparent in nationwide surveys. For example among 19 ICUs of Dutch hospitals the bacteremia rates are 5 versus 4 per 100 patient days for ICUs using versus not using SDD respectively [133]. Likewise, among >280,000 admission to 203 ICUs in the UK reporting data to the Intensive care National Audit and research center, unit acquired bacteremia occurred in 2.7 versus 2.8 percent of ICU admissions for nine ICUs that were using SDD versus 196 that were not [134]. Curiously, the nine ICUs using SDD includes three that were using SDD with a PPAP component for which the bacteremia rates was 0.1%. The bacteremia rate amongst the other six ICUs that were using SDD without a PPAP component is unknown but presumably higher than 2.7%.
The benchmark for bacteremia incidence derived here is 8.3%. The upward dispersion in bacteremia incidence among component groups from SDD RCCTs away from this benchmark is striking with all but 2 of the 27 control groups and all but 2 of 12 SDD intervention groups that did not receive PPAP being above this benchmark. This upward dispersion is apparent in the meta-regression models as positive coefficients in association with membership of either control or intervention groups of SDD studies versus the significant negative coefficient associated with exposure to the factorized PPAP component of SDD (Table 2).
The results here are in contrast with two cluster randomized trials of SDD and selective oropharyngeal decontamination (SOD) among up to 16 Dutch ICUs reported by de Smet [60] and Oostdijk [63]. However, there are two critical design aspects of these two studies which render comparisons with the findings of other studies difficult. Firstly, the VAP incidence was not reported. Secondly, a composite bacteremia end point including only five bacteremia sub-types not including Coagulase negative Staphylococci (CNS) was reported for these studies [60],[63]. As a consequence, neither the bacteremia incidence nor the counterfactual effect of SDD on bacteremia as conventionally defined is known for these studies. The inclusion of the bacteremia incidence data as reported from these two studies in a sensitivity analysis fails to change the findings here (sensitivity test three). Of note the incidence per 100 patients of bacteremia sub-types not including CNS was > 9 for the one control group and > 6 for four of eight SDD intervention groups for this [60],[63] and two other studies ([64],[74], Additional file 1: Table S3) that were otherwise not evaluable for the analysis of B-IP as a consequence of using non-standard bacteremia end-points.
Coagulase negative Staphylococci (CNS) typically account for 16%-25% of episodes of bacteremia using the CDC bacteremia definitions in series of ICU patients not receiving SDD [Additional file 3 Figure S5]. CNS bacteremia is not without risk for increased mortality and length of stay [114],[115],[135],[136]. CNS are common bacteremia isolates in this patient population but moreso among SDD recipients due to the selective effect of the SDD antibiotics. For example, among SDD recipients, 7 of 16 bacteremias in a Dutch series of 46 patients with sepsis syndrome [137], 52 of 108 bacteremia episodes in a Swiss ICU [76], 9 of 23 episodes in an Italian ICU [69], 54 of 115 episodes in a Viennese haematological ICU [138] and 9 of 26 episodes in another Dutch ICU [139] were CNS.
The finding of a higher proportion of CNS but not Pseudomonas among the bacteremia isolates of control groups is difficult to explain other than by a contextual effect associated with and arising out of the SDD intervention groups receiving the topical antibiotic component of SDD within studies of SDD which is inapparent at the level of each individual study [140].
There are several limitations of this analysis.
Only 64 of the 206 studies in the broader evidence base [122] from which these studies were derived had evaluable bacteremia incidence data available. However, the SDD summary counterfactual effects of the various interventions against both the VAP and bacteremia end points derived for the studies included here are similar to those derived elsewhere for a broader panel of studies [118]-[120]. Moreover, the summary VAP incidences here are also comparable to those in the larger panel. Also, the findings for bacteremia incidence here remain apparent in an analysis limited to studies extracted exclusively from systematic reviews (sensitivity test two).
The lack of observer blinding in some studies needs to be considered. Knowledge of treatment allocation may have influenced the taking of blood cultures to document bacteremia. Moreover, the empiric use of (non-protocolized) parenteral antibiotic therapy in each study is an important unknown as non-use may account for vulnerability at the individual level and contribute to the SDD contextual effect in the ICU at the group level in each study.
This contextual analysis is observational and is undertaken at the group level rather than the patient level. It was not possible to study the impact of unmeasured and unknown patient level risk factors for B-IP. However, the magnitude of such a putative patient level risk factor as a promoter of bacteremia incidence would need to be stronger than is magnitude of the group wide use of PPAP as a protector toward reducing bacteremia incidence (Table 2) and consistently so across all the studies and yet also be profoundly unevenly distributed, predominating in the groups of SDD RCCTs versus other groups within the broader evidence base to be able to account for the discrepancies noted here. Alternatively, there could be unpublished or missing SDD studies with control groups having a B-IP in the range of the studies of non-antibiotic methods to account for the discrepancies noted here (Table 1). As a sensitivity analysis there would need to be >20 of such studies with component groups with B-IP in the range of those seen among control groups of studies of non-antibiotic methods to rectify this discrepancy (sensitivity test one).
Conclusions
Within SDD RCCTs, the mean bacteremia incidence among concurrent component groups not exposed to PPAP is double that of the benchmark bacteremia incidence. These observations are paradoxical, as with similar observations for VAP incidences among these studies [141]. Apart from major publication bias, or the effect of major and as yet unidentified and mal-distributed patient level risk factors for both VAP and bacteremia, these profound discrepancies indicate a major contextual hazard associated with the topical component of SDD on bacteremia within RCCT’s against which protocolized parenteral antibiotic partially mitigates. The safety of SDD within the ICU environment remains a concern and inapparent outbreaks remain a possible explanation for these observations within the SDD studies [140].
Key messages
While SDD appears highly effective for infection prevention within the mechanically ventilated patient group, several paradoxical findings for the incidence and microbiology of the pneumonia end point among the SDD-RCCT studies imply a contextual hazard.
A bacteremia-IP benchmark derived from 39 non-intervention groups of mechanically ventilated patients is 8.3%.
Among SDD-RCCT studies, the mean bacteremia-IP for 27 control and 12 intervention groups that did not received protocolized parenteral antibiotic prophylaxis are each double the bacteremia-IP benchmark, respectively.
In meta-regression models, the magnitude and statistical significance of the positive effect associated with membership of either a control or an intervention group of an SDD-RCCT study on bacteremia-IP is similar to the magnitude of the negative effect associated with protocolized parenteral antibiotic prophylaxis.
These and other paradoxical discrepancies indicate a major contextual hazard associated with the topical component of SDD against which protocolized parenteral antibiotic partially mitigates.
Author’s contributions
As sole author, JH produced the design of the study, performed the statistical analysis and wrote the manuscript. JH read and approved the final manuscript.
Additional files
Abbreviations
- ICU:
-
Intensive care unit
- MV:
-
Mechanical ventilation
- SDD:
-
Selective digestive decontamination
- SOD:
-
Selective oro-pharyngeal decontamination
- VAP:
-
Ventilator associated pneumonia
- VAP-IP:
-
Ventilator associated pneumonia incidence proportion
- B-IP:
-
Bacteremia incidence proportion
- PPAP:
-
Protocolized parenteral antibiotic prophylaxis
References
Apostolopoulou E, Bakakos P, Katostaras T, Gregorakos L: Incidence and risk factors for ventilator-associated pneumonia in 4 multidisciplinary intensive care units in Athens, Greece. Respir Care. 2003, 48: 681-688.
Berg DE, Hershow RC, Ramirez CA, Weinstein RA: Control of nosocomial infections in an intensive care unit in Guatemala City. Clin Infect Dis. 1995, 21: 588-593.
Bonten MJ, Froon AH, Gaillard CA, Greve JWM, de Leeuw PW, Drent M, Buurman WA: The systemic inflammatory response in the development of ventilator-associated pneumonia. Am J Respir Crit Care Med. 1997, 156: 1105-1113.
Cade JF, McOwat E, Siganporia R, Keighley C, Presneill J, Sinickas V: Uncertain relevance of gastric colonization in the seriously ill. Intensive Care Med. 1992, 18: 210-7.
Craven DE, Kunches LM, Lichtenberg DA, Kollisch NR, Barry MA, Heeren TC, McCabe WR: Nosocomial infection and fatality in medical and surgical intensive care unit patients. Arch Intern Med. 1988, 148: 1161-8.
El-Masri MM, Hammad TA, McLeskey SW, Joshi M, Korniewicz DM: Predictors of nosocomial bloodstream infections among critically ill adult trauma patients. Infect Control Hosp Epidemiol. 2004, 25: 656-663.
Fagon JY, Chastre J, Domart Y, Trouillet JL, Pierre J, Darne C, Gibert C: Nosocomial pneumonia in patients receiving continuous mechanical ventilation. Prospective analysis of 52 episodes with use of a protected specimen brush and quantitative culture techniques. Am Rev Respir Dis. 1989, 139: 877-884.
Fagon JY, Chastre J, Vuagnat A, Trouillet JL, Novara A, Gibert C: Nosocomial pneumonia and mortality among patients in intensive care units. JAMA. 1996, 275: 866-869.
Fieselmann JF, Bock MJ, Hendryx MS, Wakefield D, Helms CM, Bentler SE: Mechanical ventilation in rural ICUs. Crit Care. 1999, 3: 23-31.
Garrouste-Orgeas M, Chevret S, Mainardi JL, Timsit JF, Misset B, Carlet J: A one-year prospective study of nosocomial bacteraemia in ICU and non-ICU patients and its impact on patient outcome. J Hosp Infect. 2000, 44: 206-213.
Garrouste-Orgeas M, Timsit JF, Tafflet M, Misset B, Zahar JR, Soufir L, Carlet J: Excess risk of death from intensive care unit—acquired nosocomial bloodstream infections: a reappraisal. Clin Infect Dis. 2006, 42: 1118-1126.
George DL, Falk PS, Wunderink RG, Leeper KV, Meduri GU, Steere EL, Glen Mayhall C: Epidemiology of ventilator-acquired pneumonia based on protected bronchoscopic sampling. Am J Respir Crit Care Med. 1998, 158: 1839-1847.
Giard M, Lepape A, Allaouchiche B, Guerin C, Lehot JJ, Robert MO, Vanhems P: Early-and late-onset ventilator-associated pneumonia acquired in the intensive care unit: comparison of risk factors. J Crit Care. 2008, 23: 27-33.
Girou E, Schortgen F, Delclaux C, Brun-Buisson C, Blot F, Lefort Y, Brochard L: Association of noninvasive ventilation with nosocomial infections and survival in critically ill patients. JAMA. 2000, 284: 2361-2367.
Guimaraes MM, Rocco JR: Prevalence of ventilator-associated pneumonia in a university hospital and prognosis for the patients affected. J Bras Pneumol. 2006, 32: 339-346.
Hébert PC, Wells G, Blajchman MA, Marshall J, Martin C, Pagliarello G, Yetisir E: A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. New Engl J Med. 1999, 340: 409-417.
Hugonnet S, Eggimann P, Borst F, Maricot P, Chevrolet JC, Pittet D: Impact of ventilator-associated pneumonia on resource utilization and patient outcome. Infect Control Hosp Epidemiol. 2004, 25: 1090-6.
Ibrahim EH, Ward S, Sherman G, Kollef MH: A comparative analysis of patients with early-onset vs late-onset nosocomial pneumonia in the ICU setting. Chest. 2000, 117: 1434-1442.
Jacobs S, Chang RW, Lee B, Bartlett FW: Continuous enteral feeding: a major cause of pneumonia among ventilated intensive care unit patients. JPEN J Parenter Enteral Nutr. 1990, 14: 353-6.
Kollef MH, Vlasnik J, Sharpless L, Pasque C, Murphy D, Fraser V: Scheduled change of antibiotic classes A strategy to decrease the incidence of ventilator-associated pneumonia. Am J Respir Crit Care Med. 1997, 156: 1040-1048.
Kunac A, Sifri ZC, Mohr AM, Horng H, Lavery RF, Livingston DH: Bacteremia and ventilator-associated pneumonia: a marker for contemporaneous extra-pulmonic infection. Surg Infect. 2014, 15: 77-83.
Laupland KB, Zygun DA, Davies HD, Church DL, Louie TJ, Doig CJ: Population-based assessment of intensive care unit-acquired bloodstream infections in adults: incidence, risk factors, and associated mortality rate. Crit Care Med. 2002, 30: 2462-2467.
Laupland KB, Kirkpatrick AW, Church DL, Ross T, Gregson DB: Intensive-care-unit-acquired bloodstream infections in a regional critically ill population. J Hosp Infect. 2004, 58 (2): 137-145.
Magnason S, Kristinsson KG, Stefansson T, Erlendsdottir H, Jonsdottir K, Kristjansson M, Gudmundsson S: Risk factors and outcome in ICU‐acquired infections. Acta Anaesthesiol Scand. 2008, 52: 1238-1245.
Nourdine K, Combes P, Carton MJ, Beuret P, Cannamela A, Ducreux JC: Does noninvasive ventilation reduce the ICU nosocomial infection risk? A prospective clinical survey. Intensive Care Med. 1999, 25: 567-573.
Osmon S, Warren D, Seiler SM, Shannon W, Fraser VJ, Kollef MH: The influence of infection on hospital mortality for patients requiring >48 h of intensive care. Chest. 2003, 124: 1021-1029.
Potgieter PD, Linton DM, Oliver S, Forder AA: Nosocomial infections in a respiratory intensive care unit. Crit Care Med. 1987, 15: 495-498.
Rello J, Ricart M, Mirelis B, Quintana E, Gurgui M, Net A, Prats G: Nosocomial bacteremia in a medical-surgical intensive care unit: epidemiologic characteristics and factors influencing mortality in 111 episodes. Intensive Care Med. 1994, 20: 94-98.
Reusser P, Zimmerli W, Scheidegger D, Marbet GA, Buser M, Gyr K: Role of gastric colonization in nosocomial infections and endotoxemia: a prospective study in neurosurgical patients on mechanical ventilation. J Infect Dis. 1989, 160: 414-421.
Salata RA, Lederman MM, Shlaes DM, Jacobs MR, Eckstein E, Tweardy D, Toossi Z, Chmielewski R, Marino J, King CH: Diagnosis of nosocomial pneumonia in intubated, intensive care unit patients. Am Rev Respir Dis. 1987, 135: 426-432.
Shorr AF, Duh MS, Kelly KM, Kollef MH: Red blood cell transfusion and ventilator-associated pneumonia: a potential link?. Crit Care Med. 2004, 32: 666-674.
Shorr AF, Jackson WL, Kelly KM, Fu M, Kollef MH: Transfusion practice and blood stream infections in critically ill patients. Chest. 2005, 127: 1722-1728.
Sofianou DC, Constandinidis TC, Yannacou M, Anastasiou H, Sofianos E: Analysis of risk factors for ventilator-associated pneumonia in a multidisciplinary intensive care unit. Eur J Clin Microbiol Infect Dis. 2000, 19: 460-463.
Torres A, Aznar R, Gatell JM, Jimenez P, Gonzalez J, Ferrer A, Celis R, Rodriguez-Roisin R: Incidence, risk, and prognosis factors of nosocomial pneumonia in mechanically ventilated patients. Am Rev Respir Dis. 1990, 142: 523-528.
Urli T, Perone G, Acquarolo A, Zappa S, Antonini B, Ciani A: Surveillance of infections acquired in intensive care: usefulness in clinical practice. J Hosp Infect. 2002, 52: 130-5.
Violan JS, Sanchez-Ramirez C, Mujica AP, Cendrero JC, Fernandez JA, de Castro FR: Impact of nosocomial pneumonia on the outcome of mechanically-ventilated patients. Crit Care (Lond). 1998, 2: 19-23.
Acosta-Escribano J, Fernández-Vivas M, Carmona TG, Caturla-Such J, Garcia-Martinez M, Menendez-Mainer A, Sanchez-Payá J: Gastric versus transpyloric feeding in severe traumatic brain injury: a prospective, randomized trial. Intensive Care Med. 2010, 36: 1532-1539.
Barraud D, Blard C, Hein F, Marçon O, Cravoisy A, Nace L, Alla F, Bollaert PE, Gibot S: Probiotics in the critically ill patient: a double blind, randomized, placebo-controlled trial. Intensive Care Med. 2010, 36: 1540-1547.
Bonten MJ, Gaillard CA, Van der Geest S, Van Tiel FH, Beysens AJ, Smeets HG, Stobberingh EE: The role of intragastric acidity and stress ulcus prophylaxis on colonization and infection in mechanically ventilated ICU patients. A stratified, randomized, double-blind study of sucralfate versus antacids. Am J Respir Crit Care Med. 1995, 152: 1825-1834.
Doig GS, Simpson F, Sweetman EA, Finfer SR, Cooper DJ, Heighes PT, Peake S: Early parenteral nutrition in critically ill patients with short-term relative contraindications to early enteral nutrition: a randomized controlled trial. JAMA. 2013, 309: 2130-2138.
Harvey SE, Parrott F, Harrison DA, Bear DE, Segaran E, Beale R, Bellingan G, Leonard R, Mythen MG, Rowan KM: Trial of the route of early nutritional support in critically ill adults. N Engl J Med. 2014, 371: 1673-1684.
Hsu CW, Sun SF, Lin SL, Kang SP, Chu KA, Lin CH, Huang HH: Duodenal versus gastric feeding in medical intensive care unit patients: a prospective, randomized, clinical study. Crit Care Med. 2009, 37: 1866-1872.
Ibrahim EH, Mehringer L, Prentice D, Sherman G, Schaiff R, Fraser V, Kollef MH: Early versus late enteral feeding of mechanically ventilated patients: results of a clinical trial. JPEN J Parenter Enteral Nutr. 2002, 26: 174-181.
Kearns PJ, Chin D, Mueller L, Wallace K, Jensen WA, Kirsch CM: The incidence of ventilator-associated pneumonia and success in nutrient delivery with gastric versus small intestinal feeding: a randomized clinical trial. Crit Care Med. 2000, 28: 1742-1746.
Laggner AN, Lenz K, Base W, Druml W, Schneeweiss B, Grimm G: Prevention of upper gastrointestinal bleeding in long-term ventilated patients. Sucralfate versus ranitidine. Am J Med. 1989, 86: 81-4.
Montecalvo MA, Steger KA, Farber HW: Nutritional outcome and pneumonia in critical care patients randomized to gastric versus jejunal tube feedings. The Critical Care Research Team Crit Care Med. 1992, 20: 1377-1387.
Oudhuis GJ, Bergmans DC, Dormans T, Zwaveling JH, Kessels A, Prins MH, Verbon A: Probiotics versus antibiotic decontamination of the digestive tract: infection and mortality. Intensive Care Med. 2011, 37: 110-117.
Reignier J, Mercier E, Le Gouge A, Boulain T, Desachy A, Bellec F, Lascarrou JB: Effect of not monitoring residual gastric volume on risk of ventilator-associated pneumonia in adults receiving mechanical ventilation and early enteral feeding. A randomized controlled trial. JAMA. 2013, 309: 249-256.
Spindler-Vesel A, Bengmark S, Vovk I, Cerovic O, Kompan L: Synbiotics, prebiotics, glutamine, or peptide in early enteral nutrition: a randomized study in trauma patients. J Parenter Enteral Nutr. 2007, 31: 119-126.
Camus C, Bellissant E, Sebille V, Perrotin D, Garo B, Legras A, Renault A, Le Corre P, Donnio PY, Gacouin A: Prevention of acquired infections in intubated patients with the combination of two decontamination regimens. Crit Care Med. 2005, 33: 307-314.
DeRiso AJ, Ladowski JS, Dillon TA, Justice JW, Peterson AC: Chlorhexidine gluconate 0.12% oral rinse reduces the incidence of total nosocomial respiratory infection and nonprophylactic systemic antibiotic use in patients undergoing heart surgery. Chest. 1996, 109: 1556-1561.
Fourrier F, Cau-Pottier E, Boutigny H, Roussel-Delvallez M, Jourdain M, Chopin C: Effects of dental plaque antiseptic decontamination on bacterial colonization and nosocomial infections in critically ill patients. Intensive Care Med. 2000, 26: 1239-1247.
Fourrier F, Dubois D, Pronnier P, Herbecq P, Leroy O, Desmettre T, Roussel-Delvallez M: Effect of gingival and dental plaque antiseptic decontamination on nosocomial infections acquired in the intensive care unit a double-blind placebo-controlled multicenter study. Crit Care Med. 2005, 33: 1728-1735.
Holzapfel L, Chevret S, Madinier G, Ohen F, Demingeon G, Coupry A, Chaudet M: Influence of long-term oro- or nasotracheal intubation on nosocomial maxillary sinusitis and pneumonia: results of a prospective, randomized, clinical trial. Crit Care Med. 1993, 21: 1132-1138.
Holzapfel L, Chastang C, Demingeon G, Bohe J, Piralla B, Coupry A: A randomized study assessing the systematic search for maxillary sinusitis in nasotracheally mechanically ventilated patients. Influence of nosocomial maxillary sinusitis on the occurrence of ventilator-associated pneumonia. Am J Respir Crit Care Med. 1999, 159: 695-701.
Segers P, Speekenbrink RG, Ubbink DT, van Ogtrop ML, Bas A: Prevention of nosocomial infection in cardiac surgery by decontamination of the nasopharynx and oropharynx with chlorhexidine gluconate. JAMA. 2006, 296: 2460-2466.
Seguin P, Tanguy M, Laviolle B, Tirel O, Malledant Y: Effect of oropharyngeal decontamination by povidone-iodine on ventilator-associated pneumonia in patients with head trauma. Crit Care Med. 2006, 34: 1514-1519.
Valencia M, Ferrer M, Farre R, Navajas D, Badia JR, Nicolas JM, Torres A: Automatic control of tracheal tube cuff pressure in ventilated patients in semirecumbent position: a randomized trial. Crit Care Med. 2007, 35: 1543-1549.
Brun-Buisson C, Legrand P, Rauss A, Richard C, Montravers F, Besbes M, Meakins JL, Soussy CJ, Lemaire F: Intestinal decontamination for control of nosocomial multiresistant gram-negative bacilli. Study of an outbreak in an intensive care unit. Ann Intern Med. 1989, 110: 873-881.
de Smet AMGA, Kluytmans JAJW, Cooper BS, Mascini EM, Benus RFJ, van der Werf TS, van der Hoeven JG, Pickkers P, Bogaers-Hofman D, van der Meer NJ, Bernards AT, Kuijper EJ, Joore JC, Leverstein-van Hall MA, Bindels AJ, Jansz AR, Wesselink RM, de Jongh BM, Dennesen PJ, van Asselt GJ, te Velde LF, Frenay IH, Kaasjager K, Bosch FH, van Iterson M, Thijsen SF, Kluge GH, Pauw W, de Vries JW, Kaan JA, Arends JP, Aarts LP, Sturm PD, Harinck HI, Voss A, Uijtendaal EV, Blok HE, Thieme Groen ES, Pouw ME, Kalkman CJ, Bonten MJ: Decontamination of the digestive tract and oropharynx in ICU patients. N Engl J Med. 2009, 360: 20-31.
Godard J, Guillaume C, Reverdy ME, Bachmann P, Bui-Xuan B, Nageotte A, Motin J: Intestinal decontamination in a polyvalent ICU. A double-blind study. Intensive Care Med. 1990, 16: 307-311.
Nardi G, Di Silvestre A, De Monte A, Massarutti D, Proietti A, Troncon MG, Zussino M: Reduction in gram-positive pneumonia and antibiotic consumption following the use of a SDD protocol including nasal and oral mupirocin. Eur J Emerg Med. 2001, 8: 203-214.
Oostdijk EA, Kesecioglu J, Schultz MJ, Visser CE, de Jonge E, van Essen EH, Bernards AT, Purmer I, Brimicombe R, Bergmans D, van Tiel F, Bosch FH, Mascini E, van Griethuysen A, Bindels A, Jansz A, van Steveninck FAL, van der Zwet WC, Fijen JW, Thijsen S, de Jong R, Oudbier J, Raben A, van der Vorm E, Koeman M, Rothbarth P, Rijkeboer A, Gruteke P, Hart-Sweet H, Peerbooms P, Winsser LJ, van Elsacker-Niele AMW, Demmendaal K, Brandenburg A, de Smet AMGA, Bonten MJM: Effects of decontamination of the oropharynx and intestinal tract on antibiotic resistance in ICUs: a randomized clinical trial. JAMA. 2014, 312: 1429-1437.
Cerdá E, Abella A, Miguel A, Lorente JA, García-Hierro P, van Saene HK, Alia A, Aranguren A: Enteral vancomycin controls methicillin-resistant Staphylococcus aureus endemicity in an intensive care burn unit: a 9-year prospective study. Ann Surg. 2007, 245: 397-407.
Fox MA, Peterson S, Fabri BM, Van Saene HK, Williets T: Selective decontamination of the digestive tract in cardiac surgical patients. Crit Care Med. 1991, 19: 1486-90.
Hammond JM, Potgieter PD: Long-term effects of selective decontamination on antimicrobial resistance. Crit Care Med. 1995, 23: 637-45.
Ledingham I, Eastaway A, Mckay I, Alcock S, Mcdonald J, Ramsay G: Triple regimen of selective decontamination of the digestive tract, systemic cefotaxime, and microbiological surveillance for prevention of acquired infection in intensive care. Lancet. 1988, 1: 785-90.
McClelland P, Murray AE, Williams PS, van Saene HK, Gilbertson AA, Mostafa SM, Bone JM: Reducing sepsis in severe combined acute renal and respiratory failure by selective decontamination of the digestive tract. Crit Care Med. 1990, 18: 935-9.
Silvestri L, Bragadin CM, Milanese M, Gregori D, Consales C, Gullo A, van Saene HFK: Are most ICU infections really nosocomial? A prospective observational cohort study in mechanically ventilated patients. J Hosp Infect. 1999, 42: 125-133.
Silvestri L, Van Saene HKF, Milanese M, Fontana F, Gregori D, Oblach L, Blazic M: Prevention of MRSA pneumonia by oral vancomycin decontamination: a randomised trial. Eur Resp J. 2004, 23: 921-926.
Stoutenbeek CP, van Saene HK, Miranda DR, Zandstra DF, Langrehr D: The effect of oropharyngeal decontamination using topical nonabsorbable antibiotics on the incidence of nosocomial respiratory tract infections in multiple trauma patients. J Trauma. 1987, 27: 357-364.
Sydow M, Burchardi H, Crozier TA: [The effect of selective decontamination on nosocomial infections, their causative agents and antibiotic resistance in long-term intubated intensive care patients]. Anasth Intensivther Notfall Med. 1990, 25: 416-23.
van Patot HT, Leusink JA, Roodenburg J, De Jongh BM, Lau HS, de Boer S, De Boer A: Selective decontamination of the digestive tract: effect of cessation of routine application at an ICU. Pharm World Sci. 1996, 18: 171-177.
Viviani M, Van Saene HK, Dezzoni R, Silvestri L, Di Lenarda R, Berlot G, Gullo A: Control of imported and acquired methicillin-resistant Staphylococcus aureus (MRSA) in mechanically ventilated patients: a dose–response study of enteral vancomycin to reduce absolute carriage and infection. Anaesth Intensive Care. 2005, 33: 361-372.
Barret JP, Jeschke MG, Herndon DN: Selective decontamination of the digestive tract in severely burned pediatric patients. Burns. 2001, 27: 439-445.
Garbino J, Lew DP, Romand JA, Hugonnet S, Auckenthaler R, Pittet D: Prevention of severe Candida infections in nonneutropenic, high-risk, critically ill patients: a randomized, double-blind, placebo-controlled trial in patients treated by selective digestive decontamination. Intensive Care Med. 2002, 28: 1708-1717.
Gaussorgues P, Salord M, Sirodot S, Tigaud S, Cagnin S, Gerard M, Robert D: Efficiency of selective decontamination of the digestive tract on the occurrence of nosocomial bacteremia in patients on mechanical ventilation receiving betamimetic therapy. Réan Soins Intens Méd Urg. 1991, 7: 169-174.
Korinek AM, Laisne MJ, Nicolas MH, Raskine L, Deroin V, Sanson-Lepors MJ: Selective decontamination of the digestive tract in neurosurgical intensive care unit patients: a double-blind, randomized, placebo-controlled study. Crit Care Med. 1993, 21: 1466-1473.
Langlois-Karaga A, Bues-Charbit M, Davignon A, Albanese J, Durbec O, Martin C, Balansard G: Selective digestive decontamination in multiple trauma patients: cost and efficacy. Pharm World Sci. 1995, 17: 12-16.
Quinio B, Albanese J, Bues-Charbit M, Viviand X, Martin C: Selective decontamination of the digestive tract in multiple trauma patients. A prospective double-blind, randomized, placebo-controlled study. Chest. 1996, 109: 765-772.
Rodriguez-Roldan JM, Altuna-Cuesta A, Lopez A, Carrillo A, Garcia J, Leon J, Martinez-Pellus AJ: Prevention of nosocomial lung infection in ventilated patients: use of an antimicrobial pharyngeal nonabsorbable paste. Crit Care Med. 1990, 18: 1239-1242.
Ruza F, Alvarado F, Herruzo R, Delgado MA, Garcia S, Dorao P, Goded F: Prevention of nosocomial infection in a pediatric intensive care unit (PICU) through the use of selective digestive decontamination. Eur J Epidemiol. 1998, 14: 719-27.
Wiener J, Itokazu G, Nathan C, Kabins SA, Weinstein RA: A randomized, double-blind, placebo-controlled trial of selective digestive decontamination in a medical-surgical intensive care unit. Clin Infect Dis. 1995, 20: 861-867.
Aerdts SJ, van Dalen R, Clasener HA, Festen J, van Lier HJ, Vollaard EJ: Antibiotic prophylaxis of respiratory tract infection in mechanically ventilated patients. A prospective, blinded, randomized trial of the effect of a novel regimen. Chest. 1991, 100: 783-791.
Blair P, Rowlands BJ, Lowry K, Webb H, Armstrong P, Smilie J: Selective decontamination of the digestive tract: a stratified, randomized, prospective study in a mixed intensive care unit. Surgery. 1991, 110: 303-309.
Cerra FB, Maddaus MA, Dunn DL, Wells CL, Konstantinides NN, Lehmann SL, Mann HJ: Selective gut decontamination reduces nosocomial infections and length of stay but not mortality or organ failure in surgical intensive care unit patients. Arch Surg. 1992, 127: 163-167.
Cockerill FR, Muller SR, Anhalt JP, Marsh HM, Farnell MB, Mucha P, Gillespie DJ, Ilstrup DM, Larson-Keller JJ, Thompson RL: Prevention of infection in critically ill patients by selective decontamination of the digestive tract. Ann Intern Med. 1992, 117: 545-553.
de La Cal MA, Cerda E, Garcia-Hierro P, Van Saene HK, Gómez-Santos D, Negro E, Lorente JA: Survival benefit in critically ill burned patients receiving selective decontamination of the digestive tract: a randomized, placebo-controlled, double-blind trial. Ann Surg. 2005, 241: 424-430.
Finch RG, Tomlinson P, Holliday M, Sole K, Stack C, Rocker G: Selective Decontamination of the Digestive Tract (SDD) in the Prevention of Secondary Sepsis in a Medical/Surgical Intensive Care Unit [Abstract]. 1991, Seventeenth international congress of chemotherapy, Berlin
Flaherty J, Nathan C, Kabins SA, Weinstein RA: Pilot trial of selective decontamination for prevention of bacterial infection in an intensive care unit. J Infect Dis. 1990, 162: 1393-1397.
Jacobs S, Foweraker JE, Roberts SE: Effectiveness of selective decontamination of the digestive tract (SDD) in an ICU with a policy encouraging a low gastric pH. Clin Intensive Med. 1992, 3: 52-58.
Kerver AJ, Rommes JH, Mevissen-Verhage EA, Hulstaert PF, Vos A, Verhoef J, Wittebol P: Prevention of colonization and infection in critically ill patients: a prospective randomized study. Crit Care Med. 1988, 16: 1087-1093.
Krueger WA, Lenhart FP, Neeser G, Ruckdeschel G, Schreckhase H, Eissner HJ, Forst H, Eckart J, Peter K, Unertl KE: Influence of combined intravenous and topical antibiotic prophylaxis on the incidence of infections, organ dysfunctions, and mortality in critically ill surgical patients: a prospective, stratified, randomized, double-blind, placebo-controlled clinical trial. Am J Respir Crit Care Med. 2002, 166: 1029-1037.
Palomar M, Alvarez-Lerma F, Jorda R, Bermejo B, for the Catalan Study Group of Nosocomial Pneumonia Prevention: Prevention of nosocomial infection in mechanically ventilated patients: selective digestive decontamination versus sucralfate. Clin Intensive Care. 1997, 8: 228-235.
Rocha LA, Martin MJ, Pita S, Paz J, Seco C, Margusino L, Villanueva R, Duran MT: Prevention of nosocomial infection in critically ill patients by selective decontamination of the digestive tract. A randomized, double blind, placebo-controlled study. Intensive Care Med. 1992, 18: 398-404.
Rolando N, Gimson A, Wade J, Philpott‐Howard J, Casewell M, Williams R: Prospective controlled trial of selective parenteral and enteral antimicrobial regimen in fulminant liver failure. Hepatol. 1993, 17: 196-201.
Sanchez Garcia M, Cambronero Galache JA, Lopez Diaz J, Cerda Cerda E, Rubio Blasco J, Gomez Aguinaga MA, Nunez Reiz A, Rogero Marin S, Onoro Canaveral JJ, Sacristan del Castillo JA: Effectiveness and cost of selective decontamination of the digestive tract in critically ill intubated patients. A randomized, double-blind, placebo-controlled, multicenter trial. Am J Respir Crit Care Med. 1998, 158: 908-916.
Stoutenbeek CP, van Saene HKF, Little RA, Whitehead A: The effect of selective decontamination of the digestive tract on mortality in multiple trauma patients: a multicenter randomized controlled trial. Intensive Care Med. 2007, 33: 261-70.
Ulrich C, Harinck-de Weerd JE, Bakker NC, Jacz K, Doornbos L, de Ridder VA: Selective decontamination of the digestive tract with norfloxacin in the prevention of ICU-acquired infections: a prospective randomized study. Intensive Care Med. 1989, 15: 424-431.
Verwaest C, Verhaegen J, Ferdinande P, Schetz M, Van den Berghe G, Verbist L, Lauwers P: Randomized, controlled trial of selective digestive decontamination in 600 mechanically ventilated patients in a multidisciplinary intensive care unit. Crit Care Med. 1997, 25: 63-71.
Zobel G, Kuttnig M, Grubbauer HM, Semmelrock HJ, Thiel W: Reduction of colonization and infection rate during pediatric intensive care by selective decontamination of the digestive tract. Crit Care Med. 1991, 19: 1242-1246.
Arnow PM, Carandang GC, Zabner R, Irwin ME: Randomized controlled trial of selective bowel decontamination for prevention of infections following liver transplantation. Clin Infect Dis. 1996, 22: 997-1003.
Bion JF, Badger I, Crosby HA, Hutchings P, Kong KL, Baker J, Hutton P, McMaster P, Buckels JA, Elliott TSJ: Selective decontamination of the digestive tract reduces gram-negative pulmonary colonization but not systemic endotoxemia in patients undergoing elective liver transplantation. Crit Care Med. 1994, 22: 40-9.
Ferrer M, Torres A, Gonzalez J, Puig de la Bellacasa J, el-Ebiary M, Roca M, Gatell JM, Rodriguez-Roisin R: Utility of selective digestive decontamination in mechanically ventilated patients. Ann Intern Med. 1994, 120: 389-395.
Georges B, Mazerolles M, Decun J-F, Rouge P, Pomies S, Cougot P: Décontamination digestive sélective résultats d'une étude chez le polytraumatisé. Réanimation Soins Intensifs Médecin d'Urgence. 1994, 3: 621-627.
Hammond JM, Potgieter PD, Saunders GL, Forder AA: Double-blind study of selective decontamination of the digestive tract in intensive care. Lancet. 1992, 340: 5-9.
Hartenauer U, Thulig B, Diemer W, Lawin P, Fegeler W, Kehrel R, Ritzerfeld W: Effect of selective flora suppression on colonization, infection, and mortality in critically ill patients: a one-year, prospective consecutive study. Crit Care Med. 1991, 19: 463-73.
Hellinger WC, Yao JD, Alvarez S, Blair JE, Cawley JJ, Paya CV, O’Brien PC: A randomized, prospective, double-blinded evaluation of selective bowel decontamination in liver transplantation. Transplantation. 2002, 73: 1904-9.
Laggner AN, Tryba M, Georgopoulos A, Lenz K, Grimm G, Graninger W, Schneeweiss B, Druml W: Oropharyngeal decontamination with gentamicin for long-term ventilated patients on stress ulcer prophylaxis with sucralfate?. Wien Klin Wochenschr. 1994, 106: 15-19.
Rolando N, Wade JJ, Stangou A, Gimson AE, Wendon J, Philpott‐Howard J, Williams R: Prospective study comparing the efficacy of prophylactic parenteral antimicrobials, with or without enteral decontamination, in patients with acute liver failure. Liver transpl Surg. 1996, 2: 8-13.
Stoutenbeek CP, van Saene HKF, Zandstra DF: Prevention of multiple organ failure by selective decontamination of the digestive tract in multiple trauma patients. The Immune Consequences of Shock, Trauma and Sepsis. Edited by: Faist E, Baue AE, Schildberg FW. 1996, Pabst Science, Lengerich, 1055-66.
Tetteroo GWM, Castelein A, Tilanus HW, Ince C, Bruining HA, Wagenvoort JHT: Selective decontamination to reduce gram-negative colonisation and infections after oesophageal resection. Lancet. 1990, 335: 704-7.
Pittet D, Tarara D, Wenzel RP: Nosocomial bloodstream infection in critically ill patients: excess length of stay, extra costs, and attributable mortality. JAMA. 1994, 271: 1598-1601.
Vallés J, León C, Alvarez-Lerma F: Nosocomial bacteremia in critically ill patients: a multicenter study evaluating epidemiology and prognosis. Spanish collaborative group for infections in intensive care units of Sociedad Espanola de Medicina Intensiva y Unidades Coronarias (SEMIUC). Clin Infect Dis. 1997, 24: 387-395.
Bonten MJ, Gaillard CA, de Leeuw PW, Stobberingh EE: Role of colonization of the upper intestinal tract in the pathogenesis of ventilator-associated pneumonia. Clin Infect Dis. 1997, 24: 309-319.
Oostdijk EA, de Smet AMG, Kesecioglu J, Bonten MJ, Dutch SOD-SDD Trialists Group: The role of intestinal colonization with gram-negative bacteria as a source for intensive care unit-acquired bacteremia. Crit Care Med. 2011, 39: 961-966.
Silvestri L, van Saene HKF, Casarin A, Berlot G, Gullo A: Impact of selective decontamination of the digestive tract on carriage and infection due to Gram-positive and Gram-negative bacteria. A systematic review of randomised controlled trials. Anaesth Intensive Care. 2008, 36: 324-338.
Silvestri L, Van Saene HKF, Milanese M, Gregori D, Gullo A: Selective decontamination of the digestive tract reduces bacterial bloodstream infection and mortality in critically ill patients. Systematic review of randomized, controlled trials. J Hosp Infect. 2007, 65: 187-203.
Liberati A, D'Amico R, Pifferi S, Torri V, Brazzi L, Parmelli E: Antibiotic prophylaxis to reduce respiratory tract infections and mortality in adults receiving intensive care. Cochrane Database Syst Rev 2004;(1):CD000022.,
Hurley JC: Prophylaxis with enteral antibiotics in ventilated patients: selective decontamination or selective cross-infection?. Antimicrob Agents Chemother. 1995, 39: 941-947.
Hurley JC: Profound effect of study design factors on ventilator-associated pneumonia incidence of prevention studies: benchmarking the literature experience. J Antimicrob Chemother. 2008, 61: 1154-1161.
Hurley JC: Ventilator associated pneumonia prevention methods using topical antibiotics: herd protection or herd peril?. Chest. 2014, 146 (4): 890-898.
Hurley JC: The perfidious effect of topical placebo: calibration of staphylococcus aureus ventilator-associated pneumonia incidence within selective digestive decontamination studies versus the broader evidence base. Antimicrob Agents Chemother. 2013, 57: 4524-4531.
Hurley JC: Lack of impact of selective digestive decontamination on pseudomonas aeruginosa ventilator-associated pneumonia: benchmarking the evidence base. J Antimicrob Chemother. 2011, 66: 1365-1373.
Garner JS, Jarvis WR, Emori TG, Horan TC, Hughes JM: CDC definitions for nosocomial infections, 1988. Am J Infect Control. 1988, 16: 128-140.
Greenland S, O’Rourke K: Meta-analysis. Modern Epidemiology. Edited by: Rothman KJ, Greenland S, Lash TL. 2008, Lippincott, Williams and Wilkins, Philadelphia, PA, 652-682. 3
Harris RJ, Bradburn MJ, Deeks JJ, Harbord RM, Altmamn DG, Sterne JAC: Metan: fixed and random effects-meta-analysis. Stata J. 2008, 8: 3-28.
Harbord RM, Higgins JPT: Meta-regression in Stata. Stata J. 2008, 8: 493-519.
Pittet D, Li N, Woolson RF, Wenzel RP: Microbiological factors influencing the outcome of nosocomial bloodstream infections: a 6-year validated, population-based model. Clin Infect Dis. 1997, 24: 1068-1078.
Agbaht K, Diaz E, Muñoz E, Lisboa T, Gomez F, Depuydt PO, Blot SI, Rello J: Bacteremia in patients with ventilator-associated pneumonia is associated with increased mortality: a study comparing bacteremic vs. nonbacteremic ventilator-associated pneumonia. Crit Care Med. 2007, 35: 2064-2070.
Klouwenberg PMK, Ong DS, Bos LD, de Beer FM, van Hooijdonk RT, Huson MA, Cremer OL: Interobserver agreement of centers for disease control and prevention criteria for classifying infections in critically ill patients. Crit Care Med. 2013, 41: 2373-2378.
Oostdijk EA, de Smet AM, Blok HE, Thieme Groen ES, van Asselt GJ, Benus RF, Bernards SAT, Bonten MJ: Ecological effects of selective decontamination on resistant gram-negative bacterial colonization. Am J Respir Crit Care Med. 2010, 181: 452-457.
van der Kooi TI, de Boer AS, Manniën J, Wille JC, Beaumont MT, Mooi BW, van den Hof S: Incidence and risk factors of device-associated infections and associated mortality at the intensive care in the Dutch surveillance system. Intensive Care Med. 2007, 3: 271-278.
Canter RR, Harvey SE, Harrison DA, Campbell MK, Rowan KM, Cuthbertson BH: Observational study of current use of selective decontamination of the digestive tract in UK critical care units. Br J Anaesth. 2014, 113 (4): 610-617.
Martin MA, Pfaller MA, Wenzel RP: Coagulase-negative staphylococcal bacteremia mortality and hospital stay. Ann Intern Med. 1989, 110: 9-16.
Digiovine B, Chenoweth C, Watts C, Higgins M: The attributable mortality and costs of primary nosocomial bloodstream infections in the intensive care unit. Am J Respir Crit Care Med. 1999, 160: 976-981.
Kieft H, Hoepelman AI, Zhou W, Rozenberg-Arska M, Struyvenberg A, Verhoef J: The sepsis syndrome in a Dutch university hospital: clinical observations. Arch Int Med. 1993, 153: 2241-2247.
Daxboeck F, Rabitsch W, Blacky A, Stadler M, Kyrle PA, Hirschl AM, Koller W: Influence of selective bowel decontamination on the organisms recovered during bacteremia in neutropenic patients. Infect Control Hosp Epidemiol. 2004, 25: 685-689.
Stoutenbeek CP, van Saene HK, Miranda DR, Zandstra DF: The effect of selective decontamination of the digestive tract on mortality in multiple trauma patients: a multicenter randomized controlled trial. Intensive Care Med. 2007, 33: 261-270.
Hurley JC: Inapparent outbreaks of ventilator-associated pneumonia: an ecologic analysis of prevention and cohort studies. Infect Control Hosp Epidemiol. 2005, 26: 374-390.
Hurley JC: Paradoxical ventilator associated pneumonia incidences among selective digestive decontamination studies versus other studies of mechanically ventilated patients. Benchmarking the evidence base. Crit Care. 2011, 15: R7-
Funding
This work was supported by the Australian Government Department of Health and Ageing through the Rural Clinical Training and Support (RCTS) program.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The author declares that he has no competing interests.
Electronic supplementary material
12879_2014_714_MOESM1_ESM.pdf
Additional file 1: VAP-IP and bacteremia-IP data for observational studies (Table S1), studies of non-antibiotic-based methods of VAP prevention (Table S2), studies of SDD– non-concurrent groups (Table S3), studies of SDD– RCCT’s (Table S4), studies of SDD-RCCT’s with Duplex design (Table S5), and Numbers of Coagulase negative Staphylococcus and Pseudomonas bacteremia isolates (Table S6). (PDF 517 KB)
12879_2014_714_MOESM3_ESM.pdf
Additional file 3: Forrest plots showing counterfactual effects as odds ratios between control versus intervention group VAP incidence (Figures S5, S7 & S9) and bacteremia incidence (Figures S6, S8 & S10) as study specific and summary effect sizes. (PDF 82 KB)
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Hurley, J.C. Topical antibiotics as a major contextual hazard toward bacteremia within selective digestive decontamination studies: a meta-analysis. BMC Infect Dis 14, 714 (2014). https://doi.org/10.1186/s12879-014-0714-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-014-0714-x