Abstract
Background
With the aging of the population worldwide, extending healthy life expectancy is an urgent issue. Muscle mass has been reported to be associated with physical independence and longevity. This study aimed to investigate the characteristics of food intake in urban community-dwelling older adults with low muscle mass.
Methods
This cross-sectional study used baseline data from the Bunkyo Health Study, which included 1618 urban community-dwelling older adults aged 65–84 years. All participants underwent measurement of body composition using bioelectrical impedance analysis and evaluation of nutrient and food intake using the brief-type self-administered diet history questionnaire. Participants were stratified by sex and divided into robust or low skeletal muscle mass index (SMI) groups according to the Asian Working Group for Sarcopenia criteria to compare differences in nutrient and food intake.
Results
The mean age and body mass index were 73.1 ± 5.4 years and 22.6 ± 3.1 kg/m2, respectively. The prevalence of low SMI was 31.1% in men and 43.3% in women. In men, all food intake, including total energy intake, was similar between the low SMI group and the robust group. In women, the low SMI group had less total energy intake, and consumed lower amounts of energy-producing nutrients (protein, fat, and carbohydrates), but there were only small differences in the intake of specific foods.
Conclusions
There were sex differences in food intake characteristics between urban community-dwelling older adults with low SMI and those who were robust. Advising women to increase their energy intake may be important in preventing muscle loss, and further research is needed in men.
Similar content being viewed by others
Introduction
The world’s population is aging rapidly. The proportion of individuals aged 65 and over worldwide (aging rate) is expected to rise to 18.7% by 2060, with further acceleration over the next 40 years [1]. The aging rate in Japan is particularly high, at 29.0% in 2022, and will reach 38.7% in 2070, making the extension of healthy life expectancy an urgent issue [1]. Sarcopenia is defined as age-related loss of muscle mass and strength. Sarcopenia is a major public health problem that hinders healthy life expectancy in an aging society, particularly as muscle mass has been reported to be associated with physical independence [2] and longevity [3].
Currently, dietary diversity and a protein intake of at least 1.0 g/kg body weight/day are recommended to prevent and improve sarcopenia [4]. In particular, regarding muscle mass, a study of older women showed that the group with the highest protein intake had higher fat-free mass [5]. Similarly, the European Society for Clinical Nutrition and Metabolism (ESPEN) recommends a protein intake of at least 1.0–1.2 g/kg body weight/day for healthy older adults and 1.2–1.5 g/kg body weight/day for older adults with low nutrition or at risk of low nutrition [6]. Furthermore, the relationship between nutrients and muscle mass has been clarified in recent years [7,8,9]. For example, it has been reported that higher total daily leucine intake is associated with muscle mass and strength in healthy older adults [10].
Against this background, it is recommended that older adults consume protein, especially essential amino acids such as leucine. However, despite known dietary variations related to place of residence, age, and gender, the specific foods and nutrients linked to low muscle mass among urban community-dwelling older adults are not fully understood. Therefore, the purpose of this study was to determine the dietary characteristics of urban community-dwelling older adults with low SMI. This study may allow us to provide more specific nutritional guidance to maintain muscle mass in urban community-dwelling older adults.
Materials and methods
Study design and participants
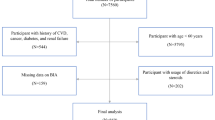
This cross-sectional study used baseline data from the Bunkyo Health Study, a prospective cohort study designed to clarify whether muscle function (muscle mass, muscle strength, and insulin sensitivity) is associated with multiple diseases that necessitate long-term care [11]. Briefly, in this study, we recruited 1,629 older adults aged 65–84 years living in Bunkyo-ku, an urban area in Tokyo, Japan. All participants underwent examinations for 2 days from October 15, 2015, to October 1, 2018. On the first day, we evaluated cognitive function using questionnaires, and also assessed muscle strength, physical performance, and gait speed. On the second day, after an overnight fast, we measured body composition. We excluded three of the 1,629 participants with unavailable data on body composition, and eight of the remaining 1,626 participants because they consumed less than 600 kcal/day (half the energy required for the low physical activity category) or more than 4,000 kcal/day (1.5 times the energy intake required for moderate physical activity category). Finally, we included 1,618 participants (679 males and 939 females) in this analysis. The study protocol was approved by the ethics committee of Juntendo University in November 2015 (Nos. 201,507 and M15-0057). This research was conducted in accordance with the principles outlined in the Declaration of Helsinki. All participants provided written informed consent and were informed that they had the right to withdraw from the trial at any time.
Skeletal muscle mass measurement
Appendicular skeletal muscle mass (ASM) was evaluated by bioelectrical impedance analysis (InBody770, InBody Japan Inc., Tokyo, Japan). The skeletal muscle mass index (SMI) was calculated by dividing ASM by the square of height (m). Low SMI was defined based on the definition of the Asian Working Group for Sarcopenia (AWGS) 2019 (< 7.0 kg/m2 in men and < 5.7 kg/m2 in women) [12].
Dietary assessment
Dietary and nutrient intake were assessed using the brief-type self-administered diet history questionnaire (BDHQ) [13, 14]. The validity of this questionnaire has been reported previously [15]. The BDHQ asks about the frequencies of dietary behaviors and the consumption of 58 foods and beverages over the past month, including the frequencies of daily consumption of 46 foods and nonalcoholic beverage items, rice, and miso soup, the frequency of consumption of five alcoholic beverages and the amount of each alcoholic beverage consumed per drinking occasion, and the frequency of daily consumption of five seasonings (salt, oil, sugar, soy sauce, and noodle soup) used in cooking and the general diet. In this study, in addition to the 58 food items included in the BDHQ, we created a new category for wheat products, defined as bread, ramen, udon, and pasta. Responses to the BDHQ were checked by research staff for completeness, and when necessary, reviewed with participants to ensure that answers were properly understood. Average daily food and nutrient intake was estimated using an ad hoc computer algorithm for the BDHQ, based on Japan’s Standard Tables of Food Composition [15].
Other measurements
Grip strength was measured twice on each side using a handgrip dynamometer (T.K.K.5401, Takei Scientific Instruments Co., Ltd., Niigata, Japan) [16]. We defined grip strength based on the average of the maximum bilateral values. We administered other physical function tests, including the 10-m walking test to assess maximum gait speed, and the Timed Up and Go test (TUG), which measures the time required for a participant to stand up from a seated position, walk 3 m, turn, walk back, and sit down again. We assessed cognitive function using the Mini-Mental State Examination-Japanese (MMSE-J) [17, 18]. Finally, we evaluated physical activity using the International Physical Activity Questionnaire, which was designed to assess different types of physical activity, such as walking and both moderate- and high-intensity activities.
Statistical analysis
Participants of each sex were divided into two groups according to the presence or absence of low SMI in each sex. IBM SPSS Statistics for Windows, version 29.0 (IBM Corp., Armonk, NY, USA) was used for the analysis. Data are expressed as medians (interquartile range) or numbers (%). Differences in the characteristics of participants in the low SMI group and the robust group were tested using the Mann-Whitney U test for continuous variables and the chi-squared test for categorical variables. Comparisons of food and nutrient intake between the low SMI group and the robust group were performed using an analysis of covariance adjusted for age, height, weight and physical activity levels. In this analysis, all the food and nutrient intake data showed a non-normal distribution, so we performed a logarithmic transformation prior to analysis. However, for copper and vitamins B1, B2, B6, and B12, which are consumed in trace amounts according to the Japanese Dietary Reference Intakes for 2020, we did not perform a logarithmic transformation [19]. All statistical tests were two-sided with a 5% significance level.
Results
The characteristics of the study participants are shown in Table 1. The mean participant age was 73.1 ± 5.4 years old. The prevalence of low SMI was 31.1% in men and 43.3% in women. Both men and women in the low SMI group were older than those in the robust group. In addition, both men and women in the low SMI group had significantly lower height, weight, and body mass index (BMI) than those in the robust group. In particular, weight was about 10 kg lower in the low SMI group in both men and women. Furthermore, women in the low SMI group had a significantly lower percentage of body fat than those in the robust group. Regarding physical function, both men and women in the low SMI group had weaker grip strength and slower performance on the TUG test and 10-m walking test than those in the robust group. In men, the MMSE score was significantly lower in the low SMI group than in the robust group.
Table 2 shows a comparison of nutrient and food intake between the low SMI and robust groups adjusted for age, height, weight and physical activity levels. In women, energy intake was significantly lower in the low SMI group than in the robust group (p = 0.002). The intake of protein (p = 0.002), fat (p = 0.001), and carbohydrates (p = 0.015) were also significantly lower in the low SMI group. In contrast, in men, there was no difference in total energy, protein, fat, or carbohydrate intake between the two groups. In women, the intake of almost all nutrients except for alcohol, sodium, sodium chloride equivalent and sucrose was significantly lower in the low SMI group than in the robust group. In men, the low SMI group showed significantly lower intake levels of manganese and Cu compared to the robust group, but no significant differences were observed in other nutrients.
In terms of food intake (Table 3), all food intake was similar between the low SMI group and the robust group, in men. In women, the intake of high-fat fish and tofu or fried tofu was higher in the robust group than in the low SMI group, and the intake of pasta and wheat products was higher in the low SMI group than in the robust group. There were no significant differences in the intake of other foods, including vegetables and beverages, in either men or women (data not shown).
Discussion
This study examined the nutritional and food intake characteristics of urban community-dwelling older adults with low muscle mass. In men, there were no significant differences in food intake, including total energy intake, between the low SMI group and the robust group. Conversely, in women, the low SMI group had less total energy intake, consumed lower amounts of energy-producing nutrients (protein, fat, and carbohydrates), high-fat fish and tofu/fried tofu than the robust group, but the low SMI group consumed significantly more pasta and wheat products than the robust group.
In the present study, there were sex-related dietary differences between robust older adults and those with low muscle mass. Sex differences have been demonstrated in nutritional behavior, styles of nutrition, dietary profiles, approach to nourishment, approach to the location of meal consumption, and the sources of nutritional knowledge; women in particular have greater trust in the benefits of healthy nutrition, are more active in controlling their weight, and choose healthier foods than men [20]. In addition, women may differ from men in their eating behaviors, regardless of age [21], and sex differences have been observed in the perception of food [20]. In this study, total energy intake and the intake of almost all nutrients were lower in the low SMI group than in the robust group in women, but there were few differences in terms of the actual foods consumed. It is suggesting that there were few differences in dietary balance and only small differences in each food intake. This suggests that women need nutritional guidance to increase their overall dietary intake and thereby prevent sarcopenia. Regarding high-fat fish, it’s worth noting that its intake was significantly lower in the low SMI group than in the robust group. High-fat fish contain n-3 fatty acids, whose intake has been reported to have a variety of beneficial effects, including preventing cardiovascular disease [22], lowering blood pressure [23], and lowering triglycerides [24]. A 6-month randomized controlled trial showed that compared to controls, subjects who received n-3 polyunsaturated fatty acids for 6 months exhibited significantly increased thigh muscle volume, handgrip strength, and one-repetition maximum strength, suggesting an effect on improving physical performance in older adults [25].
In men, there were no significant differences in food intake, including total energy intake, between the low SMI group and the robust group. However, although not statistically significant, the low SMI group tended to consume more bread and other wheat products and less rice in the carbohydrate component compared to the robust group. In addition, the low SMI group tended to consume less animal protein, including dairy products, and more plant protein than the robust group in the protein component. A survey on diet and lifestyle conducted by the Ministry of Agriculture, Forestry, and Fisheries (MAFF) reported that the older the age of Japanese people, the more bread they tend to consume as part of their staple food intake [26]. Bread requires fewer cooking steps than rice and noodles, and there is a variety of breads, such as sandwiches and sweet breads, to provide satisfaction in a single meal. This may therefore lower the hurdle to eating and make it more feasible for older adults, whose oral functions are declining. For similar reasons, it is possible that men in the low SMI group consumed higher amounts of tofu or fried tofu compared to those in the robust group. In addition, animal proteins are rich in branched-chain amino acids, which are necessary to maintain muscle mass, and a previous study in older women reported that a high intake of animal protein was significantly associated with fat-free mass [5]. Further, a meta-analysis and systematic review in middle-aged and older adults showed that dairy protein intake was associated with a significant increase in muscle mass, suggesting that dairy products are important for maintaining muscle mass [27]. Thus, protein, especially animal protein, is needed for the prevention of muscle mass loss in older adults. However, studies have shown a gender disadvantage for men compared to women in nutrition education [21]. Thus, men may need detailed guidance on specific foods to prevent sarcopenia.
A previous study reported that older adults at low risk of sarcopenia and frailty intake more energy, nutrients, and foods than older adults at high risk [28]. Another study showed that even after adjusting for age, lifestyle, physical activity, and energy intake, older adults with high SMI consumed more vegetables than those with low SMI [7]. Other studies have shown that older adults with higher dietary quality and dietary diversity have a lower risk of sarcopenia and frailty [29,30,31], and fruits and vegetables in particular have been noted to reduce these risks [32, 33]. In this study, there were no significant differences in the intake of vegetables and fruit between the low SMI and robust groups in either men or women. The National Health and Nutrition Survey showed that older adults consumed more fruits and vegetables than other age groups [34]. A recent survey reported that the average vegetable intake of older adults was 288.3 g/day in men and 273.6 g/day in women [34]. In this study, the average vegetable intake in men was 276.2 g/day in the robust group and 273.3 g/day in the low SMI group, while in women it was 268.8 g/day in the robust group and 265.82 g/day in the low SMI group; thus both groups in both sexes consumed a roughly average amount of vegetables. In addition, while a survey of fruit intake in older adults showed that approximately 40% of the population consumed less than 100 g/day [34], men in this study consumed 109.3 g/day and women consumed 110.3 g/day, suggesting that this study population is also characterized by a near-average amount of fruit intake. Thus, among urban community-dwelling older adults who consume some fruits and vegetables, their intake is not associated with low SMI, but the reason for this is unknown.
In this study, both muscle mass and body weight were about 15% lower in the low SMI group than in the robust group in men and women alike. On the other hand, percent body fat and dietary intake showed little difference between the groups. This may be due not only to food intake but also to other factors such as digestion, absorption, anabolism, and catabolism. The production of digestive enzymes generally decline with age, resulting in reduced absorption of several nutrients [35]. In addition, aging often leads to a decrease in anabolic signals and an increase in catabolic signals, contributing to muscle loss (sarcopenia) and other age-related changes [36]. This could be exacerbated by factors such as increased cytokine levels, reduced dietary energy intake, insulin resistance, and decreased growth hormone-insulin-like growth factor-I concentrations. Further, the intestinal microflora is associated with poor digestion and absorption in older adults, and this can contribute to malnutrition and other health issues. Changes in lifestyle, diet, and possibly the use of prebiotics and probiotics may help to mitigate these effects [37, 38]. Therefore, important factors for maintaining muscle mass include not only energy intake but also the management of digestion, absorption, and chronic diseases. In addition, both men and women with low SMI may need more energy intake to increase skeletal muscle mass.
There are several limitations to this study. First, the BDHQ was used for the dietary survey in this study. It is possible that some nutrients and foods were not included by the BDHQ used in this dietary survey. In addition, the study was conducted in older adults, and recall bias could not be fully eliminated. The frequency of under- and over-reporting in dietary surveys has been found to vary according to the characteristics of the subject [39, 40], and there are no reports on issues related to reporting by older adults with low muscle mass, sarcopenia, and frailty. In addition, the times at which food was eaten by the study participants were unknown. A recent study showed that protein intake at breakfast was positively correlated with muscle function in older women [41], and in particular that higher quality protein consumed at breakfast was associated with reduced muscle weakness [42]. Therefore, the timing of food intake and the specific foods eaten should be considered. The second limitation involves the subject characteristics. The study subjects were older adults living in urban areas, who may have a high educational background and high levels of health literacy. Therefore, further research is needed to generalize these results. Next, this study may be prone to Type I errors due to multiple testing. When testing differences for multiple items simultaneously, there is a risk of false positives when using a significance threshold of 0.05. Therefore, some findings, such as those shown in Table 3, should be interpreted with caution because of the potential for inflated Type I errors. Finally, as this was a cross-sectional study, it is difficult to establish causal relationships.
In conclusion, there were sex differences in dietary characteristics between urban community-dwelling older adults with low SMI and those who were robust. This suggests that dietary guidance for the prevention of muscle loss and sarcopenia in older adults should consider sex differences. Advising women to increase their total energy intake may be important in preventing muscle loss. For men, further study is needed, as there were no significant differences observed in the intake of almost all food items, including total energy intake, between the low SMI group and the robust group.
Data availability
Some or all datasets generated and/or analyzed during the current study are not publicly available; however they can be obtained from the corresponding author upon a reasonable request.
Abbreviations
- SMI:
-
Skeletal Muscle mass Index
- ESPEN:
-
European Society for Clinical Nutrition and Metabolism
- ASM:
-
Appendicular skeletal muscle mass
- AWGS:
-
Asian Working Group for Sarcopenia
- BDHQ:
-
Brief-type self-administered Diet History Questionnaire
- TUG:
-
Timed Up and Go test
- MMSE-J:
-
Mini-Mental State Examination-Japanese
- BMI:
-
Body mass index
- MAFF:
-
Ministry of Agriculture, Forestry, and Fisheries
References
Office C. White Paper on Aging Society 2023 [ https://www8.cao.go.jp/kourei/whitepaper/w-2023/zenbun/05pdf_index.html.
Dos Santos L, Cyrino ES, Antunes M, Santos DA, Sardinha LB. Sarcopenia and physical independence in older adults: the independent and synergic role of muscle mass and muscle function. J Cachexia Sarcopenia Muscle. 2017;8(2):245–50.
Srikanthan P, Karlamangla AS. Muscle mass index as a predictor of longevity in older adults. Am J Med. 2014;127(6):547–53.
Guidelines for the management of. sarcopenia 2017 [ https://minds.jcqhc.or.jp/docs/gl_pdf/G0001021/4/sarcopenia2017_revised.pdf.
Isanejad M, Mursu J, Sirola J, Kroger H, Rikkonen T, Tuppurainen M, et al. Association of protein intake with the change of lean mass among elderly women: the osteoporosis risk factor and Prevention - Fracture Prevention Study (OSTPRE-FPS). J Nutr Sci. 2015;4:e41.
Deutz NE, Bauer JM, Barazzoni R, Biolo G, Boirie Y, Bosy-Westphal A, et al. Protein intake and exercise for optimal muscle function with aging: recommendations from the ESPEN Expert Group. Clin Nutr. 2014;33(6):929–36.
Okada C, Okada E, Takimoto H. Nutrient and food group intakes and skeletal muscle index in the Japanese elderly: a cross-sectional analysis of the NHNS 2017. Public Health Nutr. 2020;23(16):2923–31.
van Dronkelaar C, van Velzen A, Abdelrazek M, van der Steen A, Weijs PJM, Tieland M, Minerals. The role of Calcium, Iron, Magnesium, Phosphorus, Potassium, Selenium, Sodium, and zinc on muscle Mass, muscle strength, and physical performance in older adults: a systematic review. J Am Med Dir Assoc. 2018;19(1):6–e113.
Ter Borg S, Luiking YC, van Helvoort A, Boirie Y, Schols J, de Groot C. Low levels of branched chain amino acids, eicosapentaenoic acid and micronutrients are Associated with low muscle Mass, strength and function in Community-Dwelling older adults. J Nutr Health Aging. 2019;23(1):27–34.
Lixandrao ME, Longobardi I, Leitao AE, Morais JVM, Swinton PA, Aihara AY et al. Daily leucine intake is positively Associated with lower limb skeletal muscle Mass and Strength in the Elderly. Nutrients. 2021;13(10).
Someya Y, Tamura Y, Kaga H, Nojiri S, Shimada K, Daida H, et al. Skeletal muscle function and need for long-term care of urban elderly people in Japan (the Bunkyo Health Study): a prospective cohort study. BMJ Open. 2019;9(9):e031584.
Chen LK, Woo J, Assantachai P, Auyeung TW, Chou MY, Iijima K, et al. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia diagnosis and treatment. J Am Med Dir Assoc. 2020;21(3):300–e72.
Kobayashi S, Murakami K, Sasaki S, Okubo H, Hirota N, Notsu A, et al. Comparison of relative validity of food group intakes estimated by comprehensive and brief-type self-administered diet history questionnaires against 16 d dietary records in Japanese adults. Public Health Nutr. 2011;14(7):1200–11.
Kobayashi S, Honda S, Murakami K, Sasaki S, Okubo H, Hirota N, et al. Both comprehensive and brief self-administered diet history questionnaires satisfactorily rank nutrient intakes in Japanese adults. J Epidemiol. 2012;22(2):151–9.
Agency SaT. Standard Tables of Food Composition in Japan, 2010,Tokyo: Ministry of Education, Culture, Sports, Science and Technology. 2010. 2010 [ https://www.mext.go.jp/en/policy/science_technology/policy/title01/detail01/1374030.htm.
Bohannon RW. Muscle strength: clinical and prognostic value of hand-grip dynamometry. Curr Opin Clin Nutr Metab Care. 2015;18(5):465–70.
Folstein MF, Folstein SE, McHugh PR. Mini-mental state. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–98.
Ideno Y, Takayama M, Hayashi K, Takagi H, Sugai Y. Evaluation of a Japanese version of the Mini-mental State examination in elderly persons. Geriatr Gerontol Int. 2012;12(2):310–6.
Health Mo. Dietary Reference Intakes for Japanese(2020) 2020 [ https://www.mhlw.go.jp/stf/newpage_08517.html.
Grzymislawska M, Puch EA, Zawada A, Grzymislawski M. Do nutritional behaviors depend on biological sex and cultural gender? Adv Clin Exp Med. 2020;29(1):165–72.
Rolls BJ, Fedoroff IC, Guthrie JF. Gender differences in eating behavior and body weight regulation. Health Psychol. 1991;10(2):133–42.
Delgado-Lista J, Perez-Martinez P, Lopez-Miranda J, Perez-Jimenez F. Long chain omega-3 fatty acids and cardiovascular disease: a systematic review. Br J Nutr. 2012;107(Suppl 2):S201–13.
Paschos GK, Magkos F, Panagiotakos DB, Votteas V, Zampelas A. Dietary supplementation with flaxseed oil lowers blood pressure in dyslipidaemic patients. Eur J Clin Nutr. 2007;61(10):1201–6.
Maki KC, Yurko-Mauro K, Dicklin MR, Schild AL, Geohas JG. A new, microalgal DHA- and EPA-containing oil lowers triacylglycerols in adults with mild-to-moderate hypertriglyceridemia. Prostaglandins Leukot Essent Fat Acids. 2014;91(4):141–8.
Smith GI, Julliand S, Reeds DN, Sinacore DR, Klein S, Mittendorfer B. Fish oil-derived n-3 PUFA therapy increases muscle mass and function in healthy older adults. Am J Clin Nutr. 2015;102(1):115–22.
Agriculture Mo. Dietary and lifestyle survey 2022 [ https://www.maff.go.jp/j/zyukyu/survey/lifestyle.html.
Matía-Martín P, Torrego-Ellacuría M, Larrad-Sainz A, Fernández-Pérez C, Cuesta-Triana F, Rubio-Herrera M. Effects of milk and dairy products on the Prevention of osteoporosis and osteoporotic fractures in europeans and non-hispanic whites from North America: a systematic review and updated Meta-analysis. Adv Nutr. 2019;10(suppl2):S120–43.
Santiago ECS, Roriz AKC, Ramos LB, Ferreira AJF, Oliveira CC, Gomes-Neto M. Comparison of calorie and nutrient intake among elderly with and without Sarcopenia: a systematic review and meta-analysis. Nutr Rev. 2021;79(12):1338–52.
Bloom I, Shand C, Cooper C, Robinson S, Baird J. Diet Quality and Sarcopenia in older adults: a systematic review. Nutrients. 2018;10(3).
Park SJ, Park J, Won CW, Lee HJ. The Inverse Association of Sarcopenia and Protein-Source Food and vegetable intakes in the Korean Elderly: the Korean Frailty and Aging Cohort Study. Nutrients. 2022;14(7).
Shibasaki K, Kin SK, Yamada S, Akishita M, Ogawa S. Sex-related differences in the association between frailty and dietary consumption in Japanese older people: a cross-sectional study. BMC Geriatr. 2019;19(1):211.
Chan R, Leung J, Woo J. A Prospective Cohort Study to Examine the Association between Dietary Patterns and Sarcopenia in Chinese Community-Dwelling Older people in Hong Kong. J Am Med Dir Assoc. 2016;17(4):336–42.
Yeung SSY, Zhu ZLY, Chan RSM, Kwok T, Woo J. Prospective Analysis of Fruit and Vegetable Variety on Health outcomes in Community-Dwelling Chinese older adults. J Nutr Health Aging. 2021;25(6):735–41.
Health Mo. National Health and Nutrition Survey 2019 [ https://www.mhlw.go.jp/stf/seisakunitsuite/bunya/kenkou_iryou/kenkou/eiyou/r1-houkoku_00002.html.
Remond D, Shahar DR, Gille D, Pinto P, Kachal J, Peyron MA, et al. Understanding the gastrointestinal tract of the elderly to develop dietary solutions that prevent malnutrition. Oncotarget. 2015;6(16):13858–98.
Roubenoff R. Catabolism of aging: is it an inflammatory process? Curr Opin Clin Nutr Metab Care. 2003;6(3):295–9.
Sanchez-Morate E, Gimeno-Mallench L, Stromsnes K, Sanz-Ros J, Roman-Dominguez A, Parejo-Pedrajas S et al. Relationship between Diet, Microbiota, and healthy aging. Biomedicines. 2020;8(8).
Sheridan PO, Bindels LB, Saulnier DM, Reid G, Nova E, Holmgren K, et al. Can prebiotics and probiotics improve therapeutic outcomes for undernourished individuals? Gut Microbes. 2014;5(1):74–82.
Murakami K, Sasaki S, Takahashi Y, Uenishi K, Yamasaki M, Hayabuchi H, et al. Misreporting of dietary energy, protein, potassium and sodium in relation to body mass index in young Japanese women. Eur J Clin Nutr. 2008;62(1):111–8.
Murakami K, Livingstone MBE, Okubo H, Sasaki S. Younger and older ages and obesity are associated with energy intake underreporting but not overreporting in Japanese boys and girls aged 1–19 years: the National Health and Nutrition Survey. Nutr Res. 2016;36(10):1153–61.
Aoyama S, Kim HK, Hirooka R, Tanaka M, Shimoda T, Chijiki H, et al. Distribution of dietary protein intake in daily meals influences skeletal muscle hypertrophy via the muscle clock. Cell Rep. 2021;36(1):109336.
Kinoshita K, Otsuka R, Nishita Y, Tange C, Tomida M, Zhang S, et al. Breakfast protein quality and muscle strength in Japanese older adults: A Community-based longitudinal study. J Am Med Dir Assoc. 2022;23(5):729–35. e2.
Acknowledgements
The authors would like to thank Liu L., Aoki T., Nakagata T., Hui H. and all staff for their contributions to data collection at the Sportology Center.
Funding
This study was supported by Strategic Research Foundation at Private Universities (S1411006) and KAKENHI (18H03184) grants from the Ministry of Education, Culture, Sports, Science, and Technology of Japan, by the Mizuno Sports Promotion Foundation, and by the Mitsui Life Social Welfare Foundation.
Author information
Authors and Affiliations
Contributions
H.K., Y.S., S.K., R.K., H.W., and Y.T. contributed to drafting the Bunkyo Health Study. Y.M. and H.K. contributed substantially to the data analysis. H.K. and Y.T. were responsible for the overall conception and design of this manuscript. Y.M., H.K., and Y.T. drafted the manuscript. TH.B., M.S., H.K., H.T., S.K., A.A., H.N., H.S., H.O., and Y.Y. contributed to the data interpretation.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was conducted in accordance with the Declaration of Helsinki, and approved by the ethics committee of Juntendo University, on 6 November 2015 (Nos. 201507 and M15-0057). All participants provided written informed consent and were informed that they had the right to withdraw from the trial at any time.
Consent for publication
Written informed consent was obtained from the participants for publication of this study.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Muroga, Y., Kaga, H., Bui, T.H. et al. Dietary characteristics of urban community-dwelling older adults with low muscle mass: the bunkyo health study: a cross-sectional study. BMC Geriatr 24, 614 (2024). https://doi.org/10.1186/s12877-024-05218-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12877-024-05218-4