Abstract
Background
The correlation between the triglyceride-glucose index (TyG) and the prognosis of ischemic stroke has been well established. This study aims to assess the influence of the TyG index on the clinical outcomes of critically ill individuals suffering from intracerebral hemorrhage (ICH).
Methods
Patients diagnosed with ICH were retrospectively retrieved from the Medical Information Mart for Intensive Care (MIMIC-IV) and the eICU Collaborative Research Database (eICU-CRD). Various statistical methods, including restricted cubic spline (RCS) regression, multivariable logistic regression, subgroup analysis, and sensitivity analysis, were employed to examine the relationship between the TyG index and the primary outcomes of ICH.
Results
A total of 791 patients from MIMIC-IV and 1,113 ones from eICU-CRD were analyzed. In MIMIC-IV, the in-hospital and ICU mortality rates were 14% and 10%, respectively, while in eICU-CRD, they were 16% and 8%. Results of the RCS regression revealed a consistent linear relationship between the TyG index and the risk of in-hospital and ICU mortality across the entire study population of both databases. Logistic regression analysis revealed a significant positive association between the TyG index and the likelihood of in-hospital and ICU death among ICH patients in both databases. Subgroup and sensitivity analysis further revealed an interaction between patients' age and the TyG index in relation to in-hospital and ICU mortality among ICH patients. Notably, for patients over 60 years old, the association between the TyG index and the risk of in-hospital and ICU mortality was more pronounced compared to the overall study population in both MIMIC-IV and eICU-CRD databases, suggesting a synergistic effect between old age (over 60 years) and the TyG index on the in-hospital and ICU mortality of patients with ICH.
Conclusions
This study established a positive correlation between the TyG index and the risk of in-hospital and ICU mortality in patients over 60 years who diagnosed with ICH, suggesting that the TyG index holds promise as an indicator for risk stratification in this patient population.
Similar content being viewed by others
Introduction
Spontaneous, nontraumatic, intracerebral hemorrhage (ICH) is a catastrophic disease making up approximately 10–20% of all types of stroke [1]. Epidemiological data indicate that 30% of ICH patients requiring intensive care unit (ICU) management and 40% of them die within 30 days [2]. Despite ongoing research and advancements in this medical field, effective therapeutic options for improving the prognosis of patients with ICH are still lacking [3]. Consequently, there is an urgent need to identify remediable factors that may impact the outcomes of ICH, as this information could potentially lead to the development of new therapeutic targets.
Insulin resistance (IR), a pathological condition where tissue does not respond normally to insulin stimulation, plays a crucial role in the development of metabolic disorders [4]. More importantly, studies have revealed that compared with peripheral tissue, IR appears earlier in the central nervous system, indicating that brain is more vulnerable to IR, especially under various pathological states such as ICH and ischemic stroke (IS) [5]. Therefore, the indicators associated with IR may be closely related to the initiation of ICH and its prognosis.
The triglyceride-glucose (TyG) index, consisting of fasting triglyceride (FTG) and fasting blood glucose (FBG), is a valuable tool for analyzing lipid and glucose metabolism [6, 7]. It is also recognized as an accurate indicator of IR [8, 9]. Some researchers have observed a positive correlation between the TyG index and the incidence and mortality rates of progressed coronary artery disease [10, 11]. Additional studies have indicated that the TyG index may have the potential to forecast negative cardiovascular events in individuals with coronary artery disease [12]. Moreover, multiple studies have demonstrated the predictive ability of the TyG index for the onset and mortality of IS [13, 14]. These findings collectively highlight the association of the TyG index with cardiovascular and cerebrovascular diseases. However, the relationship between ICH and the TyG index, as well as the prognostic role of the TyG index in this condition, remains unexplored.
Therefore, the objective of this study is to evaluate the impact of the TyG index on the prognosis of critically ill patients with ICH, which may establish its potential utility as a risk stratification tool in ICH cases.
Materials
Data sources
Data used in this study were extracted from the Medical Information Mart for Intensive Care (MIMIC-IV version 2.2) and the eICU Collaborative Research Database (eICU-CRD) [15, 16]. MIMIC-IV consists of medical records between 2008 and 2019 from over 190,000 patients who were treated in various types of ICU of the Beth Israel Deaconess Medical Center. The eICU-CRD included medical records of over 200,000 patients receiving clinical management in ICUs from over 200 medical centers between 2014 and 2015. Since data in these two databases are de-identified to hide patients’ information, the informed consent and ethics approval are not essential.
Data extraction
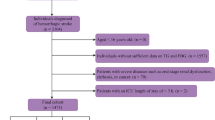
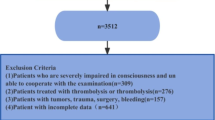
Structure query language (SQL), executed on the PostgresSQL (version 13.7.2), was utilized for data extraction from MIMIC-IV and eICU-CRD. One researcher (Yang Yang) with authorization to access both databases (Record ID: 48,776,647) conducted the data extraction. Inclusive criteria encompassed patients who were (1) aged 18 years and above; (2) diagnosed with ICH in accordance with International Classification of Diseases, 9th and 10th Revision (ICD9 and ICD10). Exclusion criteria included: (1) patients with multiple hospitalization entries, only data from the initial hospitalization due to ICH were considered; (2) patients lacking data of FTG and FBG on the first day of ICU admittance were omitted; (3) individuals who expired or were released within 24 h of ICU admission were excluded due to their significant missing data for key variables used in the regression analysis. Therefore, excluding this group of patients was necessary to ensure the reliability of the results of the regression analysis.
The following information was extracted for the final study cohort: (1) patients’ age and gender; (2) comorbidities identified by ICD-9 and ICD-10 codes; (3) initial FBG and FTG results within 24 h post-ICU admission; (4) average values of laboratory parameters within 24 h of ICU admittance; (5) minimum Glasgow Coma Scale (GCS) score on the first day of ICU admittance; (6) maximum Acute Physiology Score III (APSIII) and Sequential Organ Failure Assessment (SOFA) scores on the first day of ICU management; (7) treatment-related data that may impact the prognosis of ICH patients were extracted, which includes invasive mechanical ventilation, the use of anticoagulants, and the use of antiplatelet agents during hospitalization.
Assessment of the TyG index
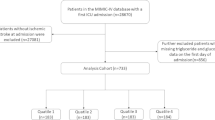
The TyG index is calculated using the formula: TyG index = ln [FTG (mg/dl) × FBG (mg/dl)/2], where FTG and FBG represent the first recorded values of FBG and FTG since ICU admission [17, 18]. In the subsequent statistical analysis, the TyG index was considered both as a continuous and categorical variable. When treated as a categorical variable, it was divided into four groups based on quartiles. The data extraction process is illustrated in Fig. 1.
Primary outcomes
The primary outcomes of interest were all-cause in-hospital mortality and ICU mortality, which were defined as deaths occurring during hospitalization and ICU staying, respectively.
Statistical analysis
Continuous variables were expressed as median (interquartile ranges) and categorical variables were described as number (percentages). Comparisons between groups were performed by Mann–Whitney U or Kruskal–Wallis test for continuous variables, and chi-squared or Fisher’s exact test for categorical ones.
In order to investigate the relationship between the TyG index and the primary outcomes, an initial analysis utilizing restricted cubic splines (RCS) with four knots was carried out to assess any potential non-linear associations between the TyG index and the risk of in-hospital and ICU mortality. If a non-linear relationship was not detected, logistic regression analysis were performed using three different models: model 1 included only the TyG index, model 2 adjusted for age and gender, and model 3 further adjusted for various potential confounders relevant to the clinical outcomes of ICH, including GCS, hypertension, congestive heart failure, white blood cell count (WBC), serum urea nitrogen (BUN), serum creatinine, red cell distribution width (RDW), serum bilirubin, serum aspartate aminotransferase (AST), prothrombin time (PT), use of anticoagulants, and use of antiplatelet agents. Additionally, to check for multicollinearity in the logistic regression analysis, a Spearman rank correlation test was carried out and the square root of the variance inflation factor (VIF) was calculated.
To explore potential variations within specific populations, subgroup analysis was conducted by stratifying patients according to gender, age (> 60 vs. ≤ 60 years), diabetes, hypertension, use of anticoagulants, and use of antiplatelet agents. The interaction between the TyG index and the other variables utilized for stratification in subgroup analysis was evaluated through likelihood ratio test. Finally, a sensitivity analysis was performed by using Cox proportional hazard regression to verify the relationship between the TyG index and in-hospital and ICU mortality. The follow-up period was measured from the date of hospital or ICU admission to the date of death during the hospitalization or ICU stay. The Cox regression model was adjusted for possible confounders as outlined in the fully adjusted model (model 3) of logistic regression mentioned above.
All statistical analysis were performed using R software (version 4.3.1). The“VIM”package was used to visualize the distribution of missing values, from which we can see that all variables had missing ratio less than 20% (Additional file 1: Figure S1). The “mice” package was adopted to address missing values by multiple imputation to obtain 5 imputation datasets in the process of logistic regression. Besides, the “corrplot” package was used to visualize the associations between continuous variables. The“plotRCS” package was used to perform RCS. The“forestploter” package was adopted to visualized the results of subgroup analysis. The “survminer” package was used to conduct Cox regression analysis. Statistically significant was set as a two tailed P < 0.05.
Results
Baseline characteristics
A total of 791 patients from MIMIC-IV and 1,113 from eICU-CRD were included in the final analysis. Among them, 418 (53%) individuals in MIMIC-IV and 627 (56%) in eICU-CRD were male. The in-hospital mortality rates were 14% in MIMIC-IV and 16% in eICU-CRD, with ICU mortality rates of 10% and 8% respectively. The median age was 72.25 (60.63, 82.59) years in MIMIC-IV and 66 (55, 77) years in eICU-CRD. Besides, the average value of TyG index was 8.72 (8.38, 9.17) in MIMIC-IV and 8.76 (8.33, 9.21) in eICU-CRD.
When dividing participants into groups based on the quartiles of the TyG index, patients in the upper quartiles had significantly higher APSIII scores, and higher proportion of invasive ventilation than those in the lower quartiles (P < 0.001 for all). Furthermore, hospital stay time, ICU stay time, in-hospital mortality, and ICU mortality all exhibited a gradual increase from the first to the fourth quartile of the TyG index. However, there was no significant difference in the mean hospital and ICU survival time of patients who died in the hospital or ICU across the quartiles of the TyG index. (Table 1 and Additional file 2: Table S1).
Baseline data of participants divided by the hospital and ICU outcomes are presented in Table 2 and Additional file 3: Table S2, respectively. Compared to in-hospital and ICU survivors, non-survivors in both the MIMIC-IV and eICU-CRD databases showed significantly higher APSIII and SOFA scores, shorter hospital stays, and a higher proportion of invasive ventilation. However, compared to in-hospital and ICU survivors, ICU stay time was shorter in non-survivors from eICU-CRD and longer in non-survivors from MIMIC-IV. Furthermore, GCS scores were lower in in-hospital and ICU non-survivors compared to survivors in eICU-CRD, but there was no significant difference in GCS scores between in-hospital and ICU survivors and non-survivors in MIMIC-IV. Interestingly, despite the potential risk of secondary hemorrhage associated with antiplatelet agents, their usage was more common among in-hospital and ICU non-survivors than survivors in MIMIC-IV. Moreover, the TyG index was notably higher in the in-hospital non-survivors compared to survivors (MIMIC-IV: 8.94 (8.51–9.48) vs. 8.70 (8.36–9.09); P < 0.001. eICU-CRD: 8.98 (8.51–9.49) vs. 8.70 (8.31–9.16); P < 0.001). Similarly, the TyG index was significantly elevated in ICU non-survivors in contrast to ICU survivors (MIMIC-IV: 9.00 (8.51–9.48) vs. 8.70 (8.36–9.10); P < 0.001. eICU-CRD: 9.09 (8.76–9.65) vs. 8.71 (8.31–9.17); P < 0.001) (Additional file 4: Figure S2).
Association between the TyG index and the primary outcomes
We initially conducted a nonlinear correlation analysis between the TyG index and the primary outcomes using RCS. Findings suggested no significant nonlinear correlation between the TyG indicator and the likelihood of either in-hospital or ICU mortality (In-hospital mortality: P for nonlinear = 0.751 in MIMIC-IV, P for nonlinear = 0.562 in eICU-CRD. ICU mortality: P for nonlinear = 0.986 in MIMIC-IV, P for nonlinear = 0.431 in eICU-CRD) (Fig. 2). Subsequently, logistic regression analysis was conducted to assess the linear relationship between the TyG index and the primary outcomes. In the fully adjusted model (model 3) that adjusted for various potential confounders related to the clinical outcomes of ICH, a positive correlation was found between the TyG index and the risk of in-hospital mortality (MIMIC-IV: OR 1.75 [95%CI 1.20–2.52], P = 0.003. eICU-CRD: OR 1.37 [95%CI 1.05–1.80], P < 0.001) and ICU mortality (MIMIC-IV: OR 2.15 [95%CI 1.45–3.17], P < 0.001. eICU-CRD: OR 1.61 [95%CI 1.13–2.27], P < 0.001). Moreover, compared to the first quartile (Q1) of the TyG index, the results of model 3 indicated that the fourth quartile (Q4) was linked to a higher risk of in-hospital mortality (MIMIC-IV: OR 2.31 [95%CI 1.18–4.67], P = 0.017. eICU-CRD: 1.73 [95%CI 1.02–3.06], P = 0.036) and ICU mortality (MIMIC-IV: OR 3.24 [95%CI 1.54–7.11], P = 0.002. eICU-CRD: 2.30 [95%CI 1.09–5.16], P = 0.034) (Table 3, Additional file 5: Table S3).
Restricted cubic spline analysis for the nonlinear association between the TyG index and the risk of, A in-hospital mortality of ICH patients from MIMIC-IV; B ICU mortality of ICH patients from MIMIC-IV; C in-hospital mortality of ICH patients from eICU-CRD; D ICU mortality of ICH patients from eICU-CRD
To assess multicollinearity in the logistic regression model, the Spearman rank correlation coefficient and VIF were calculated, respectively. Findings revealed that there was no linear correlation between the TyG index and the other continuous variables incorporated in model 3 (Additional file 6: Figure S3). Additionally, none of the variables in model 3 exhibited a square root of VIF ≥ 2 (data not shown). Taken together, these results suggest that there is no multicollinearity present in the logistic regression model, indicating the reliability of the results.
Subgroup analysis
To investigate potential variations within specific populations, logistic regression analysis was conducted across various subgroups, including gender, age, diabetes, hypertension, use of anticoagulant agents, and use of antiplatelet agents. The forest plot revealed a significant positive correlation between the TyG index and in-hospital mortality among participants over 60 years (MIMIC-IV: OR 2.58 [95%CI 1.92–4.27], P = 0.005. eICU-CRD: OR 1.56 [95%CI 1.10–2.22], P = 0.019), those without diabetes (MIMIC-IV: OR 2.20 [95%CI 1.34–3.57], P = 0.002. eICU-CRD: OR 1.69 [95%CI 1.18–2.41], P = 0.004), and those with hypertension (MIMIC-IV: OR 1.97 [95%CI 1.28–3.01], P = 0.002. eICU-CRD: OR 1.84 [95%CI 1.28–2.99], P = 0.014). Similarly, there was a positive association for ICU mortality in patients over 60 years (MIMIC-IV: OR 3.86 [95%CI 1.31–6.99], P < 0.001. eICU-CRD: OR 1.70 [95%CI 1.23–2.90], P = 0.036), those without diabetes (MIMIC-IV: OR 2.62 [95%CI 1.56–4.40], P < 0.001. eICU-CRD: OR 2.10 [95%CI 1.34–3.32], P = 0.001), and those with hypertension (MIMIC-IV: OR 2.44 [95%CI 1.54–3.84], P < 0.001. eICU-CRD: OR 1.83 [95%CI 1.13–2.97], P = 0.013). Furthermore, in both MIMIC-IV and eICU-CRD, significant interactions were found between the TyG index and patients' age, diabetic status, and history of hypertension concerning in-hospital and ICU outcomes of individuals with ICH (P for interaction < 0.05 for all) (Figs. 3 and 4).
Sensitivity analysis
To further verify the association between the TyG index and in-hospital and ICU mortality, as well as the significant interactions between the TyG index and patients' age regarding in-hospital and ICU outcomes, a sensitivity analysis was performed using Cox proportional hazard regression. Following fully adjusted, a positive correlation was observed between the TyG index and the risk of in-hospital mortality in ICH patients from both MIMI-IV and eICU-CRD datasets. The positive association between the TyG index and in-hospital and ICU mortality was also present in patients over 60 years old, those without diabetes, and those with hypertension in both databases. Importantly, significant interactions were only found between patients' age and the TyG index concerning in-hospital and ICU outcomes of ICH patients in both MIMIC-IV and eICU-CRD datasets (Table 4 and Additional file 7: Table S4). These findings collectively suggest that the TyG index has the potential to serve as a prognostic indicator for ICH in patients over 60 years of age.
Discussion
In this retrospective multicenter study, the impact of the TyG index on the prognosis of critically ill patients with ICH was evaluated, uncovering two important findings. Firstly, a positive correlation was found between the TyG index and the risk of in-hospital and ICU all-cause mortality in ICH patients. Secondly, this correlation was notably stronger in patients over 60 years old, especially in those with hypertension or lacking diabetes.
The association between the TyG index and the course of IS has been extensively studied. Wang et al. reported that individuals in the highest quartile of the TyG index face a 1.45 times greater risk of developing IS compared to those in the lowest quartile [19]. Results from a 9-year prospective study showed that keeping TyG index elevated was strongly related to an increased morbidity of IS, suggesting that monitoring and regulating the TyG index at an appropriate level could be beneficial in preventing IS [20]. Additionally, various studies have explored the capability of the TyG index in predicting the outcome of IS. Lee and colleagues found that the TyG index could forecast an adverse functional outcome three months post-reperfusion in IS patients [21]. Yang et al. noted a correlation between higher TyG index and elevated rates of both recurrence and mortality within one year following an IS event [22]. In critically ill patients, Cai W et al. found that the TyG index may assist in identifying IS patients at high risk of all-cause mortality [14]. Despite these findings highlight the significant relationship between the TyG index and IS as well as its prognosis, research on the association between the TyG index and ICH remains scarce.
To address this gap in knowledge, we conducted this study and found a positive correlation between the TyG index and the likelihood of either in-hospital or ICU mortality in individuals with ICH. The positive correlation persisted even after adjusting for potential confounders, suggesting that the TyG index could serve as an independent predictor of hospitalization outcomes in patients with ICH, potentially aiding clinicians in their decision-making process. More importantly, subgroup analysis revealed that there is a synergistic effect of old age (over 60 years), hypertension, and non-diabetic status on the TyG index’s impact on hospitalization outcomes in patients with ICH. Sensitivity analysis using Cox regression model further confirmed the synergistic effect between old age (over 60 years) and the TyG index on the in-hospital and ICU mortality of ICH patients in both MIMIC-IV and eICU-CRD databases. These results underscored the population-specific influence of the TyG index on ICH prognosis, highlighting the importance of focusing on elderly patients with ICH.
The exact mechanisms connecting the TyG index with the poor prognosis of ICH are still unclear, but evidence supports a key role of IR in this process. Patients with IR are more susceptible to hyperglycemia. A study has found that hyperglycemia could inhibit the expression of Aquaporin-4, resulting in the aggravation of vasogenic brain edema and blood–brain barrier (BBB) destruction [23]. Autophagy is a vital cellular process for maintaining homeostasis, but hyperglycemia can decrease autophagic activity in the brain during ICH, leading to the accumulation of macromolecular debris and damaged cells, ultimately causing neuronal injury [24]. In stoke rat treated with type plasminogen activator, hyperglycemia could enhance superoxide production in brain tissue and blood vessels, increasing BBB permeability in the peri-ischemic area and leading to a 3- to fivefold rise in the volume of secondary hemorrhage after thrombolysis [25]. This finding establishes a link between hyperglycemia-induced superoxide production and the increased risk of hematoma expansion in IS. The generation of reactive oxygen species has been demonstrated during ICH [26, 27]. Moreover, studies have shown that an increase in blood glucose can exacerbate hematoma expansion in a rat model of ICH [28]. Therefore, it is plausible to infer that IR related hyperglycemia may result in poor outcomes of ICH by promoting superoxide production, thereby increasing the risk of hematoma expansion.
Adequate cerebral perfusion is crucial for determining the prognosis of patients with ICH. The automatic regulation ability of cerebral blood vessels plays a significant role in maintaining appropriate cerebral perfusion during cerebral hemorrhage. Studies have indicated that elevated intracranial pressure in patients with acute ICH can impair cerebrovascular autoregulation within a two-week period [29]. The myogenic response, which refers to the ability of smooth muscle cells to react to changes in blood pressure, is essential for preserving cerebrovascular autoregulation [30]. Animal studies have proved that IR can heighten the tension of cerebrovascular myogenic response, leading to a reduction in the diameter of the cerebrovascular lumen, subsequently causing brain tissue ischemia and nerve cell injury [31]. In addition, the smooth muscle activity of distal cerebral arteries is notably higher than that of proximal ones, making them more vulnerable to IR and resulting in impaired cerebral circulation function [30].
Chronic inflammatory response is one pathogenesis of IR, and IR in turn can reflect the level of systemic inflammation in the body [32]. Interestingly, our study revealed a gradual increase in WBC across quartile intervals of the TyG index, with values exceeding the upper limit of the reference range in the fourth quartile, suggesting an escalation of systemic inflammation during the course of ICH in patients with IR. Furthermore, the integrity of BBB was compromised during ICH, allowing the infiltration of peripheral immune cells and pro-inflammatory cytokines into the central nervous system. This heightened inflammatory response in the brain can further compromise the BBB, creating a detrimental cycle [33]. In addition, study has shown that dysregulation of the insulin signaling pathway can activate NF-κB, leading to the transcription and expression of inflammatory factors in the brain, thereby exacerbating neuroinflammation [34].
Intriguingly, although the evidence presented supports the significant role of IR in linking the TyG index to the unfavorable prognosis of intracerebral hemorrhage (ICH), our study found no correlation between the TyG index and either in-hospital or ICU mortality in ICH patients with a history of diabetes, a group known to have a higher risk of IR compared to non-diabetic individuals. Explaining the cause of this paradox is challenging. One potential reason could be reverse causality [14, 35], where patients diagnosed with diabetes may be more likely to accept appropriate treatment or adopt healthy lifestyle habits. This could lead to their analytical parameters being similar to or even lower than those of non-diabetic counterparts. Consistent to this theory, in our study, no significant differences were observed in FBG, FTC, and TyG indexes between diabetic and non-diabetic individuals in both the MIMC-IV and eICU-CRD study cohorts (TyG index: 8.71 (8.38, 9.17) vs 8.77 (8.39, 9.14), P = 0.673 for MIMIC-IV. 8.75 (8.34, 9.22) vs 8.78 (8.3, 9.17), P = 0.925 for eICU-CRD). Additionally, as diabetic patients may have adopted a healthier lifestyle than their non-diabetic counterparts, their prognosis might be improved in subgroup analyses stratified by diabetes status.
The hyper-insulinemic-euglycemic clamp is considered the most accurate method for detecting IR, but its practicality is limited due to high costs, time-consuming procedures, and invasiveness. The homeostasis model assessment index for IR (HOMA-IR) is commonly used in clinical settings to assess beta-cell function and detect IR [36]. Nevertheless, its applicability is restricted in patients undergoing insulin therapy or those with ineffective beta cells [37]. Besides, HOMA-IR relies on measuring insulin levels, which are not routinely checked in clinical practice. Therefore, researchers have introduced the TyG index as a potentially reliable and cost-effective alternative marker for IR. Using hyper-insulin-normoglycemia clamp technique as the gold standard, a study showed excellent predictive efficiency of the TyG index for IR, with sensitive and specificity of 96.5% and 85.0%, respectively [8]. David and colleagues also demonstrated that the TyG index outperforms FBG and FTG in diagnosing type 2 diabetes and monitoring its progression [38]. Given that FBG and FTG measurements are available in most healthcare facilities, the TyG index has the potential to be widely utilized in blood glucose management and could serve as a valuable tool for risk assessment in patients with ICH.
The study has several limitations. First, the location and volume of hemorrhage, which are crucial factors influencing the prognosis of ICH, could not be extracted from the databases. Therefore, future research should incorporate these indicators to further validate the current findings. Secondly, specific population such as the Chinese or African are scarce in the study cohort. Consequently, the conclusions derived from this study should be cautiously interpreted in these population. Thirdly, the impact of dynamic changes in the TyG index on the prognosis of ICH patients was not assessed in this study. Given that variability in the TyG index has been linked to the incidence of IS [20], further studies are needed to investigate the cumulative effect of the TyG index on the incidence and outcome of ICH. Fourthly, exclusion of patients without FTG and FBG data on the first day of ICU admission may introduce bias if the missing data pattern is not completely random. Last but not the least, the utilization of propofol, fibrate, and glucose, along with insulin infusion prior to hospitalization, could have a notable effect on FTG and FBG levels. Nevertheless, neither MIMIC-IV nor eICU-CRD databases contains information on pre-hospitalization medications. Therefore, further investigation is required to confirm the current findings by incorporating these treatment-related information before ICU admission.
Conclusion
This study identified a positive correlation between the TyG index and in-hospital as well as ICU all-cause mortality in patients with ICH, particularly among individuals aged over 60 years with a history of hypertension. The findings indicate that the TyG index may be a useful tool for risk stratification in elderly patients with ICH, assisting clinicians in identifying high-risk individuals and providing timely intervention.
Availability of data and materials
The available data for MIMIC-IV can be accessed from the website https://mimic.physionet.org/. The available data for eICU-CRD can be accessed from the website https://eicu-crd.mit.edu/. The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- APSIII:
-
Acute Physiology Score III
- AST:
-
Serum aspartate aminotransferase
- BBB:
-
Blood–brain barrier
- BUN:
-
Serum urea nitrogen
- eICU-CRD:
-
The eICU Collaborative Research Database
- FBG:
-
Fasting blood glucose
- FTG:
-
Fasting triglyceride
- GCS:
-
Glasgow coma scale
- HOMA-IR:
-
The homeostasis model assessment index for IR
- ICD:
-
International Classification of Diseases
- ICU:
-
Intensive care unit
- ICH:
-
Intracerebral hemorrhage
- IR:
-
Insulin resistance
- IS:
-
Ischemic stroke
- MIMIC:
-
The Medical Information Mart for Intensive Care
- OR:
-
Odds ratios
- PT:
-
Prothrombin time
- RCS:
-
Restricted cubic spline
- RDW:
-
Red cell distribution width
- SOFA:
-
Sequential Organ Failure Assessment
- SQL:
-
Structure Query Language
- TyG index:
-
Triglyceride glucose index
- VIF:
-
Variance inflation factor
References
Qureshi A, Mendelow A, Hanley D. Intracerebral haemorrhage. Lancet (London, England). 2009;373(9675):1632–44.
van Asch C, Luitse M, Rinkel G, van der Tweel I, Algra A, Klijn C. Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: a systematic review and meta-analysis. The Lancet Neurology. 2010;9(2):167–76.
Keep R, Hua Y, Xi G. Intracerebral haemorrhage: mechanisms of injury and therapeutic targets. The Lancet Neurology. 2012;11(8):720–31.
Lee S, Park S, Choi C. Insulin Resistance: From Mechanisms to Therapeutic Strategies. Diabetes Metab J. 2022;46(1):15–37.
Scherer T, Sakamoto K, Buettner C. Brain insulin signalling in metabolic homeostasis and disease. Nat Rev Endocrinol. 2021;17(8):468–83.
Gui J, Li Y, Liu H, et al. Obesity- and lipid-related indices as a predictor of obesity metabolic syndrome in a national cohort study. Front Public Health. 2023;11:1073824.
Kim J, Kim J, Roh E, et al. Triglyceride and glucose index and the risk of gestational diabetes mellitus: A nationwide population-based cohort study. Diabetes Res Clin Pract. 2021;171:108533.
Guerrero-Romero F, Simental-Mendía L, González-Ortiz M, et al. The product of triglycerides and glucose, a simple measure of insulin sensitivity Comparison with the euglycemic-hyperinsulinemic clamp. J Clin Endocrinol Metabol. 2010;95(7):3347–51.
Irace C, Carallo C, Scavelli F, et al. Markers of insulin resistance and carotid atherosclerosis. A comparison of the homeostasis model assessment and triglyceride glucose index. Int J Clin Prac. 2013;67(7):665–72.
Li J, Ren L, Chang C, Luo L. Triglyceride-Glukose Index Predicts Adverse Events in Patients with Acute Coronary Syndrome: A Meta-Analysis of Cohort Studies. Horm Metabol Res. 2021;53(9):594–601.
Ding X, Wang X, Wu J, Zhang M, Cui M. Triglyceride-glucose index and the incidence of atherosclerotic cardiovascular diseases: a meta-analysis of cohort studies. Cardiovasc Diabetol. 2021;20(1):76.
Jin J, Cao Y, Wu L, et al. Triglyceride glucose index for predicting cardiovascular outcomes in patients with coronary artery disease. J Thorac Dis. 2018;10(11):6137–46.
Liu D, Yang K, Gu H, Li Z, Wang Y, Wang Y. Predictive effect of triglyceride-glucose index on clinical events in patients with acute ischemic stroke and type 2 diabetes mellitus. Cardiovasc Diabetol. 2022;21(1):280.
Cai W, Xu J, Wu X, et al. Association between triglyceride-glucose index and all-cause mortality in critically ill patients with ischemic stroke: analysis of the MIMIC-IV database. Cardiovasc Diabetol. 2023;22(1):138.
Pollard T, Johnson A, Raffa J, Celi L, Mark R, Badawi O. The eICU Collaborative Research Database, a freely available multi-center database for critical care research. Scientific data. 2018;5:180178.
Johnson A, Bulgarelli L, Shen L, et al. MIMIC-IV, a freely accessible electronic health record dataset. Scientific data. 2023;10(1):1.
Liao Y, Zhang R, Shi S, et al. Triglyceride-glucose index linked to all-cause mortality in critically ill patients: a cohort of 3026 patients. Cardiovasc Diabetol. 2022;21(1):128.
Zhang R, Shi S, Chen W, et al. Independent effects of the triglyceride-glucose index on all-cause mortality in critically ill patients with coronary heart disease: analysis of the MIMIC-III database. Cardiovasc Diabetol. 2023;22(1):10.
Wang A, Wang G, Liu Q, et al. Triglyceride-glucose index and the risk of stroke and its subtypes in the general population: an 11-year follow-up. Cardiovasc Diabetol. 2021;20(1):46.
Wang X, Feng B, Huang Z, et al. Relationship of cumulative exposure to the triglyceride-glucose index with ischemic stroke: a 9-year prospective study in the Kailuan cohort. Cardiovasc Diabetol. 2022;21(1):66.
Lee M, Kim C, Kim Y, et al. High Triglyceride Glucose Index Is Associated with Poor Outcomes in Ischemic Stroke Patients after Reperfusion Therapy. Cerebrovasc Dis. 2021;50(6):691–9.
Yang X, Wang G, Jing J, et al. Association of triglyceride-glucose index and stroke recurrence among nondiabetic patients with acute ischemic stroke. BMC Neurol. 2022;22(1):79.
Chiu C, Chen C, Shen C, et al. Hyperglycemia exacerbates intracerebral hemorrhage via the downregulation of aquaporin-4: temporal assessment with magnetic resonance imaging. Stroke. 2013;44(6):1682–9.
Liu R, Wang J, Qiu X, Wu J. Acute hyperglycemia together with hematoma of high-glucose blood exacerbates neurological injury in a rat model of intracerebral hemorrhage. Neurosci Bull. 2014;30(1):90–8.
Won S, Tang X, Suh S, Yenari M, Swanson R. Hyperglycemia promotes tissue plasminogen activator-induced hemorrhage by Increasing superoxide production. Ann Neurol. 2011;70(4):583–90.
Zhu F, Zi L, Yang P, et al. Efficient Iron and ROS Nanoscavengers for Brain Protection after Intracerebral Hemorrhage. ACS Appl Mater Interfaces. 2021;13(8):9729–38.
Ding R, Chen Y, Yang S, et al. Blood-brain barrier disruption induced by hemoglobin in vivo: Involvement of up-regulation of nitric oxide synthase and peroxynitrite formation. Brain Res. 2014;1571:25–38.
Liu J, Gao B, Clermont A, et al. Hyperglycemia-induced cerebral hematoma expansion is mediated by plasma kallikrein. Nat Med. 2011;17(2):206–10.
Ma H, Guo Z, Liu J, Xing Y, Zhao R, Yang Y. Temporal Course of Dynamic Cerebral Autoregulation in Patients With Intracerebral Hemorrhage. Stroke. 2016;47(3):674–81.
Coucha M, Abdelsaid M, Ward R, Abdul Y, Ergul A. Impact of Metabolic Diseases on Cerebral Circulation: Structural and Functional Consequences. Compr Physiol. 2018;8(2):773–99.
Butcher J, Goodwill A, Stanley S, Frisbee J. Differential impact of dilator stimuli on increased myogenic activation of cerebral and skeletal muscle resistance arterioles in obese zucker rats. Microcirculation. 2013;20(7):579–89.
Glass C, Olefsky J. Inflammation and lipid signaling in the etiology of insulin resistance. Cell Metab. 2012;15(5):635–45.
de la Monte S. Insulin Resistance and Neurodegeneration: Progress Towards the Development of New Therapeutics for Alzheimer’s Disease. Drugs. 2017;77(1):47–65.
Akhtar A, Sah S. Insulin signaling pathway and related molecules: Role in neurodegeneration and Alzheimer’s disease. Neurochem Int. 2020;135:104707.
Laura S, David N, Alejandro F, et al. The TyG index may predict the development of cardiovascular events. Eur J Clin Invest. 2015;46:189–97.
Matthews D, Hosker J, Rudenski A, Naylor B, Treacher D, Turner R. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–9.
Minh H, Tien H, Sinh C, et al. Assessment of preferred methods to measure insulin resistance in Asian patients with hypertension. J Clin Hypertens (Greenwich). 2021;23(3):529–37.
Navarro-González D, Sánchez-Íñigo L, Pastrana-Delgado J, Fernández-Montero A, Martinez J. Triglyceride-glucose index (TyG index) in comparison with fasting plasma glucose improved diabetes prediction in patients with normal fasting glucose: The Vascular-Metabolic CUN cohort. Prev Med. 2016;86:99–105.
Acknowledgements
The present study utilized data from the MIMIC-IV and eICU database. We express our gratitude to all the staff and patients who contributed to the development of the MIMIC-IV and eICU database.
Funding
This research was supported by Innovation Science Fund of Tangdu hospital, China (No. 2023BTDQN001), Shaanxi Province Key Research and Development Plan Project (2024SF-YBXM-210), and Air Force Medical University Clinical Research Program (2023LC2319).
Author information
Authors and Affiliations
Contributions
YY extracted data from MIMIC-IV and eICU-database, analyzed the data, and wrote the original draft. SL conducted literature review, analyzed the data, and wrote the original draft. JDL conducted literature review, and operated software. MM and DJ organized the data and checked the integrity of the data. YS and JWL assisted in statistical analysis. XT designed the study, and checked the final results. LL designed the study, conceptualized the research aims, and revised the paper. All authors have made an intellectual contribution to the manuscript and approved the final submission.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was conducted in accordance with the guidelines of the Helsinki Declaration. As the MIMIC-IV and the eICU-CRD database are publicly available and all data are de-identified to remove patients’ information, the requirement for informed consent of patients is not essential.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Figure S1.
The proportion and distribution of missing data for variables in (A) MIMIC-IV database and (B) eICU-CRD database.
Additional file 4: Figure S2.
The boxplot of the TyG index stratified by the in-hospital and ICU outcomes. (A) The level of the TyG index in hospital survivors and non-survivors from the MIMIC-IV database. (B) The level of the TyG index in hospital survivors and non-survivors from the eICU-CRD database. (C) The level of the TyG index in ICU survivors and non-survivors from the MIMIC-IV database. (D) The level of the TyG index in ICU survivors and non-survivors from the eICU-CRD database.
Additional file 6: Figure S3.
The correlation between continuous variables in the cohort derived from (A) MIMIC-IV and (B) eICU-CRD.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Yang, Y., Liang, S., Liu, J. et al. Triglyceride-glucose index as a potential predictor for in-hospital mortality in critically ill patients with intracerebral hemorrhage: a multicenter, case–control study. BMC Geriatr 24, 385 (2024). https://doi.org/10.1186/s12877-024-05002-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12877-024-05002-4