Abstract
Background
The role of diet quality on malnutrition in older adults is uncertain, due the paucity of the research conducted and the use of use of screening tools that did not consider phenotypic criteria of malnutrition.
Objective
To evaluate the association of two indices of diet quality, namely the Mediterranean Diet Adherence Screener (MEDAS) and the Alternative Healthy Eating Index (AHEI-2010), with malnutrition among community-dwelling older adults in Spain.
Methods
Cross-sectional analysis of data from 1921 adults aged ≥ 60 years from the Seniors-ENRICA-1 (SE-1) study, and 2652 adults aged ≥ 65 years from the Seniors-ENRICA-2 (SE-2) study. Habitual food consumption was assessed through a validated diet history. Malnutrition was defined according to the Global Leadership Initiative on Malnutrition (GLIM) phenotypic criteria. Statistical analyses were performed with logistic regression with adjustment for socioeconomic and lifestyle variables as well as for total energy and protein intake.
Results
The prevalence of malnutrition in the SE-1 study was 9.5% (95% confidence interval: 8.2 to 10.9) and 11.7% (10.5 to 13.9) in the SE-2. Adherence to the MEDAS score was associated with lower prevalence of malnutrition [pooled odds ratio for high (≥ 9 points) vs. low adherence (< 7 points): 0.64 (0.48–0.84); p-trend < 0.001]. Higher adherence to the AHEI-2010 also showed an inverse association with malnutrition (pooled odds ratio for quartile 4 vs. 1: 0.65 (0.49–0.86); p-trend 0.006). Among the individual components, higher consumption of fish and long-chain n-3 fatty acids in MEDAS and AHEI-2010, and of vegetables and nuts and legumes in AHEI-2010, and lower intake of trans-fat and sugar-sweetened beverages and fruit juice in AHEI-2010 were independently associated with lower odds of malnutrition.
Conclusion
Adherence to high diet-quality patterns was associated with lower frequency of malnutrition among older adults.
Clinical trial registry
ClinicalTrials.gov identifier: NCT02804672. June 17, 2016.; ClinicalTrials.gov NCT03541135. May 30, 2018.
Similar content being viewed by others
Introduction
Malnutrition is a syndrome characterized by energy-protein undernutrition [1]. In older adults, malnutrition has been associated with increased morbidity and mortality [2], sarcopenia [3], frailty [4], lower health-related quality of life [5], and important healthcare costs [6]. In addition, underdiagnosis and under-treatment of malnutrition are common in an increasingly aging population [7]. Unfortunately, there is not much data on its prevalence in community-living older adults [6, 8, 9]. This may be due to varying definitions of malnutrition and different diagnostic criteria [3]. Recently, a consensus has been reached to establish a common definition, known as the Global Leadership Initiative on Malnutrition (GLIM) criteria [1], which facilitates comparing results across different studies.
Malnutrition has multiple causes, including older age, living alone, impaired physical function, and a previous hospitalization [10]. Other contributing causes are aging-associated disorders, such as loss of smell, taste and appetite, mastication problems, dysphagia, or the alteration of the physiological mechanisms of thirst, as well as primary diseases that affect nutritional status [11]. Dietary strategies to prevent malnutrition have largely focused on nutrient supplementation [12]. However, there is increasing evidence suggesting that an adequate nutrient intake obtained from the habitual diet could prevent the development of physical impairment and frailty, and possibly, malnutrition [13, 14]. Since dietary recommendations based on diet patterns are easier to implement in the population [15], the identification of those with most benefit for the older population is of great interest to prevent malnutrition.
Two well-known healthy dietary patterns are the Mediterranean diet, assessed by the MEDAS score, and the Western healthy diet, represented by Alternative Healthy Eating Index (AHEI) 2010. Higher adherence to the MEDAS and AHEI-2010 scores have been associated with lower risk of chronic diseases, including cardiovascular disease [16, 17], type 2 diabetes [18] or cancer [19, 20], and death from non-traumatic diseases [21]. In addition, there is evidence that these diet patterns can prevent the frailty syndrome in older adults [22]. However, a recent cross-sectional study among Chinese population found that higher adherence to the Dietary Quality Index International, but not to the Mediterranean diet, was associated with lower likelihood of malnutrition based on the GLIM criteria [23]. Other studies have assessed different diet quality scores in relation to other definitions of malnutrition, with inconsistent results [24,25,26,27].
Therefore, the objective of our study was to evaluate the association between adherence to the MEDAS score and AHEI-2010 with malnutrition, as measured by the GLIM criteria, in two studies of community-dwelling older adults in Spain.
Methods
Study design and participants
This study used data from the Seniors-ENRICA-1 (SE-1) and Seniors-ENRICA-2 (SE-2) studies. The SE-1 was established in 2008–2010 as a cross-sectional study of a representative sample of the population of Spain aged ≥ 18 years [28]. Of them, 3289 participants aged ≥ 60y comprised the Seniors-ENRICA cohort. Information on sociodemographic data, lifestyle, health status, and morbidity were collected by a computer-assisted telephone interview. In two subsequent home visits, a physical examination was done to obtain anthropometric data. In 2012, data were updated and a battery of tests of physical and cognitive function was included; data from this wave have been used in these analyses since the physical assessment included is more comprehensive than in the baseline wave. Participants gave informed written consent, and the Clinical Research Ethics Committee of the La Paz University Hospital in Madrid approved the study (PI-2144, PI-3554).
The SE-2 study included individuals who were recruited in the years 2015–2017 through a non-probabilistic sample of convenience, with stratified cluster sampling by sex and district among all individuals aged 65 years or older with a national healthcare card, from community-dwelling residents in the city of Madrid and four surrounding towns. Baseline data were collected with a similar protocol to that used in the SE-1 [29, 30]. Study participants gave written informed consent, and the study was approved by the Clinical Research Ethics Committee of the La Paz University Hospital in Madrid (PI-1793).
Study variables
Diet
Food consumption was obtained by trained interviewers through a validated computerized diet history, which was developed from the one used in the EPIC-Spain cohort study [31, 32]. Habitual consumption of 860 foods and beverages consumed up to 1 time every 15 days was recorded, to complete a standard 1-week of consumption. Portion sizes, cooking methods, degree of food processing, and weekly and seasonal variations in food consumption were also recorded. Nutrient intakes were derived from Spanish food composition tables [32]. The validity of the diet history was evaluated by comparing its results with seven 24-h recalls over a one-year period among a subsample of participants; the observed correlations ranged between 0.27 and 0.71 across food groups and nutrients [32], which are in line with those for most instruments assessing self-reported diet in population studies [33].
Two healthy diet patterns were derived: the Mediterranean Diet Adherence Screener (MEDAS) and the Alternative Healthy Eating Index (AHEI-2010). The MEDAS score measures adherence to the Mediterranean diet in the Spanish population [34], and comprises 14 items, where 12 of them refer to the frequency of consumption of several foods, and another 2 to dietary habits typical of the Mediterranean diet in Spain. The items are scored 0 or 1 according with the compliance with the cut-off point; the global score ranges from 0 (lowest) to 14 (highest adherence) (Table S1).
The AHEI-2010 was based on a comprehensive review of foods and nutrients that had consistently been associated with lower risk of chronic disease in clinical and epidemiological investigations [35]. A value of 10 is assigned to higher consumption of healthy foods and nutrients, while a value of 0 is assigned to higher consumption of unhealthful dietary components. Intakes between the minimum and maximum levels are scored proportionately. The global score ranges from 0 (lowest) to 110 (highest diet quality) (Table S1).
Malnutrition assessment
We defined malnutrition according to the criteria by the Global Leadership Initiative on Malnutrition (GLIM) to provide a measure of this condition encompassing risk screening and diagnosis [1]. The GLIM group established two groups of criteria: phenotypic, including non-volitional weight loss, low body mass index (BMI), and reduced muscle mass; and etiological, indicating that malnutrition is due to chronic disease with inflammation, acute disease with severe inflammation, or gastrointestinal condition that adversely impacts food assimilation or absorption.
Non-volitional weight loss was defined as positive response to the question: “Have you lost 4.5 kg of weight or more in the last year?” [36]. Weight and height, measured under standardized conditions, using electronic scales (model Seca 841, precision to 0.1 kg) and portable extendable stadiometers (model Ka We 44 444Seca). Mean values of two measurements were used for the analyses. BMI was calculated as weight in kg divided by squared height in m. Participants were classified as “with low BMI” if < 20 kg/m2 among those aged < 70 years, or if < 22 kg/m2 among participants ≥ 70 years [1].
Muscle mass was measured with bioelectrical impedance analysis (BIA) (Tanita® SC-240MA, Tanita Corp., Tokyo, Japan). Skeletal muscle mass (SMM) (kg) was calculated with the equation developed by Janssen et al. [37]: ([height2/resistance * 0.401] + [sex * 3.825] + [age * -0.071]) + 5.102, where height is given in cm, resistance in ohms (from BIA), sex as 1 for males and 0 for females, and age in years. Skeletal muscle mass index (SMI) was estimated by dividing SMM by height in meters squared. Sex-specific thresholds for reduced muscle mass were < 7.26 kg/m2 for males, and < 5.25 kg/m2 for females [38]. We defined moderate-to-severe malnutrition as having at least one phenotypic criterion, since only the phenotypic criteria are proposed for severity grading, and since both studies included community-dwelling population, without acute etiological criteria that needed constant supervised care.
Other variables
In 2012 for the SE-1 and 2015–2017 for the SE-2, participants reported their sex, age, educational level, smoking status, alcohol consumption, sedentary behavior, and total energy intake. Physical activity was assessed with the validated questionnaire developed in the EPIC cohort study in Spain; participants were asked for the number of hours that they spent in a typical week during the last year on each of the following activities: walking, cycling, gardening, do-it-yourself activities at home, playing sports (running, fitness, aerobics, swimming, soccer, tennis, etc.), and climbing stairs. Total physical activity, in metabolic equivalent tasks (METs h/week), was derived from this information [39]. Sedentariness was approached by the time watching TV (h/week). Lastly, the following physician-diagnosed diseases were self-reported: musculoskeletal disease, cardiovascular disease, diabetes, cancer, chronic lung disease, and depression requiring treatment.
Statistical analyses
Of the 3289 participants from SE-1 in 2012, a total of 1921 provided valid data on diet and the variables to create the GLIM criteria. In SE-2, 2652 out from 3273 participants provided information on diet and malnutrition and were included in the analyses. Participants excluded in both cohorts were older, more often women, with lower educational level, with more comorbidity, and reporting less physical activity and more sedentary time per week than participants who accepted to be examined.
The prevalence and 95% confidence interval (CI) of malnutrition and each of its component elements were calculated in both studies. Differences in sociodemographic lifestyle and clinical characteristics by malnutrition status were assessed using the chi-square test for categorical variables and analysis of variance (ANOVA) test for quantitative variables.
Participants were classified into three groups according to their adherence to the diet quality indexes: for the MEDAS, as low (< 7 points), moderate (≥ 7 to < 9 points), and high (≥9 points); for the AHEI-2010, in quartiles. We used logistic regression models to calculate odds ratios (OR), and their 95% CI for the association between the diet indexes and malnutrition. Four consecutive multivariable models were built. The first one was adjusted for sex and age; the second model was additionally adjusted for educational level (primary, secondary and university), as a proxi of socioeconomic level, leisure-time physical activity (METs-h/week), time spent watching TV (h/week), as a proxi of sedentariness, and smoking status (current-, former- or never- smoker). The third model also included energy intake (kcal/day) and total protein intake (g/day). A fourth model was also adjusted for morbidity (musculoskeletal disease, cardiovascular disease, cancer, chronic lung disease and depression). The lowest category of adherence to the MEDAS and the first quartile in the AHEI-2010 were considered as the reference in all the models. We also examined each diet quality score as a continuous variable (per 1-SD) in association with malnutrition. We performed these analyses in each study and then pooled the results to obtain a summary OR estimate, by using inverse variance-weights and a random-effects model [40], which allowed for between-study heterogeneity.
We performed stratified analyses by sex, age, educational level, smoking status, energy intake, total protein intake, as well as presence of musculoskeletal disease, cardiovascular disease, and depression, to better understand their contribution to the examined association. Of note, physical activity, and hours of watching TV were not considered in the stratified analyses since we observed in the multivariable models that their impact modifying the study association was not as relevant as the other variables included. To test for interactions, we used likelihood-ratio tests to compare models with and without an interaction term, defined as the cross-product of the diet quality index (as continuous variable) and the stratification variable. In addition, we examined the association of each component of the diet quality indexes with malnutrition by using fully-adjusted multivariable models that were also adjusted for each of the other food components of the indexes. Finally, a sensitivity analysis was performed excluding the alcohol component from the MEDAS and AHEI-2010 to better understand its role on the studied association. Statistical significance was set at two-tailed p < 0.05. Analyses were performed with Stata (version 16.1; Stata Corp., College Station).
Results
The prevalence of malnutrition was 9.5% (95% CI: 8.2 to 10.9) in the SE-1 study and 11.7% (10.5 to 13.9) in the SE-2 (Table 1), with non-volitional weight loss being the most prevalent criterion in both studies.
Main characteristics of the study participants are presented in Table 2. Compared to those without malnutrition, those suffering this syndrome were often women, of older age and lower educational level. In the SE-2 the prevalence of smoking was higher, and in SE-1 physical activity was lower among participants with malnutrition. Also, those with malnutrition showed a higher prevalence of musculoskeletal, cardiovascular disease and depression in the SE-1, and of cardiovascular disease in the SE-2. Lastly, those with malnutrition reported lower energy and protein intake.
A higher MEDAS score was associated with lower prevalence of malnutrition in both studies (Table 3). After adjustment for age and sex, the pooled OR (95% CI) for high vs. low MEDAS adherence was 0.67 (0.51–0.88), p-trend 0.001. Additional adjustment for educational level, lifestyle, energy and protein intake and morbidity strengthened the association [model 4: OR 0.64 (0.48–0.84), p-trend < 0.001]. A higher adherence to the AHEI-2010 was also associated with lower odds of malnutrition; in model 4, the pooled OR (95% CI) for Q4 vs. Q1 was 0.65 (0.49–0.86), p-trend 0.006 (Table 4).
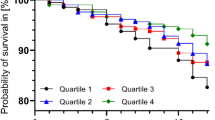
In stratified analyses by characteristics of study participants, high versus low adherence to the Mediterranean diet was associated with malnutrition in all the strata except those with small sample size (e.g., current smokers and those with cardiovascular diseases or depression). The associations were slightly stronger among women, those < 70 years, with high educational level, and those who consumed less calories (p for interaction not significant in any case) (Fig. 1). For the AHEI-2010 the associations were somewhat stronger among men, those < 70 years, with lower educational level and higher energy intake (p for interaction not significant in any case) (Fig. 2).
Some individual components of the diet quality scores were associated with malnutrition. Higher consumption of fish and long-chain n-3 fatty acids in MEDAS and AHEI-2010, vegetables and nuts and legumes in AHEI-2010, and lower intake of trans-fat and sugar-sweetened beverages and fruit juice in AHEI-2010 were independently associated with lower odds of malnutrition in pooled data (Tables S2-S3). Lastly, sensitivity analyses excluding alcohol from the MEDAS score and AHEI-2010 indexes still showed an association between adherence to these diet patterns and lower odds of malnutrition (Tables S4-S5).
Pooled odds ratios (95% confidence interval) for the association between the MEDAS score (high adherence vs. low adherence) and malnutrition, stratified by characteristics of study participants. Logistic regression models adjusted for age, sex, educational level (≤primary and secondary or university), smoking status (current, former and never smoker), leisure-time physical activity (METs-h/week), time spent watching TV (h/week), energy intake (kcal/day), total protein intake (kg/d), and morbidity (musculoskeletal disease, cardiovascular diseases, cancer, chronic lung disease, and depression), except for the stratification variable. P for interaction in all comparisons were non-significant
**Median of energy intake: 1948 kcal. Median of protein intake: 1.2 g/kg
Pooled odds ratios (95% confidence interval) for the association between AHEI-2010 score (quartile 4 vs. quartile 1) and malnutrition, stratified by characteristics of study participants. Logistic regression models adjusted for age, sex, educational level (≤primary and secondary or university), smoking status (current, former and never smoker), leisure-time physical activity (METs-h/week), time spent watching TV (h/week), energy intake (kcal/day), total protein intake(kg/day), and morbidity (musculoskeletal disease, cardiovascular disease, cancer, chronic lung disease, and depression), except for the stratification variable. P for interaction in all comparisons were non-significant
**Median of energy intake = 1948 kcal. Median of protein intake = 1.2 g/kg
Discussion
In this study, we found that a higher adherence to two healthy diet patterns decreases the odds of malnutrition among community-dwelling older adults. These results are independent of the amount of energy and protein intake and held after adjustment for chronic diseases. Our results showed that a hypothetical increase of about 2 points in adherence to the Mediterranean diet and 10 points to the AHEI-2010 is linked to a 16% lower likelihood of malnutrition. A high consumption of fish, vegetables, long chain n-3 fatty acids, and a low consumption of trans fats and sweetened beverages might drive these associations; however, the overall quality of the diet was strongly associated with malnutrition, in comparison with their individual components. The pooling of results from 2 different studies, with heterogeneous populations but with similar data collection methods, allowed for a greater external validity of the associations found.
Around 10% of older adults living in the community suffered from malnutrition (9.47% in the SE-1; 11.65% in the SE-2), which is consistent with other studies that used the GLIM criteria to diagnose malnutrition in study populations like ours [6]. By contrast, in a representative sample of community-dwelling older adults in Israel, malnutrition prevalence was lower (3.4%); this can be explained because they used a more stringent BMI cut off than our study (< 20 kg/m2 for all age groups vs. <20 for those under 70 and < 22 for those with 70 or more years) [8]. In studies where the participants were hospitalized or institutionalized, the prevalence was higher (between 20 and 30%) [41, 42], results like those obtained with simple screening tools, such as the “MUST”, to detect multimorbidity in community-dwelling adults [43].
Only a single study has previously assessed diet quality in relation to malnutrition, as defined with the GLIM criteria. This is a recently published cross-sectional analysis among Chinese community-dwelling older adults [23], where malnutrition was inversely related to the Dietary Quality Index International (DQI-I) score, a diet pattern characterized by a high consumption of vegetables and fruits and low consumption of meat and fish. By contrast with our results, this study did not find an association between the Mediterranean Diet Score (MDS) and malnutrition; this may be due to the differences in the Mediterranean diet pattern used [44], as well as a different adjustment for sociodemographic and lifestyle confounders, as well as other intrinsic characteristics of this Asian population.
Several studies have examined diet quality in relation to definitions of malnutrition other than GLIM. In a cross-sectional analysis of data from the Hellenic Longitudinal Investigation of Aging and Diet study, with urban-dwelling participants, lower adherence to the Mediterranean diet was associated with higher nutritional risk, defined with the Determine Your Nutritional Health checklist, which includes easy-to-obtain warning signs for poor nutrition: having a disease that affect diet, eating poorly, tooth loss/mouth pain, economic hardship, reduced social contact, taking multiple medicines, involuntary weight loss/gain, need assistance in self-care, and age > 80 [24]; however, no phenotypic criteria were included in this definition. In another small cross-sectional study, authors found that a low-nutrient-dense cluster of foods identified in rural older adults was associated with higher odds of obesity and low nutrient intakes [25]. Data from the Health, Aging, and Body Composition Study, with community-dwelling older adults, examined the association between the Healthy Eating Index and incidence of protein-energy malnutrition, based on low BMI and involuntary weight loss. No association was found after a follow-up of 3 to 4 years [26]. Lastly, in the Healthy Aging in Neighborhoods of Diversity across the Life Span, longitudinal data suggested that diet quality, as measured with a “mean adequacy ratio”, did predict the risk for malnutrition, determined by the Mini Nutritional Assessment [27].
There are well-known risk factors for malnutrition, including decreased appetite associated with aging, gastrointestinal malabsorption, oral health problems, decreased olfactory sensitivity [45], and psychosocial factors, such as cognitive impairment, depression, loneliness, and low educational and economic status (risk factors of malnutrition associated with diet patterns) [46]. We adjusted our analyses for many plausible confounders although we lacked information on several of them.
Strengths of this study include the assessment of habitual diet with a validated diet history and the use of two different predefined high-quality diets, in a large sample of community-living older adults. We used the definition of malnutrition using the GLIM criteria, instead of screening tools used in previous association studies. Although this definition has been implemented for use in clinical settings, its use in cohort studies with phenotypic metrics is optimal to identify malnutrition, instead of relying on self-reported questionnaire [47]. In terms of limitations, this is a cross-sectional analysis, therefore, we cannot rule out reverse causality: people with a good nutritional status may also have better general health to be able to prepare high-quality meals. Diet was self-reported; thus, some misreporting and misclassification may exist. We used a definition of the Mediterranean diet adapted to the characteristics of the Spanish diet; however, other definitions have been published. In addition, despite scientific evidence proving the AHEI-2010 is consistently associated with the lower risk of chronic diseases, the score assignment is subjective. Although we adjusted our analyses for many potential confounders, we cannot rule out the influence of those not measured, which may partially explain the differences in the estimates of the association between both cohorts. Lastly, selection bias could also play a role in our results, since participants included and excluded were different in age, sex, and diagnosed comorbidies.
Conclusion
In conclusion, adhering to the MEDAS and AHEI-2010 diet patterns was associated with less likelihood of malnutrition defined with phenotypic GLIM criteria. Measures to guarantee high diet quality among older adults seem necessary as part of the routine clinical strategy to prevent malnutrition and its consequences.
Data availability
Data describe in the manuscript, code book, and analytic code will be made available upon request pending application and approval. The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AHEI:
-
2010 Alternative Healthy Eating Index
- ANOVA:
-
Analysis of variance
- BIA:
-
Bioelectrical impedance analysis
- BMI:
-
Body mass index
- CI:
-
Confidence Interval
- DQI-I:
-
Dietary quality index international score
- GLIM:
-
Global Leadership Initiative on Malnutrition
- MDS:
-
Mediterranean diet score
- MEDAS:
-
Mediterranean Diet Adherence Screener
- METs:
-
Metabolic Equivalent Tasks
- OR:
-
Odds ratios
- SE-1:
-
Seniors-ENRICA-1
- SE-2:
-
Seniors-ENRICA-2
- SMI:
-
Skeletal muscle mass index
- SMM:
-
Skeletal muscle mass
References
Cederholm T, Jensen GL, Correia MITD, Gonzalez MC, Fukushima R, Higashiguchi T, et al. GLIM criteria for the diagnosis of malnutrition – A consensus report from the global clinical nutrition community. Clin Nutr. 2019;38:1–9. https://doi.org/10.1016/j.clnu.2018.08.002
Sanchez-Rodriguez D, Locquet M, Reginster JY, Cavalier E, Bruyère O, Beaudart C. Mortality in malnourished older adults diagnosed by ESPEN and GLIM criteria in the SarcoPhAge study. J Cachexia Sarcopenia Muscle. 2020;11:1200–11. https://doi.org/10.1002/jcsm.12574
Miller J, Wells L, Nwulu U, Currow D, Johnson MJ, Skipworth RJE. Validated screening tools for the assessment of cachexia, sarcopenia, and malnutrition: a systematic review. Am J Clin Nutr. 2018;108:1196–208. https://doi.org/10.1093/ajcn/nqy244
Rodríguez-Mañas L, Rodríguez-Sánchez B, Carnicero JA, Rueda R, García-Garcia FJ, Pereira SL, et al. Impact of nutritional status according to GLIM criteria on the risk of incident frailty and mortality in community-dwelling older adults. Clin Nutr. 2021;40:1192–8. https://doi.org/10.1016/j.clnu.2020.07.032
Damião R, Meneguci J, da Silva Santos Á, Matijasevich A, Rossi Menezes P. Nutritional risk and quality of life in community-dwelling elderly: a cross-sectional study. J Nutr Health Aging. 2018;22:111–6. https://doi.org/10.1007/s12603-017-0935-y
Rodríguez-Sánchez B, Sulo S, Carnicero JA, Rueda R, Rodríguez-Mañas L. Malnutrition prevalence and burden on healthcare resource use among spanish community-living older adults: results of a longitudinal analysis. Clin Outcomes Res CEOR. 2020;12:355–67. https://doi.org/10.2147/CEOR.S256671
Tilly J. Opportunities to improve nutrition for older adults and reduce risk of poor health outcomes. U.S. Department of Health and Human Services, Administration for Community Living 2017. https://acl.gov/sites/default/files/nutrition/Malnutrition-Issue-Brief-ACL2017.pdf
Sharman Moser S, Doyev R, Cohen B, Kurz R, Sulo S, Shalev V, et al. Prevalence and characteristics of malnutrition among community-dwelling older adults in Israel. Clin Nutr ESPEN. 2018;28:179–85. https://doi.org/10.1016/j.clnesp.2018.08.006
Yeung SSY, Chan RSM, Kwok T, Lee JSW, Woo J. Malnutrition according to GLIM criteria and adverse outcomes in community-dwelling Chinese older adults: a prospective analysis. J Am Med Dir Assoc. 2021;22:1953–e19594. https://doi.org/10.1016/j.jamda.2020.09.029
Corish CA, Bardon LA. Malnutrition in older adults: screening and determinants. Proc Nutr Soc. 2019;78:372–9. https://doi.org/10.1017/S0029665118002628
Cox NJ, Morrison L, Ibrahim K, Robinson SM, Sayer AA, Roberts HC. New horizons in appetite and the anorexia of ageing. Age Ageing. 2020;49:526–34. https://doi.org/10.1093/ageing/afaa014
Cruz-Jentoft AJ, Dawson Hughes B, Scott D, Sanders KM, Rizzoli R. Nutritional strategies for maintaining muscle mass and strength from middle age to later life: a narrative review. Maturitas. 2020;132:57–64. https://doi.org/10.1016/j.maturitas.2019.11.007
Struijk EA, Fung TT, Rodríguez-Artalejo F, Bischoff-Ferrari HA, Hu FB, Willett WC, et al. Protein intake and risk of frailty among older women in the nurses’ health study. J Cachexia Sarcopenia Muscle. 2022. https://doi.org/10.1002/jcsm.12972
Vega-Cabello V, Caballero FF, Lana A, Arias-Fernandez L, Banegas JR, Rodriguez-Artalejo F, et al. Association of zinc intake with risk of impaired physical function and frailty among older adults. J Gerontol Biol Sci Med Sci. 2022;glac014. https://doi.org/10.1093/gerona/glac014
U.S. Department of Agriculture and U.S. Department of Health and Human Services. Dietary Guidelines for Americans, 2020–2025 2020; 9th Edition; December 2020. Available at DietaryGuidelines.gov.
Martínez-González MA, Gea A, Ruiz-Canela M. The Mediterranean diet and cardiovascular health. Circ Res. 2019;124:779–98. https://doi.org/10.1161/CIRCRESAHA.118.313348
Shan Z, Li Y, Baden MY, Bhupathiraju SN, Wang DD, Sun Q, et al. Association between healthy eating patterns and risk of cardiovascular disease. JAMA Intern Med. 2020;180:1–11. https://doi.org/10.1001/jamainternmed.2020.2176
Jannasch F, Kröger J, Schulze MB. Dietary patterns and type 2 diabetes: a systematic literature review and meta-analysis of prospective studies. J Nutr. 2017;147:1174–82. https://doi.org/10.3945/jn.116.242552
Onvani S, Haghighatdoost F, Surkan PJ, Larijani B, Azadbakht L. Adherence to the healthy eating index and alternative healthy eating index dietary patterns and mortality from all causes, cardiovascular disease and cancer: a meta-analysis of observational studies. J Hum Nutr Diet off J Br Diet Assoc. 2017;30:216–26. https://doi.org/10.1111/jhn.12415
Bruno E, Manoukian S, Venturelli E, Oliverio A, Rovera F, Iula G, et al. Adherence to Mediterranean diet and metabolic syndrome in BRCA mutation carriers. Integr Cancer Ther. 2018;17:153–60. https://doi.org/10.1177/1534735417721015
Sotos-Prieto M, Bhupathiraju SN, Mattei J, Fung TT, Li Y, Pan A, et al. Association of changes in diet quality with total and cause-specific mortality. N Engl J Med. 2017;377:143–53. https://doi.org/10.1056/NEJMoa1613502
Struijk EA, Hagan KA, Fung TT, Hu FB, Rodríguez-Artalejo F, Lopez-Garcia E. Diet quality and risk of frailty among older women in the nurses’ health study. Am J Clin Nutr. 2020;111:877–83. https://doi.org/10.1093/ajcn/nqaa028
Yeung SSY, Chan RSM, Lee JSW, Woo J. Certain dietary patterns are associated with GLIM criteria among Chinese community-dwelling older adults: a cross-sectional analysis. J Nutr Sci. 2021;10:e69. https://doi.org/10.1017/jns.2021.64
Katsas K, Mamalaki E, Kontogianni MD, Anastasiou CA, Kosmidis MH, Varlamis I, et al. Malnutrition in older adults: correlations with social, diet-related, and neuropsychological factors. Nutrition. 2020;71:110640. https://doi.org/10.1016/j.nut.2019.110640
Ledikwe JH, Smiciklas-Wright H, Mitchell DC, Miller CK, Jensen GL. Dietary patterns of rural older adults are associated with weight and nutritional status. J Am Geriatr Soc. 2004;52:589–95. https://doi.org/10.1111/j.1532-5415.2004.52167.x
Hengeveld LM, Wijnhoven HA, Olthof MR, Brouwer IA, Harris TB, Kritchevsky SB, et al. Prospective associations of poor diet quality with long-term incidence of protein-energy malnutrition in community-dwelling older adults: the health, aging, and body composition (Health ABC) study. Am J Clin Nutr. 2018;107:155–64. https://doi.org/10.1093/ajcn/nqx020
Fanelli Kuczmarski M, Stave Shupe E, Pohlig RT, Rawal R, Zonderman AB, Evans MK. A longitudinal assessment of diet quality and risks associated with malnutrition in socioeconomic and racially diverse adults. Nutrients. 2019;11:2046. https://doi.org/10.3390/nu11092046
Rodríguez-Artalejo F, Graciani A, Guallar-Castillón P, León-Muñoz LM, Zuluaga MC, López-García E, et al. Rationale and methods of the study on nutrition and cardiovascular risk in Spain (ENRICA). Rev Esp Cardiol. 2011;64:876–82. https://doi.org/10.1016/j.recesp.2011.05.019
Estrada-deLeón DB, Struijk EA, Caballero FF, Ortolá R, Guallar-Castillón P, Banegas JR, et al. Association of prolonged nightly fasting with cardiovascular, renal, inflammation, and nutritional status biomarkers in community-dwelling older adults. Am J Clin Nutr. 2022;nqac021. https://doi.org/10.1093/ajcn/nqac021
Ortolá R, García-Esquinas E, Buño‐Soto A, Cabanas‐Sánchez V, Martínez‐Gómez D, Sotos‐Prieto M, et al. Associations of device‐measured sleep, sedentariness and physical activity with growth differentiation factor 15 in older adults. J Cachexia Sarcopenia Muscle. 2022;13:1003. https://doi.org/10.1002/jcsm.12924
EPIC Group of Spain. Relative validity and reproducibility of a diet history questionnaire in Spain. I. Foods. Int J Epidemiol. 1997;26(Suppl 1):S91–99. https://doi.org/10.1093/ije/26.suppl_1.s91
Guallar-Castillón P, Sagardui-Villamor J, Balboa-Castillo T, Sala-Vila A, Astolfi MJA, Pelous MDS, et al. Validity and reproducibility of a Spanish dietary history. PLoS ONE. 2014;9:e86074. https://doi.org/10.1371/journal.pone.0086074
Yuan C, Spiegelman D, Rimm EB, Rosner BA, Stampfer MJ, Barnett JB, et al. Validity of a dietary questionnaire assessed by comparison with multiple weighed dietary records or 24-hour recalls. Am J Epidemiol. 2017;185:570–84. https://doi.org/10.1093/aje/kww104
Schröder H, Fitó M, Estruch R, Martínez-González MA, Corella D, Salas‐Salvadó J, et al. A short screener is valid for assessing Mediterranean diet adherence among older Spanish men and women. J Nutr. 2011;141:1140–5. https://doi.org/10.3945/JN.110.135566
Chiuve SE, Fung TT, Rimm EB, Hu FB, McCullough ML, Wang M, et al. Alternative dietary indices both strongly predict risk of chronic disease. J Nutr. 2012;142:1009. https://doi.org/10.3945/JN.111.157222
Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol Ser A. 2001;56:M146–57. https://doi.org/10.1093/gerona/56.3.M146
Janssen I, Heymsfield SB, Baumgartner RN, Ross R. Estimation of skeletal muscle mass by bioelectrical impedance analysis. J Appl Physiol Bethesda Md 1985. 2000;89:465–71. https://doi.org/10.1152/jappl.2000.89.2.465
Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48:16–31. https://doi.org/10.1093/ageing/afy169
Pols MA, Peeters PH, Ocké MC, Slimani N, Bueno-de-Mesquita HB, Collette HJ. Estimation of reproducibility and relative validity of the questions included in the EPIC physical activity questionnaire. Int J Epidemiol. 1997;26(Suppl 1):S181–189. https://doi.org/10.1093/ije/26.suppl_1.s181
Deeks J, Altman D, Bradburn M. Meta-analyses in context. Syst Rev Health Care Lond BMJ Publ Group. 2001:285–312.
Kiesswetter E, Colombo MG, Meisinger C, Peters A, Thorand B, Holle R, et al. Malnutrition and related risk factors in older adults from different health-care settings: an enable study. Public Health Nutr. 2020;23:446–56. https://doi.org/10.1017/S1368980019002271
Maeda K, Ishida Y, Nonogaki T, Mori N. Reference body mass index values and the prevalence of malnutrition according to the global leadership initiative on malnutrition criteria. Clin Nutr. 2020;39:180–4. https://doi.org/10.1016/j.clnu.2019.01.011
Murphy JL, Aburrow A, Guestini A, Brown R, Parsons E, Wallis K. Identifying older people at risk of malnutrition and treatment in the community: prevalence and concurrent validation of the patients association nutrition checklist with ‘MUST’. J Hum Nutr Diet. 2020;33:31–7. https://doi.org/10.1111/jhn.12710
Trichopoulou A, Costacou T, Bamia C, Trichopoulos D. Adherence to a Mediterranean diet and survival in a Greek population. N Engl J Med. 2003;348:2599–608. https://doi.org/10.1056/NEJMoa025039
Dent E, Hoogendijk EO, Wright ORL. New insights into the anorexia of ageing: from prevention to treatment. Curr Opin Clin Nutr Metab Care. 2019;22:44–51. https://doi.org/10.1097/MCO.0000000000000525
Besora-Moreno M, Llauradó E, Tarro L, Solà R. Social and economic factors and malnutrition or the risk of malnutrition in the elderly: a systematic review and meta-analysis of observational studies. Nutrients. 2020;12:E737. https://doi.org/10.3390/nu12030737
Henriksen C, Paur I, Pedersen A, Kværner AS, Ræder H, Henriksen HB, et al. Agreement between GLIM and PG-SGA for diagnosis of malnutrition depends on the screening tool used in GLIM. Clin Nutr Edinb Scotl. 2022;41:329–36. https://doi.org/10.1016/j.clnu.2021.12.024
Acknowledgements
Not applicable.
Funding
This work was supported by grants from the Institute of Health Carlos III, State Secretary of R + D + I of Spain, and the European Regional Development Fund/ European Social Fund) (FIS 20/1040, 22/1111, 23/272); PLEC2022-009352 and CPP2022-009718 grants (funded by MCIN/AEI/ and “NextGenerationEU/PRTR”); PMPTA22/00107 and PMPTA23/00012 grants (ISCIII-CDTI and the European Union “NextGeneration EU/PRTR”). FACINGLCOVID-CM project (funded by Comunidad de Madrid and European Regional Development Fund “Funding REACT EU Program”). The funding agencies had no role in study design, data collection and analysis, interpretation of results, manuscript preparation or the decision to submit this manuscript for publication.
Author information
Authors and Affiliations
Contributions
AMD: Conceptualization, Methodology, Formal analysis, Data curation, Writing-Original draft preparation; Visualization; ELG: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Data curation, Writing-Reviewing and Editing, Visualization; Supervision. HYB: Validation, Formal Analysis, Data Curation, Writing-Reviewing and Editing. TFV: Data Curation, Writing-Review and Editing. VM: Writing-Reviewing and Editing; PGC: Writing-Reviewing and Editing; FRA: Writing-Reviewing and Editing.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Participants gave written informed consent, and the Clinical Research Ethics Committee of the La Paz University Hospital in Madrid approved the study (PI-2144, PI-3554, PI-1793).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Marcos-Delgado, A., Yévenes-Briones, H., Fernández-Villa, T. et al. Association between diet quality and malnutrition: pooled results from two population-based studies in older adults. BMC Geriatr 24, 417 (2024). https://doi.org/10.1186/s12877-024-04984-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12877-024-04984-5