Abstract
Background
This study aimed to evaluate the correlation between serum methylmalonic acid (MMA) levels and cognition function in patients with chronic kidney disease (CKD).
Methods
In this cross-sectional study, we included 537 CKD individuals aged ≥ 60-year-old with albuminuria from the National Health and Nutrition Examination Survey (NHANES) 2011–2014. Four cognitive tests including the Digit Symbol Substitution Test (DSST), the Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) Delayed Recall and Word Learning tests, and the Animal Fluency test (AF) were performed. Associations between MMA and cognition scores were assessed with linear regression models.
Results
MMA level was negatively associated with residual renal function and nutrition status. After multivariate adjustment, elevated serum MMA levels were independently correlated with decline of cognition in CKD patients with albuminuria.
Conclusion
Our study showed that higher serum MMA levels were independently associated with the presence of cognition dysfunction in CKD patients. The exact pathogenesis of MMA and cognition needs further research.
Similar content being viewed by others
Introduction
Chronic kidney disease (CKD) is one of the most common global causes of morbidity and mortality [1]. Cognitive impairment is commonly found in patients with CKD. A recent systematic review yielded the prevalence of cognitive decline is more common in patients with CKD compared with patients without CKD [2]. Poor cognitive function has been linked to an increasing social and financial costs [3] and mortality [4]. The underlying pathophysiology of CKD-associated cognitive dysfunction is complex, including genes, uremia toxins, vascular dysfunction, and neuroinflammation [5].
Vitamin B12 is essential to regulate DNA synthesis, methylation reactions, and genomic stability. Low vitamin B12 level is associated with neurodegenerative disease, such as Alzheimer’s disease, vascular dementia, and Parkinson’s disease [6, 7]. Metabolic vitamin B12 deficiency is common, and it is necessary to measure functional markers of vitamin B12 adequacy such as methylmalonic acid (MMA) or homocysteine [8]. MMA is probably a specific and sensitive biomarker of subclinical vitamin B12 deficiency [9, 10]. An increase in serum MMA could be caused by decreased kidney function [11]. Serum MMA may be a favorable marker to predict various chronic diseases, including cardiovascular diseases [12, 13], and neurodegenerative diseases [14]. MMA is even more strongly associated with poor cognition and physical performance than serum vitamin B12 [15, 16]. Current evidence showed a relation between serum MMA, vitamin B12 status and cognitive function in older populations [17,18,19]. However, few studies have examined the role of MMA and cognitive decline in CKD patients with albuminuria.
Therefore, in this study, we aimed to explore whether elevated serum MMA levels are related to increased risk of cognitive impairment in patients with CKD.
Methods
Data source and participants
NHANES is a nationally representative, cross-sectional survey of the noninstitutionalized US population. The protocols for conducting the NHANES were approved by the institutional review board of the National Center for Health Statistics, Centers for Disease Control and Prevention, and informed consent was obtained from all participants.
Estimated GFR (eGFR) was calculated according to the Modification of Diet in Renal Disease (MDRD) calculation [20]. CKD was defined as: CKD stages G1–G3 (eGFR 30–59 mL/min/1.73 m2 or eGFR ≥ 60 mL/min/1.73 m2 and urea albumin/creatinine ratio (UACR) ≥ 30 mg/g) and CKD stages G4 and G5 (eGFR < 30 mL/min/1.73 m2), in accordance with the Kidney Disease: Improving Global Outcome 2012 Practice Guideline for CKD [21].
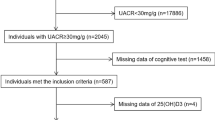
For this study, we combined data from NHANES 2011–2014. A total of 2045 CKD patients with UACR > 30 mg/g aged 60 y and older participated in the NHANES health examination. The exclusion criteria were cases with missing key data. Ultimately, 573 participants were included in this study, see Fig. 1 for detail.
Cognitive function assessment
Four separate tests formed the cognitive battery and were collected from the study participant. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) is a comprehensive set of tests used to identify Alzheimer disease by assessing the ability for new learning, delayed recall, and recognition memory [22]. The CERAD-Word Learning test (CERAD-WL) consists of 3 consecutive learning trials, and the CERAD-Delayed Recall test (CERAD-DR) occurred after the other 2 cognitive exercises were completed. The Animal Fluency (AF) test asked participants to name as many animals as they can in 1 min to assess verbal fluency [23]. The Digit Symbol Substitution Test (DSST) is designed to measure processing speed, sustained attention, and working memory [24]. According to previous study, we used the lowest 25th percentile of the scores as the cutoff points to indicate cognitive impairment [25].
Measurement of serum methylmalonic acid
Concentrations of MMA were measured in NHANES by the Division of Laboratory Sciences, National Center for Environmental Health, Centers for Disease Control and Prevention, Atlanta, GA. Detailed instructions on specimen collection and processing are discussed in the NHANES Laboratory Procedures Manual [26].
Evaluation of covariates
Information on age, education level, sex, disease status, smoking status, and drinking status was obtained using questionnaires. In addition, hemoglobin, red blood cell distribution, serum vitamin B12, folate, total cholesterol, triglyceride, albumin, uric acid, vitamin D3, blood urea nitrogen (BUN), and creatinine levels were measured at baseline. Anemia is defined as hemoglobin level < 13.0 g/dl in males and < 12.0 g/dl in females [27]. The nutritional status was assessed by geriatric nutritional risk index (GNRI), which is relatively objective and effective [28]. Major nutrition-related risk is defined as GNRI < 82; moderate nutrition-related risk is defined as 82–92; low nutrition-related risk is defined as 92–98; and no risk is defined as > 98.
Statistical analysis
We performed all statistical analyses using IBM SPSS Statistics 23. Patients were divided into four groups according to quartile of serum MMA. Categorical demographic variables were presented as numbers (weighted percentage) and compared among groups using Chi-square tests. We log-transformed MMA to normalize their distributions before statistical analysis. Relationships between MMA and covariates were examined through Spearman’s correlation analysis. Multivariable linear regression models were employed to explore the associations between MMA and each cognitive test. We adjusted for the covariates of age, sex, education level, smoke, drink, comorbidities, GNRI score, hemoglobin, albumin, cholesterol, uric acid, renal dysfunction, vitamin D3 and vitamin B12. In addition, subgroup analysis was also performed by linear regression analysis. Finally, receiver operating characteristic (ROC) curve was plotted and the area under the curve (AUC) was calculated. Two-sided p-values < 0.05 denoted significance.
Results
Baseline characteristics of the participants by the quartile of MMA levels
Table 1 presents the characteristics of the patients included in the study. A total of 573 CKD patients with albuminuria were included in the final data analysis. Patients with elevated MMA levels were likely to be older, with a lower hemoglobin, albumin, GNRI score, vitamin B12, and higher BUN, creatinine, and uric acid level. Besides, the proportion of hypertension, diabetes and anemia significantly differed among four groups. As for each cognitive test, patients in the group 4 had a relatively lower score of CERAD-WL, AF test, and DSST.
Relations of MMA with covariates
The association of MMA with other covariates was shown in Table 2. In Spearman’s correlation analysis, serum MMA level was positively associated with age, BUN, creatinine, UACR, folate, and uric acid, while negative associations were found between serum MMA leves and hemoglobin, albumin, eGFR, GNRI, and vitamin B12.
Relations of MMA with cognitive dysfunction
In our Spearman’s correlation analysis, the score of CERAD-WL, CERAD-DR, AF test, and DSST were all negatively associated with serum MMA concentrations. Furthermore, after adjusting for other confounding factors, the standardized β of MMA for CERAD-WL and AF test was − 2.191 (95%CI: -4.136, -0.246) and − 1.992 (95%CI: -3.913, -0.072), respectively. We found that an increasing level of MMA was probably an independent associated factor for a declining of cognitive function in patients with CKD (shown in Table 3).
Stratification analyses
We performed subgroup analyses and analyzed the interactions between MMA and the variables in this study (shown in Table 4). In the stratified analyses, we divided patients into subgroups according to sex, comorbidities, level of UACR, nutritional status and median value of vitamin B12. We found that the subgroups of male sex, and non-hypertension were similar to our main results. Namely, MMA negatively correlated with CERAD-WL and AF scores in male and non-hypertension patients. Besides, serum MMA was negatively associated with CERAD-WL in patients with diabetes, a relatively lower level of vitamin B12 and a higher GNRI score. MMA was also an independent associated factor for AF test in patients with malnutrition and macroalbuminuria. However, there was no significant relationship between each cognitive test and serum MMA level in the subgroups of female sex, hypertension, non-diabetes, and a higher level of vitamin B12. The results also implied that the effect of MMA on cognition function in CKD patients could be affected by URCR levels (P for interaction < 0.05). In contrast, hypertension, diabetes, vitamin B12 and nutritional status did not modify the associations between MMA and cognition function (p for interaction > 0.05).
The diagnostic value of MMA for cognition function in CKD patients
The best cutoff for the ROC curve was calculated with the Youden’s index. The optimal point of MMA for cognitive impairment was 194.5nmol/l (AUC = 0.567, sensitivity 57%, specificity 56%, P = 0.006) as measured by CERAD-WL and 181.5nmol/l (AUC = 0.565, sensitivity 65%, specificity 53%, P = 0.015) as measured by AFT, respectively.
Discussion
The final analysis results of this study showed that the increase of MMA concentration was significantly related to the decline of cognitive level in CKD patients with albuminuria. Furthermore, serum MMA level was negatively related to cognitive test score in CKD patients of male sex, patients without hypertension, and absence of vitamin B12.
CKD is defined by indicators of kidney damage—imaging or proteinuria (i.e.,UACR)—and decreased renal function for at least three months [29]. The burden of cognitive impairments in CKD has been extensively studied [30, 31], with prevalence ranging from 13–58% [32,33,34]. Cognitive impairment is an independent predictor of mortality and morbidity in end-stage renal disease patients [4, 35].
Disruption of vitamin B12 transport in the blood, or impaired cellular uptake might cause an intracellular deficiency. Diagnostic biomarkers for vitamin B12 status include decreased levels of circulating total vitamin B12, and abnormally increased levels of homocysteine and MMA. The metabolism of MMA in mitochondria could be hindered by mitochondrial methylmalonyl-CoA mutase deactivation or coenzyme active vitamin B12 deficiency, leading to an accumulating of MMA [36]. Some studies suggested that the increase of MMA was related to cognition impairment in children aged 3–16 [37], and participants aged 61–87 [38, 39]. However, a previous meta-analysis included 11 studies, and performed no correlation between the increase of MMA level and the decrease of cognitive level in the general population [40]. It is essential to definite whether a higher level of MMA might lead to cognitive decline in patients with CKD.
In our study, we included CKD patients with UACR > 30 mg/g to determine the relationship between MMA and cognitive dysfunction. We found that serum MMA level was negatively associated with cognitive score in CKD patients with albuminuria. Previous study found that impaired kidney function could increase MMA [41]. The renal disease of MMA could be induced by proximal renal tubular mitochondrial dysfunction [42]. In our study, we also found that serum MMA was positively associated with BUN, creatinine and proteinuria level, and negatively associated with eGFR. Our findings indicated that an accumulating in MMA perhaps due to a decline of renal function. Furthermore, the association between MMA and cognition dysfunction tended to be stronger in patients with macroalbuminuria in comparison to patients with microalbuminuria. Circulating level of MMA is strongly associated with elevated all-cause and cardiovascular mortality in adults [43]. Multiple survey determined MMA was inversely associated with cognitive function scores in the elderly general population [44,45,46]. The mechanisms responsible for the neurological dysfunction in methylmalonic acidemia have so far not been fully elucidated.
It is known that serum MMA levels increased with age [47]. MMA might have neurotoxicity, since its accumulating in cerebrospinal fluid [48]. A larger echogenic area of the substantia nigra was reported to be related to higher serum concentrations of MMA in Parkinson disease [49]. A study has investigated cross-sectional association between circulating MMA and brain volumes, although no significant association was observed in the fully adjusted model [50]. An elevated MMA could inhibit respiratory chain and impair energy metabolism in hippocampus tissue [51]. Furthermore, neurologic deficit in methylmalonic acidemia might be due to MMA-induced lipoperoxidation in cerebral cortex [52]. Depolarization of the plasma membrane and neuronal cell apoptosis caused by MMA might be another key mechanism [53, 54]. Serum uric acid was positively associated with MMA in this study, which was also found to be independently related to cognition dysfunction [16]. Evidence of mitochondrial reactive oxygen species generation and oxidative stress were suggested to contribute to the disorder [55]. An inverse association of albumin, hemoglobin, GNRI and MMA was observed in our study, probably indicating a poor nutrition status in patients with high MMA levels. Similar, malnutrition is also correlated with the deterioration cognitive domains [56].
In our subgroup analysis, we found MMA was an associated factor for cognitive impairment in patients with diabetes, but not patients without diabetes. Studies demonstrated that elevated MMA levels in diabetes patients acted as an indicator for peripheral neuropathy [57, 58]. Moreover, MMA accumulation was positively associated with increased mortality risk in type 2 diabetes patients [59]. Higher serum MMA was associated with the presence of cardiovascular diseases [13]. Plasma vitamin B12 was positively associated with hypertension in women [60]. In our study, we found the association of MMA and cognitive function was not significant in patients with hypertension. Hypertension has represented an important risk factor for cognitive decline, and a strict blood pressure control could prevent the progression [61, 62]. Another study explored the relation of the coexistence of high folate and low vitamin B12 status with cognitive function [63]. Having low vitamin B12/high folic acid status was associated with greater risk for cognitive impairment, when comparing to the high folate and normal vitamin B12 status. From our analysis, we did not find any significant association between MMA and cognitive score in the subgroup of high vitamin B12 group. It is reported that serum MMA and vitamin B12 concentrations do not directly correlate with each other [64]. The association of cognition with MMA is stronger than that with vitamin B12 [64]. Hence, a supplementation of vitamin B12 and ensuring optimal concentrations of both vitamin B12 and MMA might improve potential clinical prognosis. Since the predictive power of MMA for cognition deficit in CKD patients is relatively weak in this study, other longitudinal studies were needed to confirm our results.
Our study firstly demonstrated the association of MMA and cognition function in CKD patients. The use of comprehensive data from the NHANES allowed us to control for potential key confounders. This study had several limitations. First, this cross-sectional study could not establish a cause-effect association of MMA and cognition. Second, the sample size of this study was relatively small, which might lead to a bias. Third, self-reported variables and other unmeasured confounders could affect our results.
Conclusion
To sum up, higher serum MMA were associated with the presence of cognition impairment in older CKD patients. Besides, serum MMA levels negatively correlated to nutrition status, and residual renal function. Our results highlight that MMA could be a therapeutic target for cognition dysfunction in CKD patients, and further studies are needed to clarify its mechanism.
Data availability
The datasets generated and analyzed in the present study are available on the website of NHANES datasets 2011–2014 (https://wwwn.cdc.gov/nchs/nhanes).
References
Ortiz A. RICORS2040: the need for collaborative research in chronic kidney disease. Clin Kidney J. 2022;15(3):372–87.
Etgen T, Chonchol M, Förstl H, Sander D. Chronic kidney disease and cognitive impairment: a systematic review and meta-analysis. Am J Nephrol. 2012;35(5):474–82.
Lin PJ, Neumann PJ. The economics of mild cognitive impairment. Alzheimers Dement. 2013;9(1):58–62.
Griva K, Stygall J, Hankins M, Davenport A, Harrison M, Newman SP. Cognitive impairment and 7-year mortality in dialysis patients. Am J Kidney Dis. 2010;56(4):693–703.
Viggiano D, Wagner CA, Martino G, Nedergaard M, Zoccali C, Unwin R, et al. Mechanisms of cognitive dysfunction in CKD. Nat Rev Nephrol. 2020;16(8):452–69.
Vinueza Veloz AF, Carpio Arias TV, Vargas Mejía JS, Tapia Veloz EC, Piedra Andrade JS, Nicolalde Cifuentes TM, et al. Cognitive function and vitamin B12 and D among community-dwelling elders: a cross-sectional study. Clin Nutr ESPEN. 2022;50:270–6.
Moore E, Mander A, Ames D, Carne R, Sanders K, Watters D. Cognitive impairment and vitamin B12: a review. Int Psychogeriatr. 2012;24(4):541–56.
Spence JD. Metabolic vitamin B12 deficiency: a missed opportunity to prevent dementia and stroke. Nutr Res. 2016;36(2):109–16.
Vashi P, Edwin P, Popiel B, Lammersfeld C, Gupta D. Methylmalonic acid and homocysteine as indicators of vitamin B-12 Deficiency in Cancer. PLoS ONE. 2016;11(1):e0147843.
Obeid R, Jung J, Falk J, Herrmann W, Geisel J, Friesenhahn-Ochs B, et al. Serum vitamin B12 not reflecting vitamin B12 status in patients with type 2 diabetes. Biochimie. 2013;95(5):1056–61.
Ganji V, Kafai MR. Population prevalence, attributable risk, and attributable risk percentage for high methylmalonic acid concentrations in the post-folic acid fortification period in the US. Nutr Metab (Lond). 2012;9(1):2.
Polytarchou K, Dimitroglou Y, Varvarousis D, Christodoulis N, Psachoulia C, Pantziou C, et al. Methylmalonic acid and vitamin B12 in patients with heart failure. Hellenic J Cardiol. 2020;61(5):330–7.
Wang X, Li W, Xiang M. Increased serum methylmalonic acid levels were associated with the presence of cardiovascular diseases. Front Cardiovasc Med. 2022;9:966543.
Levin J, Bötzel K, Giese A, Vogeser M, Lorenzl S. Elevated levels of methylmalonate and homocysteine in Parkinson’s disease, progressive supranuclear palsy and amyotrophic lateral sclerosis. Dement Geriatr Cogn Disord. 2010;29(6):553–9.
Wolffenbuttel BHR, Wouters H, de Jong WHA, Huls G, van der Klauw MM. Association of vitamin B12, methylmalonic acid, and functional parameters. Neth J Med. 2020;78(1):10–24.
Bailey RL, Carmel R, Green R, Pfeiffer CM, Cogswell ME, Osterloh JD, et al. Monitoring of vitamin B-12 nutritional status in the United States by using plasma methylmalonic acid and serum vitamin B-12. Am J Clin Nutr. 2011;94(2):552–61.
Doets EL, van Wijngaarden JP, Szczecińska A, Dullemeijer C, Souverein OW, Dhonukshe-Rutten RA, et al. Vitamin B12 intake and status and cognitive function in elderly people. Epidemiol Rev. 2013;35:2–21.
Tangney CC, Tang Y, Evans DA, Morris MC. Biochemical indicators of vitamin B12 and folate insufficiency and cognitive decline. Neurology. 2009;72(4):361–7.
Lewis MS, Miller LS, Johnson MA, Dolce EB, Allen RH, Stabler SP. Elevated methylmalonic acid is related to cognitive impairement in older adults enrolled in an elderly nutrition program. J Nutr Elder. 2005;24(3):47–65.
Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130(6):461–70.
Inker LA, Astor BC, Fox CH, Isakova T, Lash JP, Peralta CA, et al. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for the evaluation and management of CKD. Am J Kidney Dis. 2014;63(5):713–35.
Morris JC, Heyman A, Mohs RC, Hughes JP, van Belle G, Fillenbaum G, et al. The Consortium to establish a Registry for Alzheimer’s Disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer’s disease. Neurology. 1989;39(9):1159–65.
Clark LJ, Gatz M, Zheng L, Chen YL, McCleary C, Mack WJ. Longitudinal verbal fluency in normal aging, preclinical, and prevalent Alzheimer’s disease. Am J Alzheimers Dis Other Demen. 2009;24(6):461–8.
Brody DJ, Kramarow EA, Taylor CA, McGuire LC. Cognitive performance in adults aged 60 and over: National Health and Nutrition Examination Survey, 2011–2014. Natl Health Stat Rep. 2019;126:1–23.
Chen SP, Bhattacharya J, Pershing S. Association of Vision Loss with Cognition in older adults. JAMA Ophthalmol. 2017;135(9):963–70.
Mineva EM, Zhang M, Rabinowitz DJ, Phinney KW, Pfeiffer CM. An LC-MS/MS method for serum methylmalonic acid suitable for monitoring vitamin B12 status in population surveys. Anal Bioanal Chem. 2015;407(11):2955–64.
Coronado Daza J, Martí-Carvajal AJ, Ariza García A, Rodelo Ceballos J, Yomayusa González N, Páez-Canro C, et al. Early versus delayed erythropoietin for the anaemia of end-stage kidney disease. Cochrane Database Syst Rev. 2015;2015(12):Cd011122.
Bouillanne O, Morineau G, Dupont C, Coulombel I, Vincent JP, Nicolis I, et al. Geriatric nutritional risk index: a new index for evaluating at-risk elderly medical patients. Am J Clin Nutr. 2005;82(4):777–83.
Stevens PE, Levin A. Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med. 2013;158(11):825–30.
Brodski J, Rossell SL, Castle DJ, Tan EJ. A systematic review of cognitive impairments Associated with kidney failure in adults before natural age-related changes. J Int Neuropsychol Soc. 2019;25(1):101–14.
Berger I, Wu S, Masson P, Kelly PJ, Duthie FA, Whiteley W, et al. Cognition in chronic kidney disease: a systematic review and meta-analysis. BMC Med. 2016;14(1):206.
Kurella Tamura M, Xie D, Yaffe K, Cohen DL, Teal V, Kasner SE, et al. Vascular risk factors and cognitive impairment in chronic kidney disease: the chronic renal insufficiency cohort (CRIC) study. Clin J Am Soc Nephrol. 2011;6(2):248–56.
Shea YF, Lee MC, Mok MM, Chan FH, Chan TM. Prevalence of cognitive impairment among peritoneal dialysis patients: a systematic review and meta-analysis. Clin Exp Nephrol. 2019;23(10):1221–34.
Gupta A, Mahnken JD, Johnson DK, Thomas TS, Subramaniam D, Polshak T, et al. Prevalence and correlates of cognitive impairment in kidney transplant recipients. BMC Nephrol. 2017;18(1):158.
Kallenberg MH, Kleinveld HA, Dekker FW, van Munster BC, Rabelink TJ, van Buren M, et al. Functional and cognitive impairment, Frailty, and adverse Health outcomes in older patients reaching ESRD-A systematic review. Clin J Am Soc Nephrol. 2016;11(9):1624–39.
Green R, Allen LH, Bjørke-Monsen AL, Brito A, Guéant JL, Miller JW, et al. Vitamin B(12) deficiency. Nat Rev Dis Primers. 2017;3:17040.
Kvestad I, Hysing M, Shrestha M, Ulak M, Thorne-Lyman AL, Henjum S, et al. Vitamin B-12 status in infancy is positively associated with development and cognitive functioning 5 y later in Nepalese children. Am J Clin Nutr. 2017;105(5):1122–31.
Vogiatzoglou A, Smith AD, Nurk E, Drevon CA, Ueland PM, Vollset SE, et al. Cognitive function in an elderly population: interaction between vitamin B12 status, depression, and apolipoprotein E ε4: the Hordaland Homocysteine Study. Psychosom Med. 2013;75(1):20–9.
Vogiatzoglou A, Refsum H, Johnston C, Smith SM, Bradley KM, de Jager C, et al. Vitamin B12 status and rate of brain volume loss in community-dwelling elderly. Neurology. 2008;71(11):826–32.
Wang C, Zhang Y, Shu J, Gu C, Yu Y, Liu W. Association between Methylmalonic Acid and Cognition: a systematic review and Meta-analysis. Front Pediatr. 2022;10:901956.
Vogiatzoglou A, Oulhaj A, Smith AD, Nurk E, Drevon CA, Ueland PM, et al. Determinants of plasma methylmalonic acid in a large population: implications for assessment of vitamin B12 status. Clin Chem. 2009;55(12):2198–206.
Manoli I, Sysol JR, Li L, Houillier P, Garone C, Wang C, et al. Targeting proximal tubule mitochondrial dysfunction attenuates the renal disease of methylmalonic acidemia. Proc Natl Acad Sci U S A. 2013;110(33):13552–7.
Wang S, Liu Y, Liu J, Tian W, Zhang X, Cai H, et al. Mitochondria-derived methylmalonic acid, a surrogate biomarker of mitochondrial dysfunction and oxidative stress, predicts all-cause and cardiovascular mortality in the general population. Redox Biol. 2020;37:101741.
McCracken C, Hudson P, Ellis R, McCaddon A. Methylmalonic acid and cognitive function in the Medical Research Council cognitive function and ageing study. Am J Clin Nutr. 2006;84(6):1406–11.
Lildballe DL, Fedosov S, Sherliker P, Hin H, Clarke R, Nexo E. Association of cognitive impairment with combinations of vitamin B12-related parameters. Clin Chem. 2011;57(10):1436–43.
Clarke R, Birks J, Nexo E, Ueland PM, Schneede J, Scott J, et al. Low vitamin B-12 status and risk of cognitive decline in older adults. Am J Clin Nutr. 2007;86(5):1384–91.
Lewerin C, Ljungman S, Nilsson-Ehle H. Glomerular filtration rate as measured by serum cystatin C is an important determinant of plasma homocysteine and serum methylmalonic acid in the elderly. J Intern Med. 2007;261(1):65–73.
Hoffmann GF, Meier-Augenstein W, Stöckler S, Surtees R, Rating D, Nyhan WL. Physiology and pathophysiology of organic acids in cerebrospinal fluid. J Inherit Metab Dis. 1993;16(4):648–69.
Obeid R, Schadt A, Dillmann U, Kostopoulos P, Fassbender K, Herrmann W. Methylation status and neurodegenerative markers in Parkinson disease. Clin Chem. 2009;55(10):1852–60.
van der Zwaluw NL, Brouwer-Brolsma EM, van de Rest O, van Wijngaarden JP et al. Veld PH, Kourie DI,. Folate and Vitamin B(12)-Related Biomarkers in Relation to Brain Volumes. Nutrients. 2016;9(1).
Pettenuzzo LF, Ferreira Gda C, Schmidt AL, Dutra-Filho CS, Wyse AT, Wajner M. Differential inhibitory effects of methylmalonic acid on respiratory chain complex activities in rat tissues. Int J Dev Neurosci. 2006;24(1):45–52.
Malfatti CR, Royes LF, Francescato L, Sanabria ER, Rubin MA, Cavalheiro EA, et al. Intrastriatal methylmalonic acid administration induces convulsions and TBARS production, and alters Na+,K+-ATPase activity in the rat striatum and cerebral cortex. Epilepsia. 2003;44(6):761–7.
Kowaltowski AJ, Maciel EN, Fornazari M, Castilho RF. Diazoxide protects against methylmalonate-induced neuronal toxicity. Exp Neurol. 2006;201(1):165–71.
Han L, Wu S, Han F, Gu X. Insights into the molecular mechanisms of methylmalonic acidemia using microarray technology. Int J Clin Exp Med. 2015;8(6):8866–79.
Stepien KM, Heaton R, Rankin S, Murphy A, Bentley J, Sexton D et al. Evidence of oxidative stress and secondary mitochondrial dysfunction in metabolic and non-metabolic disorders. J Clin Med. 2017;6(7).
Duan LP, Zheng ZX, Zhang YH, Dong J. [Association of malnutrition-inflammation-cardiovascular disease with cognitive deterioration in peritoneal dialysis patients]. Beijing Da Xue Xue Bao Yi Xue Ban. 2019;51(3):510–8.
Sun AL, Ni YH, Li XB, Zhuang XH, Liu YT, Liu XH, et al. Urinary methylmalonic acid as an indicator of early vitamin B12 deficiency and its role in polyneuropathy in type 2 diabetes. J Diabetes Res. 2014;2014:921616.
Wile DJ, Toth C. Association of metformin, elevated homocysteine, and methylmalonic acid levels and clinically worsened diabetic peripheral neuropathy. Diabetes Care. 2010;33(1):156–61.
Wang S, Wang Y, Wan X, Guo J, Zhang Y, Tian M, et al. Cobalamin Intake and related biomarkers: Examining associations with mortality risk among adults with type 2 diabetes in NHANES. Diabetes Care. 2022;45(2):276–84.
Tamura T, Kuriyama N, Koyama T, Ozaki E, Matsui D, Kadomatsu Y, et al. Association between plasma levels of homocysteine, folate, and vitamin B(12), and dietary folate intake and hypertension in a cross-sectional study. Sci Rep. 2020;10(1):18499.
Gorelick PB, Nyenhuis D, Materson BJ, Calhoun DA, Elliott WJ, Phillips RA, et al. Blood pressure and treatment of persons with hypertension as it relates to cognitive outcomes including executive function. J Am Soc Hypertens. 2012;6(5):309–15.
Williamson JD, Pajewski NM, Auchus AP, Bryan RN, Chelune G, Cheung AK, et al. Effect of intensive vs standard blood pressure control on probable dementia: a Randomized Clinical Trial. JAMA. 2019;321(6):553–61.
Bailey RL, Jun S, Murphy L, Green R, Gahche JJ, Dwyer JT, et al. High folic acid or folate combined with low vitamin B-12 status: potential but inconsistent association with cognitive function in a nationally representative cross-sectional sample of US older adults participating in the NHANES. Am J Clin Nutr. 2020;112(6):1547–57.
Samson ME, Yeung LF, Rose CE, Qi YP, Taylor CA, Crider KS. Vitamin B-12 malabsorption and renal function are critical considerations in studies of folate and vitamin B-12 interactions in cognitive performance: NHANES 2011–2014. Am J Clin Nutr. 2022;116(1):74–85.
Acknowledgements
Not applicable.
Funding
This study was supported by the Xuanwu Hospital Huizhi talent leader training program to Aihua Zhang.
Author information
Authors and Affiliations
Contributions
A.H.Z contributed to the study concept and design; J.L.Z, L.Y.W, Y.J.P, and S.Y.W contributed to data collection; J.L.Z, and A.H.Z contributed to the statistical analysis; J.L.Z contributed to the original draft; A.H.Z contributed to the review draft. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All participants submitted written informed consent and were approved by the NCHS Research Ethics Review Board (Continuation of Protocol #2011–17).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhang, J., Wu, L., Wang, S. et al. Increased serum methylmalonic acid levels were associated with the presence of cognitive dysfunction in older chronic kidney disease patients with albuminuria. BMC Geriatr 24, 159 (2024). https://doi.org/10.1186/s12877-024-04759-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12877-024-04759-y