Abstract
Introduction
The association between chronic pain and frailty might indicate that pain is an independent driver of frailty but might alternatively be explained by inclusion within frailty identification tools of morbidities that commonly lead to chronic pain. This research examines the extent to which the association of pain with frailty might be attributed to morbidities.
Methods
A cross-sectional analysis of older people in a UK cohort with or at risk of musculoskeletal problems or frailty (Investigating Musculoskeletal Health and Wellbeing study), used multivariable logistic regression and Z-tests to assess the degrees of associations of pain (McGill Pain Rating Index), and painful and non-painful morbidity counts with frailty (modified FRAIL questionnaire).
Results
Data were from 2,185 participants, 56% female, median age 73 (range 60 to 96) years. 430 (20%) participants were classified as frail. In a fully adjusted standardised model, pain (aOR 2.07 (95%CI 1.83 to 2.33) and ‘any’ morbidity aOR (1.74 (95%CI 1.54 to 1.97) were both significantly associated with frailty. When morbidity was subclassified as painful or non-painful, painful (aOR 1.48 (95%CI 1.30 to 1.68) and non-painful (aOR1.39 (95%CI 1.24 to 1.56)) morbidities each were associated with frailty, as also was pain (aOR 2.07 (95%CI 1.83 to 2.34, p < 0.001).
Conclusions
Pain is associated with frailty, over and above any effect of painful and non-painful morbidities. This forms the justification for future research which focuses on pain management in the identification, prevention, and treatment of frailty.
Similar content being viewed by others
Introduction
Frailty is a vulnerability state in older people that is characterised by a loss of homeostatic resilience as a consequence of multi-organ, age-associated decline [1]. Frailty is conceptualised using cumulative deficit models such as the Frailty Index [2]. Alternatively, frailty may be defined as a phenotype which groups clinical characteristics for example the Frailty Phenotype [3]. This research uses a hybrid frailty identification tool based on both models (FRAIL [4]). The prevalence of frailty in the UK is estimated at 15% of people over 65 years [5]. People with frailty have higher mortality, higher hospitalisation rates and are more disabled than those who are non-frail.
Frailty, whether classified according to cumulative deficit [2, 6,7,8,9,10] or phenotype models [3, 7, 11,12,13] or the hybrid FRAIL [4], is associated with chronic pain (pain unresolved after three months [14]). Musculoskeletal conditions are the most common causes of chronic pain affecting over a third of the UK population [15, 16]. An estimated 8.5 million people in the UK have osteoarthritis [15].
Frailty is associated also with multi-morbidity (two or more long-term health conditions [17]). In fact, multi-morbidity is an integral part of frailty identification tools based on the cumulative deficit model [2, 6,7,8,9,10] and FRAIL [4]. Accumulated deficits including those from morbidities represent multi-organ decline, and are associated with frailty classification [2, 18].
An association between chronic pain and frailty is identified both using the Fried phenotype model of frailty (which does not directly include morbidities) [3, 7, 11,12,13], and using the cumulative deficit models of frailty [2, 6,7,8,9,10]. This suggests that the association of pain with frailty is not purely a statistical phenomenon resulting from inclusion of morbidity counts in frailty identification tools. This raises the possibility that chronic pain might itself contribute to the frailty state. If so, chronic pain would be an additional variable that could be used in to identify, predict and measure frailty. Furthermore, attempts to ameliorate or manage chronic pain could potentially prevent or reverse frailty states. Current frailty interventions focus on other aspects such as exercise and nutrition [19].
We set out to examine the extent to which association of chronic pain with frailty might be attributed to morbidities.
Methods
We performed cross-sectional analysis of data from participants recruited to the Investigating Musculoskeletal Health & Wellbeing (IMH&W) study, based in the East Midlands, UK (20).
Participants and data sources
To participate in the IMH&W study participants were aged 18 years and over, and able to give informed consent and had completed baseline postal IMH&W questionnaires (n = 5,500). The eligible data extracted for this study in July 2020 included participants aged ≥ 60 years who completed all 5 items of the FRAIL questionnaire.
IMH&W recruited participants through multiple pathways, including inviting people with or at risk of musculoskeletal problems or frailty [20]. To enhance representation of participants with frailty in IMH&W, one recruitment pathway involved patients from General Practitioners (GP) registers with an electronic Frailty Index (eFI) score of ≥ 0.12 (threshold for mild frailty) [21]. The eFI comprises 36 deficits, consisting of morbidities, symptoms, activity/ mobility restrictions, social vulnerability, and care requirements [21].
This research was conducted under the ethics approval given for the IMH&W study by the Central London Research Ethics Committee (ref.18/LO/0870).
Variables
The outcome variable is frailty, the predictors are morbidities and pain. Age, sex, and BMI were included as potential confounders. All variables are described below.
Frailty
The IMH&W survey included the 5 self-report items comprising the FRAIL questionnaire [4]. Items were each scored as 0: not present, or 1: present. The 5 items were: Fatigue (feeling tired all or most of the time in the last month); Resistance (difficulty ascending 10 steps without aid) and Ambulation (difficulty walking several hundred yards without aids) each scored as no or yes; Illnesses (≥ 5 from 11 specified conditions) and Loss of weight (> 5% during a year). The FRAIL items are used to classify people into non-frail (0 to 2 items) or frail (3 to 5 items). In the current study, to remove the overlap between FRAIL and morbidities, we modified FRAIL (“mFRAIL”). This omission of the illnesses (morbidity) item permitted examination of the contribution of morbidities to a frailty classification that approximates the phenotype model. Participants were classified using mFRAIL as non-frail (0 to 2 items) or frail (3 to 4 items).
The mFRAIL tool was assessed for validity using Receiver Operating Characteristic (ROC) curve analysis. The area under the ROC curve using the original FRAIL cutoff of 3/5 items = 0.997 and mFRAIL cut-off of 3/4 items was 0.986, p = 0.004.
Morbidities
We generated an ‘any’ morbidity count variable (unweighted) which comprised the 11 conditions included in the illnesses item in the original FRAIL questionnaire (as above), plus 8 morbidities from the Charlson Comorbidity Index (CCI) [22] and 7 from the Functional Comorbidity Index (FCI) [23]. We ascertained these morbidities from:
1. A checklist of conditions prefaced with the question; ‘has a doctor told you that you have any of these conditions or problems,’ including the 11 FRAIL conditions and 7 other conditions (Additional Fig. 1).
2. Free text for “other conditions”, classified using criteria developed by consensus between WJC, DAW, and JRFG. Additional Table 1 shows the criteria for classifying “other” morbidities from free text. We performed this classification using an algorithm we developed using Excel and a series of logic and lookup commands. Two reviewers (WJC, SS) independently checked a sample of 100 participants and confirmed its reliability [ICC = 0.94 (95% CI 0.91 to 0.96), p < 0.001].
3. Participants’ self-reported medications by free text and/or prescriptions. WJC, DAW and JRFG reviewed and edited the list of participant medications to include only those medications specifically used for conditions included in our morbidities list, based on information in the British National Formulary (Additional Table 2). Morbidities were coded by algorithm, as above, according to self-reported use of these specific medications for each participant. For example, if a participant reported insulin, we inferred they had diabetes mellitus even if they had not listed it in the checklist or free text. Two reviewers (WJC, SS) independently checked a sample of 100 participants and confirmed its reliability [ICC = 0.98 (95% CI 0.97 to 0.99), p < 0.001].
We counted each morbidity once, drawing first from the morbidity checklist, secondly from free text and finally by inference from medication lists. Additional Table 3 shows the morbidities identified by this process.
We also classified the 26 morbidities as either ‘painful’ or ‘non-painful’ morbidities according to the International Association for the Study of Pain, Classification of Chronic Pain list of conditions for which pain management is routinely considered part of appropriate treatment [24].(Additional Table 3).
Pain
In our primary analysis, pain was measured using the McGill Pain Rating Index [25, 26], to represent pain of any type or source. This instrument comprises 78 pain descriptors in 20 sets of words, which categorise pain into a common intensity dimension, in which a higher score indicates greater pain [25]. The descriptor rank value was based on the word position within each set. The Pain Rating Index equals the sum of the descriptor rank values, ranging from 1 to 78. Only one word may be ticked per set, if no words apply, a zero score was allocated. In confirmatory analysis we used a 0–10 Numerical Rating Scale (NRS) of joint pain intensity. Participants responded to the question: “over the past four weeks, how intense was your average pain or the average aching in your most bothersome joint, where 0 is “no pain”, and 10 is “pain as bad as could be”?”
Other variables
Age (in years), sex (male/female), and body mass index class (BMI) (underweight/normal/pre-obese/obese) were included in all multivariable analyses. Weight (kg), and height (m) were from questionnaire self-report, from which BMI (kg/m2) was calculated. BMI was then classified using WHO categories [27]; obese sub-categories were collapsed into a single ‘obese’ category of BMI > 30. BMI was treated as categorical because those regarded as either underweight or obese might be less healthy than those with normal BMI.
Statistical analysis
Data were summarised using medians and ranges for non-normally distributed variables, and n (%) for dichotomous variables. Normality was assessed graphically using histograms and statistically using the Shapiro-Wilk test. None of the assessed variables were found to be normally distributed. Differences between groups were evaluated using Mann-Whitney U tests for continuous variables, and Chi-squared test for categorical variables. Correlations were assessed using Spearman rho and bootstrapped (10,000) to derive confidence intervals. Cases with any missing data were excluded in regression analysis.
We validated the mFRAIL threshold by exploring the internal structure of FRAIL using Cronbach’s alpha and Receiver Operator Curve (ROC) analysis of mFRAIL score against original FRAIL classification.
Multivariable logistic regression analyses were used to examine the extent to which association of chronic pain with frailty can be attributed to morbidities. Change was assessed by comparing the association of chronic pain with frailty alongside when ‘any’ morbidity count was added to the model. Standardized coefficients permitted comparison of variables with different scales. They represent the change in the dependent variable’s standard deviation associated with a one-standard-deviation increase in the predictor variable.
To examine the extent to which association of morbidities with frailty can be attributed to pain, we used multivariable logistic regression analyses. We investigated associations between painful and non-painful morbidity count with frailty.
Age, sex, and BMI class were selected a priori, other co-variables were included if p < 0.05 in prior bivariate analysis.
Categorical variables were classified as a binary outcome (absent/present) in all logistic regression models. Coefficients were standardised by generating z scores ((value-mean)/ standard deviation)) to permit a direct comparison of the magnitude and impact of different variables on the dependent variable. Z-tests were used to compare the strengths of regression coefficients or the changes between coefficients in separate models [28], where Z values ≥ ± 2 were interpreted as significantly different coefficients.
Statistical analyses were undertaken using STATA SE v16 (StataCorp LLC), using p ≤ 0.05 to indicate statistical significance.
Results
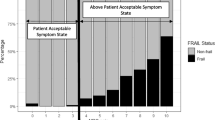
There were 5,550 baseline data records checked for eligibility, with 2,185 participants whose data met the eligibility criteria for this study. Their characteristics are shown in Table 1. Median age was 73 (range 60 to 96) years, and 1,202 (55%) were female. FRAIL and mFRAIL (FRAIL with illnesses excluded) classified respectively 430 (20%) and 418 (19%) as frail. Use of mFRAIL led to a re-classification of only 12/430 (3%) participants classified by FRAIL. The median (IQR) Pain Rating Index was 13 (7 to 22). Data missingness was: Pain Rating Index n = 233 (11%), sex n = 1, and BMI n = 29 (1%), with no missing data for frailty, age, or morbidity counts. A flow diagram with details of missing data which were excluded from analysis are shown in Additional Fig. 2.
Morbidities
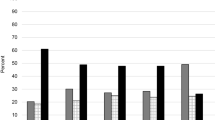
Participants reported median (range) 3 (0 to 12) ‘any’ morbidities, 2 (0 to 8) painful morbidities, and 1 (0 to 5) non-painful morbidities. Only 96 (4%) participants reported no morbidities, and 1,297 (59%) participants had at least one painful plus one non-painful morbidity. The frequencies of morbidity counts are shown in Table 2. The most frequently reported painful and non-painful morbidities were arthritis 1,477 (68%) and hypertension 856 (39%), respectively. Higher ‘any’ morbidity count was associated with being female, pre-obese or obese. Higher painful morbidity count was associated with being female, older, or obese. Higher non-painful morbidity count was associated with obesity (Additional Table 4).
Bivariate associations of frailty with pain, morbidity, and covariates
Frailty (mFRAIL) was associated with pain, morbidity counts and co-variates.
In those classified as frail, the median Pain Rating Index was 22 (IQR 13 to 33) compared to 11 (IQR 6 to 19) in those who were non-frail. Pain Rating Index was associated with mFRAIL frailty classification (OR 2.23, 95% CI 2.00 to 2.50, p < 0.001).
‘Any’ (OR 2.04, 95% CI 1.83 to 2.28, p < 0.001), painful (OR 1.89, 95% CI 1.69, 2.10, p < 0.001) and non-painful (OR 1.50, 95% CI 1.36 to 1.67, p < 0.001) morbidity counts were each associated with mFRAIL frailty classification.
Age (OR 1.02, 95% CI 1.00 to 1.03, p = 0.045), female sex (OR 1.91, 95% CI 1.52 to 2.39, p < 0.001), and BMI class (underweight OR 3.21 95% CI 1.42 to 7.25, p = 0.005, pre-obese OR 1.50 95% CI 1.11 to 2.03, p = 0.008, obese OR 2.96 95% CI 2.21 to 3.97, p < 0.001) also each was associated with mFRAIL frailty classification.
The extent to which association of chronic pain with frailty can be attributed to morbidities
In multivariable analysis, higher pain was associated with mFRAIL frailty classification (aOR 2.21 (95% CI 1.96 to 2.49), p < 0.001, when adjusted for age, sex, and BMI class (Table 3). When ‘any’ morbidity count was added to the model there was a non-significant (Z = 0.76) reduction in the contribution of pain to frailty classification (aOR 2.07 (95% CI 1.83 to 2.33), p < 0.001). When instead of ‘any’ morbidity, painful (aOR 1.48 (95% CI 1.30 to 1.68)) and non-painful (aOR 1.39 (95% CI 1.24 to 1.56)) morbidity counts were together included in the model, the contribution of pain to frailty classification was similar (aOR 2.07 (95% CI 1.83 to 2.34), p < 0.001), Z=-0.002) (Table 3).
Similar findings were found in secondary analyses using NRS joint pain scores instead of the Pain Rating Index: pain (aOR 3.32, (95%CI2.79, 3.95), p < 0.001), painful (aOR 1.37, (95%CI 1.20, 1.56), p < 0.001) and non-painful (aOR 1.39, (95%CI 1.24, 1.58), p < 0.001) morbidities were significantly associated with mFRAIL frailty classification (Additional Table 5).
The extent to which association of morbidities with frailty can be attributed to pain
Higher ‘any’ morbidity count (aOR 1.98 (95% CI 1.77 to 2.23, p < 0.001)); higher painful (aOR 1.84, 95% CI 1.65 to 2.06, p < 0.001) and higher non-painful (aOR 1.49, 95% CI 1.34 to 1.66, p < 0.001) morbidity counts were associated with mFRAIL frailty classification in separate multivariable regression models, each of which included age, sex, and BMI class as covariates (Table 4). Both painful and non-painful morbidity counts remained significantly associated with mFRAIL frailty classification when they were included in a single age-, sex-, and BMI- adjusted model (painful morbidity count aOR 1.67, (95% CI 1.49 to 1.88, p < 0.001, non-painful morbidity count aOR 1.38, (95% CI 1.24 to 1.55, p < 0.001). When Pain Rating Index was added to this model, painful and non-painful morbidity counts remained significantly associated with mFRAIL frailty classification (Table 3), although the effect of painful morbidity count was slightly reduced and became similar to that of non-painful morbidities.
Discussion
We found that pain, painful and non-painful morbidity counts were all associated with frailty when included in a single multivariable model. Inclusion of morbidities in any model did not substantially reduce the relationship between chronic pain and frailty, indicating that this relationship is unlikely to be explained entirely by morbidities.
Our findings confirm and help to elucidate the previously demonstrated association between pain and frailty [6, 7, 10,11,12, 29]. Pain is associated with morbidities, but multimorbidity alone does not explain the effect of pain on frailty classification. It might be that pain or the process through which pain is experienced leads to frailty, or that loss of resilience inherent in the frailty state predisposes people to chronic pain. Others have described the relationship between morbidities and frailty [1, 18, 30,31,32,33], however, they have not explored this in the context of the relationship between pain and frailty. This research confirms the relationship between morbidities and frailty, and, to our knowledge, this is the first study to explore morbidity classification by pain.
A clinical implication of our findings, given that chronic pain is highly prevalent [15, 16], is that effective pain management might have great potential to prevent or reduce frailty in the community. Another implication, for those who study frailty, is that chronic pain might be a factor that could be used in the identification, measurement, and prediction of frailty.
Our study has both strengths and limitations. Although different results might have been obtained in different populations, our sample was representative of the IMH&W cohort, was large and had a high prevalence of painful and non-painful morbidities, pain, and frailty, enabling detailed exploration of these relationships. Our sample had an approximately equal male to female distribution and included people from a range of socioeconomic backgrounds. However, IMH&W itself selectively recruited people with or at risk of frailty or musculoskeletal problems and displayed little ethnic diversity.
Different results might have been obtained using different frailty classification tools and future work should include other frailty classification tools to confirm our findings. IMH&W was a postal questionnaire survey and so it was not possible for us to use in-person measurement of gait speed and grip strength to classify frailty phenotype [3]. We modified the FRAIL classification criteria by removal of the illness item in order to investigate the role of morbidities in the relationship between pain and frailty. However, mFRAIL and FRAIL only classified 12 (3%) participants differently, suggesting that mFRAIL and FRAIL have similar validity for frailty classification. FRAIL has previously been shown to be a valid tool for frailty classification [4, 34], which performs comparably with other frailty tools [35, 36]. Our findings, however, suggest that mFRAIL and FRAIL might not fully describe frailty, and other frailty classifications might give different results.
We obtained similar findings using two different pain measurement tools (Pain Rating Index and NRS). However, pain is a complex, multidimensional symptom and other pain measurement tools could give different results. It remains possible that aspects of pain (e.g., lower limb joint pain) result in an overclassification of frailty due to the inclusion in frailty classification tools of physical activity.
A strength of our study is that we found an association between pain and frailty, even after using an extensive list of morbidities to measure morbidity counts. We acknowledge the imprecision of classifying morbidities as either painful or non-painful using IASP criteria for conditions where pain management should be considered. Pain may be reported in conditions such as stroke that were classified as non-painful. Future research might assess effects of differentially weighting specific morbidities, although our findings suggest that weighting painful and non-painful morbidities differently would be unlikely to substantially affect frailty classification, nor the association of pain with frailty.
We acknowledge the risk of residual confounding due to the inclusion of inter-correlated variables in our multivariable models.
Longitudinal and interventional study designs are required to determine causality of the relationships we observed between pain, morbidities, and frailty. Future research should explore mechanisms by which pain might lead to frailty, for example by reducing physical activity, impairing appetite and nutrition, or through neuro-endocrine dysregulation. Randomised controlled trials would be required to test whether interventions that improve pain (even if not directly addressing underlying morbidities) can prevent or reverse frailty. A range of interventions that can reduce chronic pain (e.g., psychological, pharmacological, surgical, physical) might be explored in populations with or at risk of frailty, aiming not only to reduce pain, but also to facilitate transition to a non-frail state, or prevent transition into frailty.
In conclusion, chronic pain and multi-morbidity are both associated with frailty. The relationship of pain with frailty cannot be explained by morbidities, and the relationship between morbidities and frailty is not explained solely by pain. Further research is required to understand the complex relationship between pain and frailty. Interventions to mitigate the effect of chronic pain upon frailty should not be focussed solely upon treating underlying morbidities, but also manage chronic pain irrespective of its aetiology.
Data availability
The data that support the findings of this study are available from the authors upon reasonable request and with permission of the University of Nottingham. Enquires should be sent to David.Walsh@nottingham.ac.uk.
References
Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. The Lancet. 2013;381(9868):752–62.
Rockwood K, Mitnitski A. Frailty in Relation to the Accumulation of deficits. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2007;62(7):722–7.
Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol a-Biol. 2001;56(3):M146–M56.
Morley JE, Malmstrom TK, Miller DK. A simple frailty questionnaire (FRAIL) predicts outcomes in middle aged African americans. J Nutr Health Aging. 2012;16(7):601–8.
Reeves D, Pye S, Ashcroft DM, Clegg A, Kontopantelis E, Blakeman T, et al. The challenge of ageing populations and patient frailty: can primary care adapt? BMJ. 2018;362:k3349.
Wade KF, Marshall A, Vanhoutte B, Wu FC, O’Neill TW, Lee DM. Does pain predict frailty in older men and women? Findings from the English Longitudinal Study of Ageing (ELSA). The Journals of Gerontology: Series A. 2017;72(3):403–9.
Rodríguez-Sánchez I, García-Esquinas E, Mesas AE, Martín-Moreno JM, Rodríguez-Mañas L, Rodríguez-Artalejo F. Frequency, intensity and localization of pain as risk factors for frailty in older adults. Age Ageing. 2019;48(1):74–80.
Shega JW, Dale W, Andrew M, Paice J, Rockwood K, Weiner DK. Persistent pain and frailty: a case for homeostenosis. J Am Geriatr Soc. 2012;60(1):113–7.
Chen C, Winterstein AG, Fillingim RB, Wei Y-J. Body weight, frailty, and chronic pain in older adults: a cross-sectional study. BMC Geriatr. 2019;19(1):1–10.
Chaplin WJ, McWilliams DF, Millar BS, Gladman JRF, Walsh DA. The bidirectional relationship between chronic joint pain and frailty: data from the investigating Musculoskeletal Health and Wellbeing cohort. BMC Geriatr. 2023;23(1):273. 2023/05/05.
Megale RZ, Ferreira ML, Ferreira PH, Naganathan V, Cumming R, Hirani V et al. Association between pain and the frailty phenotype in older men: longitudinal results from the Concord Health and Ageing in Men Project (CHAMP). Age and ageing. 2018 01 May;47(3):381–7.
Bindawas SM, Vennu V, Stubbs B. Longitudinal relationship between knee Pain Status and Incident Frailty: data from the Osteoarthritis Initiative. Pain Med. 2018;19(11):2146–53.
Veronese N, Maggi S, Trevisan C, Noale M, De Rui M, Bolzetta F et al. Pain increases the risk of developing Frailty in older adults with Osteoarthritis. Pain Med [Observational Study]. 2017 03 01;18(3):414–27.
Fayaz A, Croft P, Langford RM, Donaldson LJ, Jones GT. Prevalence of chronic pain in the UK: a systematic review and meta-analysis of population studies. BMJ Open. 2016;6(6):e010364.
Versus Arthritis. The State of Musculoskeletal Health 2021. Annual Report. Versus Arthritis, 2021.
Havelin J, King T. Mechanisms underlying bone and Joint Pain. Curr Osteoporos Rep. 2018;16(6):763–71.
Barnett K, Mercer SW, Norbury M, Watt G, Wyke S, Guthrie B. Epidemiology of multimorbidity and implications for health care, research, and medical education: a cross-sectional study. The Lancet. 2012;380(9836):37–43.
Fried LP, Xue QL, Cappola AR, Ferrucci L, Chaves P, Varadhan R et al. Nonlinear multisystem physiological Dysregulation Associated with Frailty in Older Women: implications for etiology and treatment. The journals of Gerontology Series A: Biological sciences and Medical sciences. 2009;64A(10):1049–57.
Travers J, Romero-Ortuno R, Bailey J, Cooney MT. Delaying and reversing frailty: a systematic review of primary care interventions. Br J Gen Pract., Millar B, McWilliams DF, Abhishek A, Akin-Akinyosoye K, Auer DP, Chapman V et al. Investigating musculoskeletal health and wellbeing; a cohort study protocol. BMC Musculoskelet Disord. 2020;21(1):1–10.
Millar B, McWilliams DF, Abhishek A, Akin-Akinyosoye K, Auer DP, Chapman V, et al. Investigating musculoskeletal health and wellbeing; a cohort study protocol. BMC Musculoskelet Disord. 2020;21(1):1–10.
Clegg A, Bates C, Young J, Ryan R, Nichols L, Ann Teale E, et al. Development and validation of an electronic frailty index using routine primary care electronic health record data. Age Ageing. 2016;45(3):353–60.
Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–83.
Groll DL. The development of a functional Comorbidity Index. University of Toronto; 2004.
International Association for the Study of Pain. Classification of Chronic Pain, Second Edition (Revised)., 1994 [updated 2012; cited 2022 10/2022]; Second:[Subcommittee on Taxonomy.]. Available from: https://www.iasp-pain.org/publications/free-ebooks/classification-of-chronic-pain-second-edition-revised/.
Melzack R. The McGill Pain Questionnaire: major properties and scoring methods. Pain. 1975;1(3):277–99.
Melzack R, Torgerson WS. On the language of pain. Anesthesiology. 1971;34(1):50–9.
World Health Organization. WHO/Europe | Nutrition - Body mass index - BMI. World Health Organisation; 2021. https://www.euro.who.int/en/health-topics/disease-prevention/nutrition/a-healthy-lifestyle/body-mass-index-bmi. [cited 2021 01/03/2021]; BMI classification].
Clogg CC, Petkova E, Haritou A. Statistical methods for comparing regression coefficients between models. Am J Sociol. 1995;100:1261–93.
Saraiva MD, Suzuki GS, Lin SM, de Andrade DC, Jacob-Filho W, Suemoto CK. Persistent pain is a risk factor for frailty: a systematic review and meta-analysis from prospective longitudinal studies. Age Ageing. 2018;47(6):785–93.
Villacampa-Fernandez P, Navarro-Pardo E, Tarin JJ, Cano A. Frailty and multimorbidity: two related yet different concepts. Maturitas. 2017;95:31–5.
Morley JE. Frailty and Sarcopenia the new geriatric giants. Rev Invest Clin. 2016;68:59–67.
Theou O, Rockwood MRH, Mitnitski A, Rockwood K. Disability and co-morbidity in relation to frailty: how much do they overlap? Arch Gerontol Geriatr. 2012;55(2):e1–e8.
Dent E, Morley JE, Cruz-Jentoft AJ, Woodhouse L, Rodríguez-Mañas L, Fried LP, et al. Physical Frailty: ICFSR International Clinical Practice guidelines for Identification and Management. The journal of nutrition, health & aging; 2019.
Susanto M, Hubbard RE, Gardiner PA. Validity and Responsiveness of the FRAIL Scale in Middle-Aged Women. Journal of the American Medical Directors Association., Aprahamian I, Cezar NODC, Izbicki R, Lin SM, Paulo DLV, Fattori A et al. Screening for Frailty With the FRAIL Scale: A Comparison With the Phenotype Criteria. J Am Med Dir Assoc. 2017;18(7):592-6.
Aprahamian I, Cezar NODC, Izbicki R, Lin SM, Paulo DLV, Fattori A, et al. Screening for Frailty With the FRAIL Scale: A Comparison With the Phenotype Criteria. J Am Med Dir Assoc. 2017;18(7):592-6.
Ravindrarajah R, Lee DM, Pye SR, Gielen E, Boonen S, Vanderschueren D, et al. The ability of three different models of frailty to predict all-cause mortality: results from the European Male Aging Study (EMAS). Arch Gerontol Geriatr. 2013;57(3):360–8.
Acknowledgements
The authors would like to thank the participants for taking part in the IMH&W study. Also, the members of the NIHR Nottingham BRC Musculoskeletal Theme’s Public and Patient Involvement and Engagement Advisory Group who helped provide feedback in the IMH&W design.
Funding
This work was supported by the National Institute for Health Research (NIHR) through the NIHR Nottingham Biomedical Research Centre and by Versus Arthritis (Centre initiative grant number 20777). The views expressed are those of the author(s) and not necessarily those of the funders.
Author information
Authors and Affiliations
Contributions
WJC was lead data analyst, interpreted the data, and contributed to study conception and design. WJC drafted the manuscript. BSM contributed to study design and data acquisition. HRL contributed to study design, study conception and data analysis. SMS contributed to data analysis. DMcW contributed to data analysis, study conception, study design and data interpretation. JRFG and DAW both contributed to study conception, study design, data analysis, data interpretation. DMcW, JRFG and DAW made substantive revisions to the manuscript drafts DAW also made contributions to data acquisition. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
DMcW declares grant and research support from Pfizer Ltd, GlaxoSmithKline Plc and Lilly Ltd. DAW declares a personal financial interest in his employment by the University of Nottingham, who receive funding for his salary from the UK Government, Sherwood Forest Hospitals NHS Foundation Trust and UKRI. In the past 36 months, DAW declared the following non-personal financial interests: consultancy through his employment with the University of Nottingham to Pfizer Ltd, Eli Lilly, AbbVie Ltd, Galapagos and Reckitt Benckiser Health Ltd, and GlaxoSmithKline Plc, and responsibilities for investigator-led grants outside the work in this presentation held by the University of Nottingham from Pfizer Ltd and Lilly Ltd. The other authors do not have any competing interests.
Ethics approval and consent to participate
Ethical approval for the Investigating Musculoskeletal Health & Wellbeing study was by the Central London Research Ethics Committee (ref.18/LO/0870 IRAS 227758). Clinicaltrials.gov NCT03696134 registered 4/10/2018. The study was conducted according to the Declaration of Helsinki the principles of Good Clinical Practice (GCP) and the UK Policy Framework for Health and Social Care Research, 2018. Written informed consent was obtained from all participants prior to participation and they were all assured that they could withdraw their consent at any time without consequences.
Consent for publication
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Chaplin, W.J., Lewis, H.R., Shahtaheri, S.M. et al. The association of painful and non-painful morbidities with frailty: a cross sectional analysis of a cohort of community dwelling older people in England. BMC Geriatr 24, 158 (2024). https://doi.org/10.1186/s12877-023-04602-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12877-023-04602-w