Abstract
Background
The benefits of physical activity (PA) and adequate sleep are well documented, and their importance strengthens with the increasing prevalence of chronic diseases and multimorbidity (MM). Interventions to promote physical activity and sleep that use commercial activity trackers may be useful non-pharmacological approaches to managing individual health; however, limited evidence exists on their use to improve physical activity in older adult patients with MM.
Methods
This study aims to measure the effects of behavioral change techniques (BCTs) delivered by a wearable device on physical activity and quality of sleep (QS) in older adult patients with MM. We designed an open-label randomized controlled trial with participants recruited through primary care and a specialist outpatient clinic. Participants must be more than 65 years old, have MM, and have access to smartphones. All eligible participants will receive PA promotion content and will be randomly assigned to wear a smartwatch. The primary outcome will be the participants’ PA measurement at baseline and at six months using the International Physical Activity Questionnaire - Short Form (IPAQ-SF). Secondary outcomes will include changes in the participants’ frailty status, biometric measurements, quality of life, and biopsychosocial assessments. A sample size of 40 participants per arm was calculated to detect group differences, with 50 participants planned to recruit and randomize into each arm.
Discussion
This study aims to contribute to a better understanding of PA patterns and the impact of wearable-based PA interventions in patients with MM. In addition, we aim to contribute to more knowledge about the relationship between PA patterns, Patient Reported Outcomes Measures (PROMs), and healthcare resource utilization in patients with MM. To achieve this, the study will leverage a locally developed PROMs registry and assess data from participants’ medical records, in order to understand the added impact of wearable data and medical information data on predicting PROMs and unplanned hospital admissions.
Trial registration
NCT05777291
Similar content being viewed by others
Background
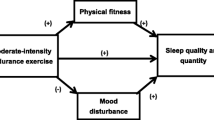
Concurrently, the harmful effects of physical inactivity, which refers to the level of Physical Activity (PA) that fails to meet the guidelines, and the benefits of meeting these guidelines are well established [1,2,3]. PA and exercise, the latter being a planned, structured, repetitive version of physical activity that promotes physical fitness maintenance or development, are key components of lifestyle medicine [1]. The benefits of PA and exercise extend beyond improving mental health and quality of life and contribute to the prevention and treatment of several chronic diseases, including but not limited to obesity, cardiovascular conditions, diabetes, and various types of cancer [4,5,6].
Lack of sleep has been suggested to play a significant role in the development of major non-communicable diseases (NCDs), which motivates sleep habits to be considered an important modifiable health risk factor and a key component of a healthy lifestyle [7]. Additionally, most manifestations of NCDs worsen with age [8]. As part of lifestyle medicine, further changes in PA and sleep may constitute useful non-pharmacological approaches to managing individual health in older adults [9, 10]. While some aspects of behavior change are still not fully understood, the effectiveness of behavioral change techniques (BCTs) in both altering and sustaining health behaviors, including physical activity and sleep, has been proven, even among the geriatric population [11, 12].
Activity monitoring bracelets, smartwatches, and other wearables may serve as convenient delivery channels for these BCTs and could assist older adult patients in implementing the previously mentioned changes [13, 14]. A recent systematic review showed strong evidence of the positive impact of wearables on increasing physical activity among the general population, although effect on physiological and psychosocial measures was not clear [15]. Despite these findings, limited evidence exists on older adult patients with multimorbidity, both on objectively measured PA as well as on the efficacy of using activity trackers to improve PA [16]. Commercial off-the-shelf activity trackers allow users to self-monitor their daily PA, including the number of steps, type of PA, and amount of sleep. Fitbit (Fitbit Inc, San Francisco, CA, USA) activity trackers have previously been utilized as both measurement and intervention tools, however, it is unclear how they are being integrated into PA intervention studies, and their use in Multimorbidity (MM) patients remains limited [16, 17]. Moreover, in order to access the impact of the PA the patient’s view must be considered. This can be measured using PROMs which are often not considered in these studies [18, 19].
Methods/Design
Aims of the study
The main purpose of this study is to measure the effect of the use of BCTs delivered by a wearable device in addition to an intervention to promote PA and quality of sleep (QS) in reported activity by an older adult population with MM. We also aim to assess PA measurements experienced by patients with MM, their relationship with patient-reported outcomes, and adverse clinical outcomes.
Design and setting of the study
This study will be an open-label randomized control trial, reported in line with the Consolidated Standards of Reporting Trails (CONSORT) statement and checklist and Template for Intervention Description and Replication (TIDieR) checklist [20, 21]. Participants will be recruited through primary care and specialist outpatient clinic at Hospital da Luz Lisboa, a private tertiary hospital in Lisbon, Portugal.
Inclusion and exclusion criteria
Patients may be included if they are more than 65 years old and have MM. We will use a previously proposed definition of MM as the presence of two or more of the following chronic conditions: hypertension, depression or anxiety, chronic musculoskeletal conditions causing pain or limitation, arthritis and/or rheumatoid arthritis, osteoporosis, asthma, chronic obstructive pulmonary disease (COPD), ischemic heart disease, peripheral artery disease, heart failure, cerebrovascular diseases, chronic stomach or colon conditions, chronic hepatitis, diabetes mellitus, thyroid disorders, any active cancer in the previous five years, chronic kidney disease, chronic urinary conditions, hyperlipidemia, and obesity [22]. In addition, participants must have access to their smartphones. Exclusion criteria will include: patients who are sufficiently physically active, defined with International Physical Activity Questionnaire - Short Form (IPAQ-SF) > 150 min aerobic physical activity per week, an existing absolute contraindication for PA according to the American College of Sports Medicine (acute myocardial infarction within two days, ongoing unstable angina, uncontrolled cardiac arrhythmia with hemodynamic compromise, active endocarditis, symptomatic severe aortic stenosis, decompensated heart failure, acute pulmonary embolism, pulmonary infarction, deep venous thrombosis, acute myocarditis or pericarditis, acute aortic dissection, physical disability that precludes safe and adequate testing), poor comprehension of Portuguese language, disabling neurological disorder (defined as mRankin score \(\ge\) 4), severe psychiatric illness, learning disability, dementia and cognitive impairment, registered blind, housebound or resident in a nursing home, non-ambulant, advanced cancer, and scheduled for surgery within five months after the first consultation. Orthopedic or rheumatologic diseases with severe impairment and chronic pain syndromes with inherently reduced mobility will also not be included.
Study intervention
All Participants who are eligible and consented to participate in the study shall receive PA promotion content in accordance to the Swedish model [23]. This method consists of five core components: (1) person-centered health promotion consultation; (2) a written prescription of physical activity; (3) a prescription guided by evidence-based knowledge on physical activity in the prevention and treatment of health conditions; (4) a follow-up of the written prescription; and (5) collaboration between the healthcare service and physical activity organizers outside healthcare. It is also emphasized that the method should be tailored to the local conditions in healthcare organizations. More specifically, we will recommend PA and provide informative flyers according to the Portuguese National Healthcare Authority and the World Health Organization recommendations for older age people. In addition to PA promotion counseling, all patients will be randomly allocated to either the intervention or the control groups using block randomization generated by a computer-generated random number sequenceFootnote 1. The allocation sequence will be generated by a research assistant who will not be involved in the data collection process. Eligible participants will be randomly assigned in a 1:1 ratio to one of the following intervention arms: Arm A (experimental arm), use of the device activity watch (Fitbit Sense), and a sleep monitoring mattress (Withings SA, France - Sleep Analyzer); Arm B (control): non-use of the devices. Participants randomized to wearable device BCT (Arm A) will be provided goal setting, self-monitoring and feedback. On the first visit they will undergo a brief training session where they’ll learn how to effectively use both devices, sync their data, and interpret the feedback they receive. Arm A participants will receive weekly feedback based on their activity and sleep data trough their devices. This might include suggestions for increasing step counts or tips for better sleep hygiene. The study team will provide a communication channel available for participants to address any technical issues or queries they might have regarding device usage. They will be instructed to wear the wearable device all the time (except when charging) and use the mattress while sleeping. Patients who do not use the device for at least 60% of the time will be excluded from the trial. Compliance of divide use will be regularly monitored by the study team.
Study measures and outcomes
All participants will have two study visits: baseline and after 6-month follow-up (Fig. 1). Measurements will include self-reported data: IPAQ-SF, INTERMED-Self Assessment (IMSA) and Short Form Health Survey (SF-12); study team measurements: Vivifrail testFootnote 2, Body Mass Index (BMI), abdominal perimeter and calf perimeter measurements; and Electronic Health Records (EHRs) information retrieval (diagnoses, medications, and unplanned health visits before and during the study period). Information on the treatment allocation will not be provided to the data collectors. The data collection forms only contain unique identifier codes assigned to each participant.
The primary outcome will be the participants’ PA measurement assessed at baseline and at 6 months using the IPAQ-SF. Secondary outcomes will include changes in participants’ frailty status, biometric measurements, quality of life, and biopsychosocial assessments at baseline and at 6 months (Table 1).
Statistical analysis
A sample size of 40 participants per arm was calculated to be sufficient to detect a group difference, with the alpha set at 0.05, and the power set at 0.80. Protecting against a dropout rate of 20% over the 6-month study duration, 50 participants will be recruited and randomized into each arm (A or B). We will conduct an exploratory analysis of the recorded data. This will be based on descriptive statistics and will be used to characterize the universe of data and identify outliers and general trends. Agreement between IPAQ-SF and wearable activity will be assessed trough Cohen’s kappa. We will use McNemar’s test to compare groups at baseline and 6-month follow-up regarding the study outcomes. In addition, we will assess the impact of wearable data and PROMs in predicting unplanned hospital admissions using classification accuracy, absolute error, and ROC AUC analysis.
Discussion
We will employ the IPAQ-SF to measure the level of activity in all patients and therefore detect differences between patients who will be using wearables and those who will not. Despite conflicting evidence regarding the correlation with objectively measured PA, this test is highly reported in the literature and has been previously validated in Portuguese. We expect that the results of this study will contribute to better knowledge about the PA patterns of patients with MM as well as the impact of PA intervention in this population, a topic that is underexplored in the literature [16].
In addition, we aim to explore the influence of different PA patterns on other important outcomes such as PROMs and healthcare resource utilization. For this purpose, we will make use of a PROMs registry that has been developed locally for the purpose of data entry. We aim to understand the added impact of wearable data on medical information data for predicting PROMs and unplanned hospital admissions. Data from participants’ medical records will be assessed in the following fields: previous diagnosis, previous hospital episode types and specialties, previous medical procedures, biometrics (weight, height, and BMI), clinical notes, laboratory results, and drugs prescribed or administered in an inpatient setting. The recorded data will be anonymized and stored locally. We aim to understand whether there is any added impact of using this information for future modeling.
Availability of data and materials
Not applicable.
Abbreviations
- BCTs:
-
Behaviour change techniques
- BMI:
-
Body mass index
- COPD:
-
chronic obstructive pulmonary disease
- CONSORT:
-
Consolidated Standards of Reporting Trails
- EHRs:
-
Electronic Health Records
- IMSA:
-
INTERMED-Self Assessment
- IPAQ-SF:
-
International Physical Activity Questionnaire - Short Form
- MM:
-
Multimorbidity
- NCDs:
-
Non-communicable diseases
- PA:
-
Physical activity
- PROMs:
-
Patient Reported Outcome Measures
- QS:
-
Quality of sleep
- SF-12:
-
Short Form Health Survey
- TIDieR:
-
Template for Intervention Description and Replication
References
Thivel D, Tremblay A, Genin PM, Panahi S, Rivière D, Duclos M. Physical Activity, Inactivity, and Sedentary Behaviors: Definitions and Implications in Occupational Health. Front Public Health. 2018;6:288. https://doi.org/10.3389/fpubh.2018.00288.
Ding D, Ramirez Varela A, Bauman AE, Ekelund U, Lee IM, Heath G, et al. Towards better evidence-informed global action: lessons learnt from the Lancet series and recent developments in physical activity and public health. Br J Sports Med. 2020;54(8):462–8. https://doi.org/10.1136/bjsports-2019-101001.
Lavie CJ, Ozemek C, Carbone S, Katzmarzyk PT, Blair SN. Sedentary Behavior, Exercise, and Cardiovascular Health. Circ Res. 2019;124(5):799–815. https://doi.org/10.1161/CIRCRESAHA.118.312669.
Luan X, Tian X, Zhang H, Huang R, Li N, Chen P, et al. Exercise as a prescription for patients with various diseases. J Sport Health Sci. 2019;8(5):422–41. https://doi.org/10.1016/j.jshs.2019.04.002.
Pedersen BK, Saltin B. Exercise as medicine - evidence for prescribing exercise as therapy in 26 different chronic diseases. Scand J Med Sci Sports. 2015;25:1–72. https://doi.org/10.1111/sms.12581.
Lear SA, Hu W, Rangarajan S, Gasevic D, Leong D, Iqbal R, et al. The effect of physical activity on mortality and cardiovascular disease in 130 000 people from 17 high-income, middle-income, and low-income countries: the PURE study. Lancet. 2017;390(10113):2643–54. https://doi.org/10.1016/S0140-6736(17)31634-3.
Dalmases M, Benítez I, Sapiña-Beltran E, Garcia-Codina O, Medina-Bustos A, Escarrabill J, et al. Impact of sleep health on self-perceived health status. Sci Rep. 2019;9(1):7284. https://doi.org/10.1038/s41598-019-43873-5.
Vasiliadis HM, Pitrou I, Grenier S, Berbiche D, Hudon C. Psychological Distress, Cognition, and Functional Disability Trajectory Profiles of Aging in Primary Care Older Adults. Clin Gerontol. 2022;1–13. https://doi.org/10.1080/07317115.2022.2060158.
Hannan M, Kringle E, Hwang CL, Laddu D. Behavioral Medicine for Sedentary Behavior, Daily Physical Activity, and Exercise to Prevent Cardiovascular Disease: A Review. Curr Atheroscler Rep. 2021;23(9):48. https://doi.org/10.1007/s11883-021-00948-x.
Gupta CC, Vincent GE, Coates AM, Khalesi S, Irwin C, Dorrian J, et al. A Time to Rest, a Time to Dine: Sleep, Time-Restricted Eating, and Cardiometabolic Health. Nutrients. 2022;14(3):420. https://doi.org/10.3390/nu14030420.
Ahmed S, Heaven A, Lawton R, Rawlings G, Sloan C, Clegg A. Behaviour change techniques in personalised care planning for older people: a systematic review. Br J Gen Pract. 2021;71(703):e121–7. https://doi.org/10.3399/bjgp20X714017.
Kettle VE, Madigan CD, Coombe A, Graham H, Thomas JJC, Chalkley AE, et al. Effectiveness of physical activity interventions delivered or prompted by health professionals in primary care settings: systematic review and meta-analysis of randomised controlled trials. BMJ. 2022;e068465. https://doi.org/10.1136/bmj-2021-068465.
Arroyo AC, Zawadzki MJ. The Implementation of Behavior Change Techniques in mHealth Apps for Sleep: Systematic Review. JMIR mHealth uHealth. 2022;10(4):e33527. https://doi.org/10.2196/33527.
Direito A, Carraça E, Rawstorn J, Whittaker R, Maddison R. mHealth Technologies to Influence Physical Activity and Sedentary Behaviors: Behavior Change Techniques, Systematic Review and Meta-Analysis of Randomized Controlled Trials. Ann Behav Med. 2017;51(2):226–39. https://doi.org/10.1007/s12160-016-9846-0.
Ferguson T, Olds T, Curtis R, Blake H, Crozier AJ, Dankiw K, et al. Effectiveness of wearable activity trackers to increase physical activity and improve health: a systematic review of systematic reviews and meta-analyses. Lancet Digit Health. 2022;4(8):e615–26. https://doi.org/10.1016/S2589-7500(22)00111-X.
Jørgensen LB, Bricca A, Bernhardt A, Juhl CB, Tang LH, Mortensen SR, et al. Objectively measured physical activity levels and adherence to physical activity guidelines in people with multimorbidity-A systematic review and meta-analysis. PLoS ONE. 2022;17(10):e0274846. https://doi.org/10.1371/journal.pone.0274846.
St Fleur RG, St George SM, Leite R, Kobayashi M, Agosto Y, Jake-Schoffman DE. Use of Fitbit Devices in Physical Activity Intervention Studies Across the Life Course: Narrative Review. JMIR mHealth uHealth. 2021;9(5):e23411. https://doi.org/10.2196/23411.
Holmes MM, Stanescu S, Bishop FL. The Use of Measurement Systems to Support Patient Self-Management of Long-Term Conditions: An Overview of Opportunities and Challenges. Patient Relat Outcome Measures. 2019;10:385–94. https://doi.org/10.2147/PROM.S178488.
Clarkson P, Stephenson A, Grimmett C, Cook K, Clark C, Muckelt PE, et al. Digital tools to support the maintenance of physical activity in people with long-term conditions: A scoping review. Digit Health. 2022;8:20552076221089776. https://doi.org/10.1177/20552076221089778.
Moher D, Hopewell S, Schulz KF, Montori V, Gotzsche PC, Devereaux PJ, et al. CONSORT 2010 Explanation and Elaboration: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340(mar23 1):c869. https://doi.org/10.1136/bmj.c869.
Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ. 2014;348(mar07 3):g1687. https://doi.org/10.1136/bmj.g1687.
Fortin M, Almirall J, Nicholson K. Development of a research tool to document self-reported chronic conditions in primary care. London: SAGE Publications Sage UK; 2017.
Onerup A, Arvidsson D, Blomqvist S, Daxberg EL, Jivegård L, Jonsdottir IH, et al. Physical activity on prescription in accordance with the Swedish model increases physical activity: a systematic review. Br J Sports Med. 2019;53(6):383–8. https://doi.org/10.1136/bjsports-2018-099598.
Acknowledgements
Not applicable.
Funding
The project IntelligentCare - Intelligent Multimorbidity Management System (Reference LISBOA-01-0247-FEDER-045948) is co-financed by the ERDF - European Regional Development Fund through the Lisbon Portugal Regional Operational Program - LISBOA 2020 and by the Portuguese Foundation for Science and Technology - FCT under CMU Portugal.
Author information
Authors and Affiliations
Contributions
The study concept and design was conceived by BN, EDH, HVP, JSC, DF, PF, JMM and NS. BN, ED, JSC will conduct patient screening and recruitment. JMM, BG, PM and JM developed the platform to collect the wearables data. BG and JM will perform the data collection from EHR. FC defined and validated to Portuguese the PROMS used in the study. Analysis will be performed by BN, PF, JMM, FL and NS. BN prepared the first draft of the manuscript. All authors provided edits and critiqued the manuscript for intellectual content.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study designed is in accordance with Declaration of Helsinki and has ethical approval from the local IRB from Hospital da Luz Lisboa (CES/02/2023/JAG). All participants will provide written informed consent and all study personnel have received training in the ethical conduct of human subject research.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Neves, B., Haghighi, E., Pereira, H. et al. Impact of a wearable-based physical activity and sleep intervention in multimorbidity patients: protocol for a randomized controlled trial. BMC Geriatr 23, 853 (2023). https://doi.org/10.1186/s12877-023-04511-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12877-023-04511-y