Abstract
Background
Low mobility during an acute hospitalization is frequent and associated with adverse effects, including persistent functional decline, institutionalization and death. However, we lack effective interventions to improve mobility that are scalable in everyday practice. The INTOMOB trial – INtervention to increase MOBility in older hospitalized medical patients – will test the effect of a multilevel intervention to improve mobility of older hospitalized patients on functional mobility.
Methods
The INTOMOB multicenter superiority parallel cluster randomized controlled trial will enroll in total 274 patients in Swiss hospitals. Community-dwelling adults aged ≥ 60 years, admitted to a general internal medicine ward with an anticipated length of hospital stay of ≥ 3 days, will be eligible for participation. Unit of randomization will be the wards. A multilevel mobility intervention will be compared to standard of care and target the patients (information and exercise booklets, mobility diary, iPad with exercise videos), healthcare professionals (e-learning, oral presentation, mobility checklist), and environment (posters and pictures on the wards). The primary outcome will be life-space level, measured by the University of Alabama at Birmingham Study of Aging Life-Space Assessment (LSA), at 30 days after enrollment. The LSA is a measure of functional mobility, i.e., how far participants move from bedroom to outside town. Secondary outcomes include, among others, LSA at 180 days, mobility and falls during hospitalization, muscle strength at discharge, and falls, emergency room visits, readmissions, and death within 180 days.
Discussion
This study has the potential to improve outcomes of older hospitalized patients through an intervention that should be scalable in clinical practice because it fosters patient empowerment and does not require additional resources. The tools provided to the patients can help them implement better mobility practices after discharge, which can contribute to better functional outcomes. The choice of a functional patient-reported outcome measure as primary outcome (rather than a “simple” objective mobility measure) reinforces the patient-centeredness of the study.
Trial registration
clinicaltrials.gov (NCT05639231, released on December 19 2022); Swiss National Clinical Trial Portal (SNCTP000005259, released on November 28 2022).
Similar content being viewed by others
Introduction
Background and rationale
Up to 80% of patients are able to ambulate independently during an acute hospitalization, while bed rest is not indicated in up to 60% of bed rest episodes [1, 2]. However, low mobility of hospitalized patients is a widespread problem: 83% of hospitalization time is spent in bed, and only 3% walking or standing [1, 2]. Low hospital mobility is associated with cascading adverse effects, including muscle atrophy and contractures, bone loss, falls, delirium, depression, constipation, prolonged length of hospital stay, disability, institutionalization, and death [1,2,3,4,5]. Only 30% of patients with functional decline during hospitalization recover within one year, while two thirds die or are institutionalized [6, 7]. Nevertheless, higher hospital mobility was associated with better outcomes in older medical patients, such as fewer readmissions and institutionalizations [8,9,10,11].
The consequences of low hospital mobility therefore impose a burden on patients, healthcare systems, and society, and constitute a priority to address. However, previous studies that focused on older patients during an acute medical hospitalization present several limitations: most were conducted in a single center, assessed only mobility (and not functional / patient-relevant) outcomes, did not follow patients after hospital discharge, or were not randomized [8,9,10,11,12,13,14]. Moreover, they often incompletely considered barriers and facilitators to mobility or required additional resources [10, 11], rendering them not easily scalable in everyday practice.
We thus still need to provide clinicians and patients with a scalable and effective way to improve older patient mobility on general internal medicine wards with the goal of reducing the adverse consequences of low hospital mobility.
Objectives
The primary objective of the INTOMOB trial is to test the effect of the INTOMOB intervention, compared to standard of care, on patient functional mobility, assessed by the life-space level, measured by the University of Alabama at Birmingham Study of Aging Life-Space Assessment (LSA), at 30 days after enrollment. The LSA is a measure of functional mobility, i.e., how far participants move from bedroom to outside town. The hypothesis is that patients randomized to the INTOMOB intervention will have a higher LSA at 30 days after enrollment, compared to those randomized to standard of care.
The secondary objectives are to evaluate the effect of the intervention on functioning, quality of life, depression, pressure ulcers, delirium, mobility, muscle strength, fear of/concerns about falling, prescribing of fall-risk increasing drugs, falls, new institutionalization, discharge destination, emergency room visits, readmissions, death, and satisfaction with hospital stay. The acceptability of the intervention for the patients and of the healthcare professionals (HCPs) is also assessed.
Trial design
This is a multicenter superiority parallel cluster randomized controlled trial with a 180-day follow-up. The randomization occurs at the ward level (= clusters), while data are analyzed at individual patient level. The trial includes four assessments: baseline, discharge (-1 to +2 days), 30 ± 5 days (D30) after enrollment, and 180 ± 5 days (D180) after enrollment. The primary outcome is assessed at D30, while secondary outcomes are assessed at discharge, D30 and/or D180. A cluster design is chosen because the tested intervention targets not only the patients, but also the HCPs and the hospital environment (wards). The trial was preceded by a pilot study that assessed the feasibility and acceptability of the study intervention and procedures that were then adapted as required [15].
Methods
This study protocol is presented in accordance with the SPIRIT-Outcomes 2022 checklist [16], and corresponds to the last version (version 5.0) submitted to the ethical committee, dated from April 19, 2023.
Study setting
The INTOMOB trial is conducted on acute general internal medicine wards of three hospitals, covering different linguistic regions of Switzerland (and thus representing cultural differences): Bern University Hospital (mostly German-speaking, with 130 general internal medicine beds), Baden Cantonal Hospital (German-speaking, with 123 general internal medicine beds) and Fribourg Cantonal Hospital (mostly French-speaking, with 130 general internal medicine beds). Of note, the pilot study that preceded the trial was conducted in Tiefenau Hospital in Bern instead of Baden Cantonal Hospital. The site change is due to an unforeseen closure of Tiefenau Hospital.
The multicenter approach is chosen to increase generalizability of findings by covering different linguistic and cultural regions and hospitals of different sizes and with various already existing practices regarding hospital mobility. Only general internal medicine wards are included to ensure patients are comparable, and because other units (e.g., orthopedics, neurology) often have specific mobilization protocols that could interfere with the intervention.
Study population and eligibility criteria
Patient inclusion criteria are: age ≥ 60 years, being ambulatory during the two weeks preceding admission (self-report), community-dwelling for at least the 30 days prior to enrollment, understanding French or German, and planned length of hospital stay ≥ 3 days after enrollment. Exclusion criteria are: medical contraindication to walk, wheelchair-bound, end-of-life, severe psychiatric disorder (severe depression, schizophrenia, psychosis), delirium (according to the Confusion Assessment Method) [17], and severe visual impairment. Patients with cognitive impairment (based on clinical judgment) can be included if a proxy can provide consent and actively support the patient during the study. The rationale for including patients with cognitive impairment is that they are also particularly vulnerable to adverse outcomes of low mobility and more likely to stay in bed, however frequently excluded from trials [18]. Including such patients can increase generalizability of study results.
Intervention and control procedures
The INTOMOB intervention was developed based on barriers and facilitators identified in the literature [7] and through a mixed methods study conducted by the authors with 200 patients recently hospitalized on general internal medicine wards in Switzerland, as well as 142 HCPs (nursing staff, physicians, physiotherapists) working on those wards [19]. The intervention had to be scalable, i.e., not to require additional resources (e.g., more staff) that might prevent its implementation in clinical practice. The INTOMOB intervention and study procedures were adapted based on the results of a pilot study conducted between December 2022 and March 2023. Feasibility, acceptability and scalability of the intervention were assessed during the pilot study, and the comfort, practicability and acceptability of a wrist-worn and an ankle-worn accelerometer were compared [15].
The intervention targets are: 1) the patients; 2) the HCPs (nursing staff and medical residents); and 3) the hospital environment (Fig. 1). The preeminent component of the INTOMOB intervention is the patient part, which encompasses: 1) an information booklet that highlights the preservation of functionality and autonomy as the main goal of hospital mobility (Supplement 1); 2) a customizable diary allowing patients to write their mobility goals, results, and difficulties (Supplement 2); 3) a booklet with 29 mobility exercises (in lying, sitting, and standing position) that includes pictures and explanations (Supplement 3); and 4) an iPad 10.2’’ provided to the patients during hospitalization with access to videos for each mobility exercise of the booklet. The videos are stored on a hidden website so that they cannot be found by patients or HCPs of the control group.
The HCP intervention includes an e-learning covering mobility evaluation and recommendations, motivational communication (including two short videos depicturing examples of appropriate and inappropriate patient-HCP communication regarding mobility), barriers and facilitators to hospital mobility, goal setting and evaluation, and presentation of the INTOMOB intervention (e-learning as PowerPoint in Supplement 4a-b). Completion of the e-learning is monitored by the investigators to ensure adherence. The study is presented regularly by the study investigators via a PowerPoint and oral presentation to the HCPs (Supplement 5). In addition, a mobility checklist (Supplement 6) is provided to the nursing staff as a pocket card, hung in resident and nursing offices and in the wards, and placed on carts, to remind the HCPs to assess and address mobility. Nursing staff is the main focus of the HCP intervention given that it does not change wards like medical residents do. Physiotherapists are not directly targeted by the intervention but are informed about it. They can support the patients with the intervention materials, while not using them in the control group.
The environment intervention is designed to foster mobility by making hallways more welcoming to patients. We hang up in the hallways posters on topics of interest to older persons (sleep, nutrition, hospital staff, mobility at hospital and after hospitalization, walking aids, polypharmacy, famous people), as well as pictures of landscapes, flowers, animals and famous people with brief information on them (Supplement 7a-e).
The intervention is compared to standard of care: patients receive standard of care regarding mobility by nursing staff and physicians, as well as physiotherapy if deemed necessary by hospital staff. HCPs on control wards do not complete the e-learning and do not receive the checklist or the presentation. The environment is not modified on control wards.
Recruitment and assessments
All patients admitted to the ward are screened by the research team for eligibility based on electronic health records and information from the ward staff. Potentially eligible patients are informed by a research team member and asked for consent to participate. Participants can choose if they additionally agree for data reuse for potential ancillary studies. Given the cluster design, randomization arm is known to the research team before approaching the patients. To preserve blinding, participants of the control wards receive information on the study, but not on the INTOMOB intervention. Those participants are informed orally at the end of follow-up about other aspects of the study. Informed consent forms are available in Supplements 8a-b.
The trial includes four assessments, conducted by the research team: 1) baseline; 2) discharge; 3) D30; 4) D180. The schedule of patient-level assessments is detailed in Table 1. The baseline visit is conducted on the ward at enrollment. The discharge visit is conducted on the ward within one day prior to discharge. In case discharge is not well-planned, items not requiring patient examination can be completed by phone call (or on-site in case of a transfer within the hospital) within two days after discharge, to avoid delaying discharge. D30 and D180 assessments are conducted by phone with the study participants or, if not directly reachable, with a contact provided by the patients or with their general practitioner. For patients with cognitive impairment, assessments are conducted with the proxy.
Mobility is objectively assessed in both trial arms using the ActiGraph wrist-worn accelerometer during hospitalization [31]. This device was chosen following feedback of patients and HCPs who participated in the pilot study.
Study outcomes
The primary outcome is life-space level at D30 measured by the LSA [21] or the LSA in Institutionalized Settings (LSA-IS) for patients living in an institution (hospital, rehabilitation, nursing home, other institution) [22]. The LSA is a self-reported assessment of mobility capacity and frequency, as well as mobility independence (equipment/personal assistance), over the last four weeks (Table 1 Fig. 2a). It is a validated and comprehensive tool with good test–retest reliability (interclass correlation 0.96) to evaluate the whole continuum of mobility, reflecting functional mobility [21]. The LSA can be easily used during a phone interview and correlates closely with physical functioning [32,33,34,35,36,37,38]. It has been used in several studies on mobility, allowing future comparison [13]. The LSA-IS is a validated adaptation of the LSA assessing the same aspects of mobility, but for the last day (Fig. 2b). The LSA and the LSA-IS use a similar scale, ranging from 0 to 120 points, and can be merged. A 5-point change is considered a minimal important change [39].
Secondary outcomes (detailed in Table 2) include functioning, quality of life, depression, pressure ulcer, delirium, mobility, muscle strength, fear of falling, prescribing of fall-risk increasing drugs, falls, new institutionalization, emergency room visits, discharge destination, readmissions, death, satisfaction with hospital stay, and acceptability of the intervention. In addition to patient-level assessments, acceptability of the intervention is assessed through a survey and semi-structured interviews in a sample of HCPs of the intervention group.
Finally, perspectives on hospital mobility of patients and HCPs of the intervention group are assessed through the survey that was used in the mixed methods study conducted in the preparatory phase of the trial, and assesses determinants of intention and behavior, based on barriers and facilitators to mobility and on the Health Action Process Approach, which specifies the mechanisms leading to an intention and then to a behavior (Supplement 9) [40, 41].
Randomization
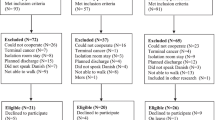
Randomization, done by computer through a blinded statistician before start of the trial, occurs at the ward level (= cluster unit) in a 1:1 ratio, with a block size of two and stratification by hospital and by ward size (>30 vs. <=30 beds). HCPs working on, and patients admitted to an intervention ward, receive the intervention procedure. HCPs working on, and patients admitted to a control ward, receive the control procedure. Figure 3 shows a flow chart of the trial design.
Blinding
The study is partially blinded: complete blinding is difficult due to the nature of the intervention, the communication between HCPs working on different wards (= clusters), and staff rotation. Patient blinding occurs through a differential patient information (see section on recruitment). HCP intervention focuses on nursing staff to minimize contamination bias because medical residents change wards more frequently. Outcome assessors are blinded for D30 and D180 assessments. Data analysis is blinded.
Sample size
Based on the trial by Brown et al. [13], reporting a 10.9-point difference in the LSA between intervention and control patients hospitalized on medical wards and aged 65 or older (N = 100), we calculated that 232 patients (116 in each arm) in 12 clusters (6 in each arm, mean cluster size: 19.3 patients) provides 80% power to detect a 10-point difference in the LSA (standard deviation 19 points) with an intraclass correlation coefficient of 0.05 (based on intraclass correlation coefficients from previous cluster trials) [42], a coefficient of variation for cluster size of 0.5, and a 0.05 two-sided alpha level. We increased the number of patients to 274 (137 in each arm; in 12 clusters in total) to allow for a 15% attrition rate.
Data management, safety and monitoring
For each participant, an electronic case report form with appropriate coded identification is maintained in a REDCap® (Research Electronic Data Capture) study database, a secure web application for building and managing online surveys and databases. Only authorized research team can access the database with personal login data and specific access rights. The final dataset is accessible to the primary investigator and statisticians.
Adverse events are systematically collected. The investigators comply with all regulations concerning safety set by the ethical committee, and report serious adverse events occurring during the trial within 24 h to the ethical committee, for which a causal relationship with the intervention cannot be excluded. Intervention discontinuation occurs in case of significant risk for a study participant. On-site and central data monitoring is part of the quality control activities implemented for this study and is performed according to separate monitoring plans. All involved parties keep participant data strictly confidential. Audit or monitoring visits by independent monitors or members of the ethical committee are possible at any time point of the trial. Participants are informed about that.
Statistical analysis plan
Baseline characteristics will be presented using descriptive summary statistics. Continuous variables will be presented as mean with standard deviation or median with quartiles, as appropriate. Categorical variables will be presented as absolute and relative frequencies.
The primary analysis will be an intention-to-treat analysis including all randomized patients, to compare the LSA between intervention and control groups at D30. The secondary analysis will be a per-protocol analysis, excluding patients that violated any eligibility criteria, did not receive the allocated procedure, withdrew consent, or were discharged less than 3 days after enrollment.
The LSA will be analyzed using generalized estimating equations (GEEs) with Gaussian distribution, exchangeable correlation structure, and robust standard errors with small sample correction. This model accounts for the cluster design and for the small number of clusters, and will be adjusted for baseline LSA. Absolute difference between groups will be presented with 95% confidence interval (CI).
Continuous secondary outcomes will be analyzed similarly. Model residuals will be inspected visually for normality using quantile–quantile-plots. For non-normal distributions, we will apply an adequate distribution in GEEs (e.g., negative binomial or gamma). Absolute differences will be presented with 95% CI.
Count outcomes (number of falls/emergency room visits/readmissions) will be analyzed using GEE with negative binomial distribution, log link, exchangeable correlation structure, and robust standard errors. Relative differences will be presented as rate ratio with 95% CI.
Time-to-event outcomes (mortality/time to first readmission/emergency room visit/institutionalization) will be analyzed using Cox regression. To account for clustering, we will use shared frailties for clusters and robust standard errors. Relative differences will be presented as hazard ratio with 95% CI.
Categorical outcomes (discharge destination) will be analyzed using multinomial logistic regression with robust standard errors to account for clustering. Relative differences will be presented as odds ratio with 95% CI.
We will conduct predefined subgroup analyses by age (60–80 vs. > 80 years), sex, length of stay, baseline LSA, baseline activities of daily living/instrumental activities of daily living, and number of comorbidities (cutoffs for continuous variables based on the median).
In sensitivity analyses, we will use generalized linear mixed-effects models instead of GEEs to account for clustering. For outcomes measured at D30 and D180, we will use a repeated-measures mixed-effects model. Should a GEE model not converge, we will use a mixed-effects model for that specific outcome as primary analysis approach. Because cluster randomization may lack the excellent balancing in characteristics between groups seen in individual-level randomization, we will adjust each model for additional pre-defined patient-level variables (age, sex, body mass index, number of limitations in activities of daily living/instrumental activities of daily living, fear of/concerns about falling, and baseline muscle strength) to account for case-mix differences between groups in a sensitivity analysis. Moreover, we will assess imbalances of patient characteristics between groups. If we observe imbalances in covariates not considered in the sensitivity analyses, we will perform additional adjustments to assess the robustness of our results.
If the primary outcome is missing in more than 5% of patients, we will employ multiple imputation in the primary analysis and additionally perform an available case analysis as sensitivity analysis disregarding missing data.
Patient and HCP acceptability of the intervention will be analyzed using descriptive summary statistics for the quantitative questions. The qualitative questions will be analyzed using a mixed deductive and inductive approach. Quantitative and qualitative results will be integrated using joint displays to draw meta-inferences from the mixed data, describing the results as confirming, expanding, or divergent. The results will be used to improve the intervention.
We will analyze the survey on patient and HCP perspectives on hospital mobility as in the preparatory phase. We will assess correlations between variables using Pearson correlation coefficients, and use the variance inflation factor to assess multicollinearity for variables with a correlation coefficient ≥ 0.60. We will conduct hierarchical regressions to assess the determinants of intention and behavior, and present the results as beta-coefficients with 95% CI. We will use delta R-squared (R2) to assess the improvement of the models through the different steps of the hierarchical regression. We will compare the results of intervention patients and HCPs with the results obtained in INTOMOB preparatory phase.
We will report results in accordance with the 2010 Consolidated Standards of Reporting Trials statement extension to cluster-randomized trials [43]. Any deviation from the original statistical plan will be described and justified in the final report. There is no interim analysis planned, i.e., there are no stopping rules on the individual or trial level. We will use Stata/MP (StataCorp LP, College Station, Texas) and R (R Project for Statistical Computing; r-project.org) for quantitative data analysis, and MAXQDA for qualitative data analysis (VERBI Software, Berlin, Germany).
Potential limitations and risk for bias
Although patient cross-over occurs rarely in the participating hospitals (based on local data, about 1–2% of all admitted patients are transferred between wards), the following strategy has been decided in such cases: if patients move from an intervention to a control or non-randomized ward, they are encouraged to pursue the patient intervention. Conversely, if patients from a control ward move to an intervention ward, they do not receive the patient intervention, but might be influenced by the environment/HCP intervention. However, since the core part of the intervention is the patient intervention and cross-overs remain rare, we deem that it will not introduce a major bias. Any such cross-over will be accounted for in the per-protocol analysis.
Contamination bias is possible due to medical resident rotations. To minimize this, HCPs are asked to avoid talking about the intervention with colleagues of other wards, and the focus of HCP intervention is the nursing staff instead of the medical residents.
Selection bias is possible because randomization arm is known before approaching the patients. To minimize this, the study team is instructed to approach all patients fulfilling inclusion criteria similarly. Recruitment and acceptance rates are monitored and compared between the intervention and control groups. Misbalance of patient characteristics or recruitment numbers are analyzed and discussed with the recruitment team.
Finally, with hospital stays getting shorter and shorter, patients might not stay long enough to fully benefit from the intervention (although we include only patients with a planned length of stay of ≥ 3 days). However, this represents also real-life practice.
Ethical considerations and dissemination
The protocol was approved by the local ethical committees (“Ethikkommission für die Forschung am Menschen – Universität Bern”, "Ethikkommission Nordwest- und Zentralschweiz (EKNZ)", "Commission cantonale d'éthique de la recherche sur l'être humain du canton de Vaud (CER-VD") and the study registered before pilot study initiation, on the Clinical Trials Registry platform of the National Institute of Health (NIH) – clinicaltrials.gov (NCT05639231, first release on December 19, 2022) – and on the Swiss National Clinical Trial Portal (SNCTP000005259, first release on November 28, 2022). Any important protocol modifications will be submitted to the ethical committee for approval before implementation.
The results of the trial will be communicated at scientific meetings and in peer-reviewed journals. The guidelines of the International Committee of Medical Journal Editors will be applied to define authorship eligibility for any publication related to the trial [44]. Participants can be informed of the study results after completion of the analyses. Statistical codes and participant-level data can be made available in an anonymized form upon appropriate request.
Discussion
Low hospital mobility is a widespread problem and a major issue to tackle urgently, in light of its numerous cascading adverse effects, especially detrimental for the older population. Many published studies on older patient hospital mobility have limitations, such as being monocentric or not randomized, lacking follow-up after discharge, lacking assessment of patient-relevant outcomes, or testing an intervention that is not scalable in clinical practice, so they did not lead to practice changes on a larger scale [8,9,10,11,12,13,14]. INTOMOB aims to circumvent these limitations by proposing a multicenter, superiority cluster randomized controlled trial testing a multilevel patient-empowering scalable intervention, with a 6-month follow-up and a focus on functional patient-relevant outcomes.
Strengths, innovation and expected impact
INTOMOB has several strengths. First, it is conducted in three hospitals of different sizes and with different existing practices regarding mobility, and in different linguistic/cultural regions, which will increase generalizability. Second, patients with cognitive impairment, an understudied and vulnerable population [18], can be included in the study. Third, the intervention addresses the barriers and the facilitators to hospital mobility, and was developed to be scalable, which is primordial for successful implementation in everyday practice. Finally, the intervention and study procedures were pilot-tested, allowing for a seamless conduction of the trial.
The main innovation of the INTOMOB intervention is its comprehensive approach by targeting not only the patients, but also the environment and the HCPs, allowing for multifaceted, global patient care. Moreover, the intervention was designed to avoid overloading the HCPs, which should ensure its scalability in everyday clinical practice.
If the INTOMOB intervention is successful, it will provide a way to improve mobility and functional outcomes of older hospitalized patients without requiring additional resources that are not available in clinical practice. The INTOMOB study has the potential to offer a scalable solution for broad-scale implementation of best practices to improve quality of care of the increasing older and vulnerable patient population.
Availability of data and materials
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
Abbreviations
- CI:
-
Confidence interval
- D30:
-
30 (± 5) Days after enrollment
- D180:
-
180 (± 5) Days after enrollment
- GEE:
-
Generalized estimating equation
- HCP:
-
Healthcare professional
- LSA:
-
Life-space Assessment
- REDCap:
-
Research electronic data capture
References
Brown CJ, Redden DT, Flood KL, Allman RM. The underrecognized epidemic of low mobility during hospitalization of older adults. J Am Geriatr Soc. 2009;57(9):1660–5.
Brown CJ, Friedkin RJ, Inouye SK. Prevalence and outcomes of low mobility in hospitalized older patients. J Am Geriatr Soc. 2004;52(8):1263–70.
Creditor MC. Hazards of hospitalization of the elderly. Ann Intern Med. 1993;118(3):219–23.
Markey DW, Brown RJ. An interdisciplinary approach to addressing patient activity and mobility in the medical-surgical patient. J Nurs Care Qual. 2002;16(4):1–12.
Kalisch BJ, Lee S, Dabney BW. Outcomes of inpatient mobilization: a literature review. J Clin Nurs. 2014;23(11–12):1486–501.
Barnes DE, Mehta KM, Boscardin WJ, Fortinsky RH, Palmer RM, Kirby KA, et al. Prediction of recovery, dependence or death in elders who become disabled during hospitalization. J Gen Intern Med. 2013;28(2):261–8.
Mani H, Möri C, Mattmann M, Liechti F, Inauen J, Aujesky D, et al. Barriers and facilitators to mobility of patients hospitalised on an acute medical ward: a systematic review. Age Ageing. 2022;51(7):1–11.
Hamilton AC, Lee N, Stilphen M, Hu B, Schramm S, Frost F, et al. Increasing mobility via in-hospital ambulation protocol delivered by mobility technicians: a pilot randomized controlled trial. J Hosp Med. 2019;14(5):272–7.
Moreno NA, de Aquino BG, Garcia IF, Tavares LS, Costa LF, Giacomassi IWS, et al. Physiotherapist advice to older inpatients about the importance of staying physically active during hospitalisation reduces sedentary time, increases daily steps and preserves mobility: a randomised trial. J Physiother. 2019;65(4):208–14.
Hastings SN, Sloane R, Morey MC, Pavon JM, Hoenig H. Assisted early mobility for hospitalized older veterans: preliminary data from the STRIDE program. J Am Geriatr Soc. 2014;62(11):2180–4.
Wood W, Tschannen D, Trotsky A, Grunawalt J, Adams D, Chang R, et al. A mobility program for an inpatient acute care medical unit. Am J Nurs. 2014;114(10):34–40 (quiz 1-2).
Raymond MJ, Jeffs KJ, Winter A, Soh SE, Hunter P, Holland AE. The effects of a high-intensity functional exercise group on clinical outcomes in hospitalised older adults: an assessor-blinded, randomised-controlled trial. Age Ageing. 2017;46(2):208–13.
Brown CJ, Foley KT, Lowman JD Jr, MacLennan PA, Razjouyan J, Najafi B, et al. Comparison of posthospitalization function and community mobility in hospital mobility program and usual care patients: a randomized clinical trial. JAMA Intern Med. 2016;176(7):921–7.
Teodoro CR, Breault K, Garvey C, Klick C, O’Brien J, Purdue T, et al. STEP-UP: study of the effectiveness of a patient ambulation protocol. Medsurg Nurs. 2016;25(2):111–6.
Bergsma D, Panait C, Leist P, et al. Feasibility and Acceptability of an INtervention TO Increase MOBility in Older Hospitalized Medical Patients (INTOMOB): A Mixed-Methods Pilot Study. Gerontol Geriatr Med. 2023;9. https://doi.org/10.1177/23337214231202148.
Butcher NJ, Monsour A, Mew EJ, Chan A-W, Moher D, Mayo-Wilson E, et al. Guidelines for reporting outcomes in trial protocols: the spirit-outcomes 2022 extension. JAMA. 2022;328(23):2345–56.
Inouye SK, van Dyck CH, Alessi CA, Balkin S, Siegal AP, Horwitz RI. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113(12):941–8.
Shepherd V, Wood F, Griffith R, Sheehan M, Hood K. Protection by exclusion? The (lack of) inclusion of adults who lack capacity to consent to research in clinical trials in the UK. Trials. 2019;20(1):474.
Herzog PJ, Herzog-Zibi RDL, Mattmann M, et al. Perspectives of patients and clinicians on older patient mobility on acute medical wards: a qualitative study. BMC Geriatr. 2023;23:558. https://doi.org/10.1186/s12877-023-04226-0.
Kondrup J, Rasmussen HH, Hamberg O, Stanga Z. Nutritional risk screening (NRS 2002): a new method based on an analysis of controlled clinical trials. Clin Nutr. 2003;22(3):321–36. https://theactigraph.com/. Accessed 14 Apr 2023.
Baker PS, Bodner EV, Allman RM. Measuring life-space mobility in community-dwelling older adults. J Am Geriatr Soc. 2003;51(11):1610–4.
Hauer K, Ullrich P, Heldmann P, Hummel S, Bauer JM, Werner C. Validation of the interview-based life-space assessment in institutionalized settings (LSA-IS) for older persons with and without cognitive impairment. BMC Geriatr. 2020;20(1):534.
Mahoney FI, Barthel DW. Functional evaluation: the Barthel index. Md State Med J. 1965;14:61–5.
Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9(3):179–86.
EurolQol Group. EuroQol--a new facility for the measurement of health-related quality of life. Health Policy. 1990;16(3):199–208.
Kroenke K, Spitzer RL, Williams JB. The Patient Health Questionnaire-2: validity of a two-item depression screener. Med Care. 2003;41(11):1284–92.
National Pressure Injury Advisory Panel, European Pressure Ulcer Advisory Panel and Pan Pacific Pressure Injury Alliance. Prevention and Treatment of Pressure Ulcers: Quick Reference Guide. Emily Haesler (Ed.). Cambridge Media: Perth; 2014.
de Morton NA, Davidson M, Keating JL. The de Morton Mobility Index (DEMMI): an essential health index for an ageing world. Health Qual Life Outcomes. 2008;6:63.
Yardley L, Beyer N, Hauer K, Kempen G, Piot-Ziegler C, Todd C. Development and initial validation of the Falls Efficacy Scale-International (FES-I). Age Ageing. 2005;34(6):614–9.
Hendriks AA, Vrielink MR, Smets EM, van Es SQ, De Haes JC. Improving the assessment of (in)patients’ satisfaction with hospital care. Med Care. 2001;39(3):270–83.
ActiGraph. https://theactigraph.com/. Accessed 14 Apr 2023.
Brown CJ, Kennedy RE, Lo AX, Williams CP, Sawyer P. Impact of Emergency Department Visits and Hospitalization on Mobility Among Community-Dwelling Older Adults. Am J Med. 2016;129(10):1124.e9-.e15.
Fisher SR, Graham JE, Ottenbacher KJ, Deer R, Ostir GV. Inpatient walking activity to predict readmission in older adults. Arch Phys Med Rehabil. 2016;97(9 Suppl):S226–31.
Peel C, Sawyer Baker P, Roth DL, Brown CJ, Brodner EV, Allman RM. Assessing mobility in older adults: the UAB study of aging life-space assessment. Phys Ther. 2005;85(10):1008–119.
Kennedy RE, Williams CP, Sawyer P, Lo AX, Connelly K, Nassel A, et al. Life-space predicts health care utilization in community-dwelling older adults. J Aging Health. 2019;31(2):280–92.
Iyer AS, Wells JM, Bhatt SP, Kirkpatrick DP, Sawyer P, Brown CJ, et al. Life-Space mobility and clinical outcomes in COPD. Int J Chron Obstruct Pulmon Dis. 2018;13:2731–8.
Kennedy RE, Sawyer P, Williams CP, Lo AX, Ritchie CS, Roth DL, et al. Life-space mobility change predicts 6-month mortality. J Am Geriatr Soc. 2017;65(4):833–8.
Lo AX, Brown CJ, Sawyer P, Kennedy RE, Allman RM. Life-space mobility declines associated with incident falls and fractures. J Am Geriatr Soc. 2014;62(5):919–23.
Kennedy RE, Almutairi M, Williams CP, Sawyer P, Allman RM, Brown CJ. Determination of the minimal important change in the life-space assessment. J Am Geriatr Soc. 2019;67(3):565–9.
Schwarzer R. Health action process approach (HAPA) as a theoretical framework to understand behavior change. Actualidades en Psicología. 2016;30(121):119–30.
Schwarzer R. Modeling health behavior change: How to predict and modify the adoption and maintenance of health behaviors. Appl Psychol. 2008;57(1):1–29.
Thompson DM, Fernald DH, Mold JW. Intraclass correlation coefficients typical of cluster-randomized studies: estimates from the Robert Wood Johnson Prescription for Health projects. Ann Fam Med. 2012;10(3):235–40.
Campbell MK, Piaggio G, Elbourne DR, Altman DG. Consort 2010 statement: extension to cluster randomised trials. BMJ. 2012;345:e5661.
https://www.icmje.org/recommendations/browse/roles-and-responsibilities/defining-the-role-of-authors-and-contributors.html. Accessed 1 May 2023.
World Medical Association. Declaration of Helsinki. 1964 (amended 2013). https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/. Accessed 26 June 2022.
International Council on Harmonization (ICH) E6(R2) Guideline for Good Clinical Practice. 2016. https://www.ema.europa.eu/en/ich-e6-r2-good-clinical-practice. Accessed 26 June 2022.
International Council on Harmonization (ICH) E2A Clinical Safety Data Management: Definitions and Standards for Expedited Reporting. 1995. https://www.ema.europa.eu/en/ich-e2a-clinical-safety-data-management-definitions-standards-expedited-reporting. Accessed 26 June 2022.
Acknowledgements
We thank Tabea Stoller, Lynn Pantano and Yvonne Stauffer for their contributions in the development of the intervention.
Funding
This study is funded by the Ambizione Grant PZ00P3_201672 from the Swiss National Science Foundation (SNSF). The sponsor is the Insel Gruppe AG, Freiburgstrasse 8, 3010 Bern, Switzerland. The funding body and the sponsor have no role in the study design, analysis or interpretation of the data, or writing of the manuscripts for publication.
Author information
Authors and Affiliations
Contributions
CEA conceived the project. CEA, DB, BM and JS contributed to the development of the intervention. CB, JG, MM and FDL provided constructive feedback in the development of the intervention and pilot phase of the study. DB, MM and MMW are involved in patient recruitment. BM and CEA wrote the first draft of the manuscript. All authors critically revised the manuscript and have approved its final version for publication.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study is conducted in compliance with the Declaration of Helsinki [45], the International Council on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use – Good Clinical Practice [46, 47] and the Swiss human research legislation (Swiss Federal Human Research Act 810.30 and Ordinance on Clinical Trials with the exception of Clinical Trials of Medical Devices 810.305). Patient participation is voluntary and written informant consent is obtained by all participants or their proxy in case of cognitive impairment. The protocol was approved by the local ethical committees (“Ethikkommission für die Forschung am Menschen – Universität Bern”, "Ethikkommission Nordwest- und Zentralschweiz (EKNZ)", "Commission cantonale d'éthique de la recherche sur l'être humain du canton de Vaud (CER-VD") and the study registered before pilot study initiation, on the Clinical Trials Registry platform of the National Institute of Health (NIH) – clinicaltrials.gov (NCT05639231, first release on December 19, 2022) – and on the Swiss National Clinical Trial Portal (SNCTP000005259, first release on November 28, 2022).
Consent for publication
Study participants are informed that the results of the study will be published in peer-reviewed journals and provide written informed consent on that.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Supplement 1.
Information booklet.
Additional file 3: Supplement 3.
Exercise booklet.
Additional file 4: Supplement 4.
a. E-learning residents. b. E-learning nursing staff.
Additional file 5: Supplement 5.
Slides for HCP oral presentation.
Additional file 6: Supplement 6.
Checklist.
Additional file 7: Supplement 7.
a. Posters. b. Landscapes - environment intervention. c. Flowers - environment intervention. d. Animals - environment intervention. e. - Famous people - environment intervention.
Additional file 8: Supplement 8.
a. - ICF -control. b. - ICF - intervention.
Additional file 9: Supplement 9.
Perspectives on mobility survey.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Mooser, B., Bergsma, D., Liechti, F.D. et al. Impact of an INtervention to increase MOBility in older hospitalized medical patients (INTOMOB): Study protocol for a cluster randomized controlled trial. BMC Geriatr 23, 705 (2023). https://doi.org/10.1186/s12877-023-04285-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12877-023-04285-3