Abstract
Background
Distal transradial access (dTRA) has been suggested to have great advantages over cTRA. However, there is a lack of preliminary data on dTRA in patients undergoing emergency coronary angiography (CAG) or percutaneous coronary intervention (PCI). To explore the feasibility and safety of distal transradial access in patients with acute chest pain.
Methods
A total of 1269 patients complaining of acute chest pain in our emergency department from January 2020 to February 2022 were retrospectively included. The patients who met the inclusion criteria were divided into the conventional transradial access (cTRA) group (n = 238) and the dTRA group (n = 158). Propensity score matching was used to minimize the baseline differences.
Results
The cannulation success rate in the dTRA group was significantly lower than that in the cTRA group (87.41% vs. 94.81%, p < 0.05). No significant differences in the puncture time and total procedure time were noted between the two groups (p > 0.05). Compared with the cTRA group, the hemostasis duration was significantly shorter [4(4, 4) h vs. 10(8, 10) h, p < 0.001) and the incidence of minor bleeding (BARC Type I and II) was significantly lower in the dTRA group than that in the cTRA group (0.85% vs. 5.48%, p = 0.045). Asymptomatic radial artery occlusion was observed in six patients (5.83%) in the cTRA group and one patient (1.14%) in the dTRA group (p = 0.126). The subgroup analysis of ST-elevation myocardial infarction (STEMI) showed no significant differences in the puncture time, D-to-B time or total procedure time between the two groups.
Conclusions
The dTRA for emergency CAG or PCI has an acceptable success rate and puncture time, a shorter hemostasis time, and a downward trend in RAO rate compared to the cTRA. The dTRA did not increase the D-to-B time in emergency coronary interventions in STEMI patients. On the contrary, a low incidence of RAO by the dTRA created an opportunity for future coronary interventions in non-culprit vessels in the same access.
Trial registration
Retrospectively registered in Chinese Clinical Trial Registry (registry number: ChiCTR2200061104, date of registration: June 15, 2022).
Similar content being viewed by others
The diagnosis of acute chest pain is a challenge for attending physicians in the emergency department due to a wide spectrum of diseases associated with chest pain, including acute coronary syndrome (ACS), acute pulmonary embolism (APE), aortic dissection (AD), and harmless muscular tension [1]. In addition to a 12-channel electrocardiogram (ECG) and laboratory tests, cardiac imaging, including echocardiography and coronary angiography (CAG), plays a key role in the diagnosis. Conventional transradial access (cTRA) for CAG and percutaneous coronary intervention (PCI) has been widely accepted as the default vascular access, owing to less bleeding complications, early ambulation and lower all-cause mortality in patients with ACS [2, 3]. However, postprocedure radial artery occlusion (RAO) is a concern, with an incidence of approximately 5-8% [4], which precludes the use of the same artery for future transradial access (TRA) procedures, coronary artery bypass grafting surgery and hemodialysis arteriovenous fistula [5]. Recently, a novel puncture access site in the anatomical snuffbox (AS), namely, distal radial access (DRA), was first introduced by Kiemeneij for coronary catheterization in 2017 [6]. Some advantages of distal transradial access (dTRA) over cTRA were demonstrated, such as the lower incidence of RAO, shorter hemostasis duration, lower risk of bleeding complications and better comfort of the patient [7]. However, there is a lack of preliminary data on dTRA in patients with acute chest pain, who often require rapid diagnosis by CAG or treatment with potent anti-thrombotic agents or PCI. In this study, we aimed to investigate the real-world feasibility and safety of the dTRA in patients with acute chest pain who underwent emergency CAG or PCI using propensity score matching (PSM).
Methods
We retrospectively analyzed a total of 1269 consecutive patients who complained of acute chest pain in our emergency department, which has a chest pain unit, from January 2020 to February 2022. We included the patients with acute chest pain who undergo emergency CAG or PCI for further study. The exclusion criteria were as follows: (1) patients undergoing CAG or PCI via femoral artery access without a first attempt on conventional radial access (CRA) or DRA; (3) age < 18 years; (4) patients without a palpable pulse in the conventional radial artery or the AS area; (5) infection of the puncture site; (6) a history of radial artery puncture and cannulation; and (7) patients with insufficient data. According to the puncture site, the enrolled patients were divided into the cTRA group and the dTRA group. This study involving human participants was carried out in accordance with the 1975 Helsinki Declaration, as revised in 2000 (5), and was approved by the Ethics Committee of Affiliated Wujin Hospital of Jiangsu University (Ethics approval number:201938). Informed consent was obtained from all enrolled patients in the study.
A thorough clinical evaluation of patients with acute chest pain was performed, including medical history, physical examination, 12-channel ECG, cardiac troponin I (cTnI), D-dimer, cardiac imaging and further focused diagnostics. Once the patient was diagnosed with ACS or was suspected to have myocardial ischemia, emergency CAG or PCI was initiated. The patient with confirmed ACS immediately received 300 mg of aspirin, 180 mg of ticagrelor or 300–600 mg of clopidogrel and 3000–5000 U of unfractionated heparin. The use of antiplatelet agents and unfractionated heparin in patients with suspected myocardial ischemia depended on the clinical decision of physicians.
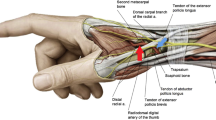
The procedures were performed by 4 experienced radial operators who performed at least 300 CAG or PCI procedures via the cTRA per year and who had more than 5 months of training on the dTRA. The number of included cases in cTRA (dTRA) for each of the 4 operators was 60 (30), 42 (58), 42 (54) and 44 (66), respectively. The right radial artery was the primary access side. The choice of puncture site being at the proximal 3 cm of the wrist’s transverse striation for cTRA or at the proximal part of the AS or the first intermetacarpal space for dTRA was primarily based on the operator’s personal preference.
After disinfection, local anesthesia was achieved using approximately 2-3 ml of 2% lidocaine hydrochloride. The radial artery at the proximal part of the AS (an angle of 60-70°) or the first intermetacarpal space (an angle of 20–30°) or the wrist (an angle of 30-45°) was punctured using a 20G ago-cannula-needle. Following successful puncture, an angled tip of 0.025″ plastic guide wire (Terumo Corporation, Tokyo, Japan) was inserted into the cannula. A 0.014″ coronary guide wire was used as an alternative when resistance was encountered due to the excessive tortuosity of the radial artery. Then, a 6 French sheath (Terumo Corporation, Tokyo, Japan) was advanced into the artery through the plastic guide wire. The puncture time was recorded from the beginning of the first attempt to puncture to the moment of sheath insertion. Cannulation success was defined as completion of the sheath insertion in the same access. If unfractionated heparin was not administered in the emergency department, 3000 U of unfractionated heparin was injected through the sheath. A dose of 100 mcg of nitroglycerine was given to prevent radial spasm unless there was hemodynamic compromise. Then, emergency CAG was conducted. In the case of PCI, additional unfractionated heparin (70–100 U/kg) was administered according to the patient’s body weight. The total procedural time was defined as the duration from the beginning of local anesthesia to the end of CAG or PCI.
Following the emergency CAG or PCI procedure, hemostasis was immediately obtained using cohesive elastic bandage with a 4 × 4 cm piece of sterile gauze for the dTRA during the sheath removal. After 2–4 h, the bandage was loosened to check for hemostasis by nurses and was recompressed for additional 30-60 min in case bleeding until hemostasis. In the cTRA, hemostasis was achieved with an air-filled closure device. Briefly, when the sheath was pulled out, the compression device was placed over the puncture site at inflation of 8-12 ml of air into the air compartment without compromising the radial artery patency, which can be accessed by reverse Barbeau test. After 4 h, the deflation of 2 ml of air was performed per hour until the compression was completely released. Once bleeding, extra 2 ml of air was inflated into the air compartment for additional 60-90 min. The hemostasis duration was defined as the duration from the removal of the sheath to complete decompression. The bleeding complication was defined according to the BARC criteria [8]. Minor bleeding complications included BARC I type and II type. Access-related hematoma was assessed according to the Early Discharge After Transradial Stenting of Coronary Artery (EASY) trial classification [9]. The blood flow of the radial artery at the forearm and the AS was assessed by vascular ultrasonography (HS1 Plus, L18-4, Konica Minolta, Japan) during the follow-up period, which ranged from 2 to 24 months. RAO was determined by the absence of a color Doppler blood flow signal. The D-to-B time was expressed as the duration from hospital arrival to balloon inflation at the culprit lesion.
Demographics, medical history, procedure-related characteristics and follow-up data of the two groups were collected. The data were as follows: sex, age, medical history, the cause of acute chest pain, body mass index (BMI), mean artery pressure (MAP), heart rate (HR), hemoglobin (HB), platelet (PLT), creatinine (Cr), alanine aminotransferase (ALT), albumin (A), triglyceride (TG), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), puncture time, cannulation success rate, fluoroscopy time, total procedural time, contrast dosage, incidence of cardiogenic shock (CS), hemostasis duration, access-related complications including hematoma, bleeding, thumb numbness, arteriovenous fistula, pseudoaneurysm and RAO and the prognosis of disease. In addition, the causes of access failure were analyzed, including puncture failure and guide wire insertion failure.
Continuous variables were reported as the means ± standard deviations (SD) or median (interquartile ranges), as appropriate. For normal distribution, the differences between the two groups were compared using unpaired t tests. For nonnormal distributions, the differences between the two groups were analyzed with the Mann-Whitney U test. Categorical variables were expressed as counts and percentages, which were analyzed with Pearson’s chi-square test or Fisher's exact test.
To minimize biased estimates, the baseline differences and potential confounders were adjusted by 1:1 PSM using the nearest neighbor matching algorithm with a caliper of 0.1 and without replacement. The covariates in the PSM model included sex, age, diabetes mellitus (DM), smoking, hypertension, ACS, BMI, MAP, HR, HB, PLT, Cr, ALT, HDL-C, LDL-C, transient hypotension, CS and use of anticoagulation preprocedure. Comparison of the standardized mean difference was used to evaluate the match quality. Standardized differences less than 10% indicated a relatively good match quality. All data were analyzed using SPSS version 22.0 (IBM Co., Armonk, NY, USA). A p value < 0.05 was considered statistically significant.
Results
A total of 1269 patients complaining of acute chest pain visited our emergency department from January 2020 to February 2022. Patients were excluded based on the inclusion and exclusion criteria. Therefore, a total of 396 patients (n = 238 for the cTRA group; n = 158 for the dTRA group) were included for further analysis. Pairs of patients in the two groups were successfully matched by 1:1 PSM. (Fig. 1).
Some of the clinical baseline data of the enrolled patients before and after matching are summarized in Table 1. There were significant differences in the percentages of smoking and CS and the levels of LDL-C and HDL-C between the two groups before matching. In the study, a standardized mean difference less than 10% revealed high-quality matching and an adequate balance of covariate distribution between the two groups (Fig. 2). There were no significant differences in 18 confounding variables between the two groups after matching (Fig. 2, Table 1).
The clinical baseline data of 18 confounding variables before and after matching between the two groups. After matching, the standardized mean difference was less than 10% between the two matched groups. EH, essential hypertension; DM, diabetes mellitus; ACS, acute coronary syndrome; PLT, platelet; Cr, creatinine; LDL-C, low-density lipoprotein cholesterol; CS, cardiogenic shock; HR, heart rate; BMI, body mass index; MAP, mean artery pressure; HDL-C, high-density lipoprotein cholesterol; HB, hemoglobin; ALT, alanine aminotransferase
The time from the onset of acute chest pain to visiting the emergency department in the dTRA group was significantly longer than that in the cTRA group [5 (3, 10) h vs. 4 (2, 6) h, p < 0.01]. The cannulation success rate in the dTRA group was significantly lower than that in the cTRA group (87.41% vs. 94.81%, p < 0.05). Interestingly, no significant differences in the puncture time and total procedure time were noted between the two groups [2 (1, 3) min vs. 2 (1, 3) min, p = 0.625; 45 (30, 60) min vs. 40 (30, 55) min, p = 0.276, respectively). There were no significant differences in the procedure methods, percentage of ACS, number of stents, contrast dosage or 90-day mortality between the two groups (p > 0.05). Moreover, no significant differences were observed in constituent ratios of ACS [ST-elevation myocardial infarction (STEMI), non-STEMI (NSTEMI) and unstable angina (UA)] and culprit vessel [left main artery (LM), left anterior descending artery (LAD), left circumflex artery (LCX) and right coronary artery (RCA)] between the two groups (p > 0.05) (Table 2).
The difference in the percentage of STEMI was not significant between the cTRA group and the dTRA group [90 (66.67%) vs. 84 (62.22%), p > 0.05]. No significant difference in the cannulation success rate was reported between the two groups [86 (95.56%) vs. 76 (90.48%), p = 0.186]. The subgroup analysis based on the presence of STEMI showed that there were no significant differences in puncture time, D-to-B time or total procedure time between the two groups (p > 0.05). However, the fluoroscopy time in the dTRA group was significantly longer than that in the cTRA group [11.53 (7.43, 17.54) min vs. 9.13 (7.24, 14.12) min, p = 0.039] (Fig. 3).
Comparison of time associated with emergency procedures in patients with ST-elevation myocardial infarction between the matched groups. No significant differences were noted in puncture time (a), fluoroscopy time (b), D to B time (c) and total procedure time (d) between the matched subgroups. D to B, door-to-balloon; cTRA, conventional transradial access; dTRA, distal transradial access
Switched or crossover access was observed in 7 out of 135 patients (5.19%) in the cTRA group and in 17 (12.59%) in the dTRA group (p = 0.032). The cTRA was actually completed in 146 patients and the dTRA in 118 patient (Fig. 4). There were no significant differences in baseline data between the two groups after crossover (supplementary Table). The major causes of puncture procedure failure, including puncture failure and guide wire insertion failure, are listed in Table 3. In the dTRA group, resistance was encountered in 16 cases of tortuosity of the radial artery during wire guide insertion. Of these, sheath introduction was achieved via the 0.014″ Runthrough NS guide wire (Terumo Corporation, Tokyo, Japan) in 8 cases.
The hemostasis duration in the cTRA group was significantly longer than that in the dTRA group [10 (8, 10) h vs. 4 (4, 4) h, p < 0.001]. The incidence of minor bleeding (BARC type I and II) was significantly higher in the cTRA group than in the dTRA group (5.48% vs. 0.85%, p = 0.045). No significant difference was noted in hematoma or numbness (p > 0.05). Local numbness in the puncture site was experienced in 2 (1.37%) patients in the cTRA group and 1 (0.85%) in the dTRA group, which spontaneously improved within 2 weeks. No pseudoaneurysms or arteriovenous fistulas were observed in our study (Table 4).
A total of 103 of 146 patients (70.54%) in the cTRA group and 88 of 118 patients (74.58%) in the dTRA group were followed up by vascular ultrasonography during the follow-up period (p = 0.467). No significant difference was noted in the average follow-up period between the two groups [12 (7, 17) months vs. 11 (5, 15) months, p = 0.144). Asymptomatic proximal RAO was observed in 6 patients (5.83%) in the cTRA group and distal RAO in 1 patients (1.14%) in the dTRA group (p = 0.126) during the follow-up period (Fig. 5).
Color Doppler images of the proximal RA and the distal RA. Long axis (a) and short axis (b) images of a normal proximal RA (white arrow). Long axis (c) and short axis (d) images of a normal distal RA (white arrow). Long axis (e) and short axis (f) images of an occluded proximal RA (white arrow). Long axis (g) and short axis (h) images of an occluded distal RA (white arrow). The distal RA lies on the dorsal surface of the scaphoid (arrowhead). RA, radial artery
The possible factors associated with puncture complications and the hemostasis duration included CS, hypotension, use of anticoagulation preprocedure and postprocedure, unfractionated heparin dosage and the types of antiplatelet agents. No significant difference was noted in CS, hypotension or use of anticoagulation preprocedure between the two groups after matching (Table 1). Similarly, no significant difference was found in the unfractionated heparin dosage, the types of antiplatelet agents and the use of anticoagulation postprocedure (p > 0.05, Table 5).
Discussion
In this retrospective observational study, the principal findings are as follows: i) the success rate of the emergency CAG or PCI via the dTRA was high and acceptable, despite some conversion of the access site; ii) the hemostasis duration in the dTRA group was significantly shorter than that in the cTRA group; iii) the dTRA did not delay the D-to-B time and total procedure time in patients with STEMI. To our knowledge, this was the first study to investigate the feasibility and safety of the dTRA for emergency CAG or PCI in patients complaining of acute chest pain.
Time is life for patients with acute chest pain, especially STEMI. A large-scale, multicenter, randomized trial suggested that cTRA in patients with ACS was associated with better clinical outcomes than transfemoral access (TFA) [10]. Therefore, the cTRA was recommended over the TFA in patients with STEMI by the 2017 ESC guidelines [11]. Recently, a novel puncture site, AS, has attracted more attention due to less bleeding, a short hemostasis duration and a low incidence of RAO [12]. However, a review demonstrated that most procedural indications (96.2%) avoided the dTRA in an emergency context due to concerns about possible clinical adverse events [13]. Only a few studies showed no significant difference in adverse cardiac events in the dTRA and cTRA [13, 14]. In our study, no significant difference was observed in cardiac mortality between the two groups (2.22% vs. 3.70%, p > 0.05) despite a longer time from onset to visit [4 (2, 6) h vs. 5 (3, 10) h, p = 0.002] and an increased fluoroscopy time in the subgroup analysis in STEMI due to more use of 0.014″ working guide wire in the dTRA group [11.53 (7.43, 17.54) min vs. 9.13 (7.24, 14.12) min, p = 0.039], which preliminarily indicated the effectiveness and feasibility of the dTRA in emergency CAG or PCI.
The cannulation success rate is an important index on which most studies have focused. Previous studies have reported a dTRA cannulation success rate of 70% to 99.2%, most of which were lower than that of the cTRA [12, 15]. The cannulation success rate of the dTRA was 89% in the pilot study of Kimeneij. In another study regarding STEMI, the success rate of the dTRA was 92.8% [16]. A recent meta-analysis showed that the cannulation success rate in dTRA was not significantly different compared with cTRA (p = 0.1) [17]. However, in our trial, the cannulation success rate of the dTRA was significantly lower than that of the cTRA (87.41% vs. 94.81%, p = 0.032). Meanwhile, the 87.41% cannulation success rate of the dTRA in the present study was low compared to that in previous studies. The possible explanations are as follows: 1) patients with acute chest pain were included in our study, and all of them underwent emergency CAG or PCI. In the emergency context, acute pain and the first unsuccessful puncture can induce more spasms of the small radial artery in the AS, resulting in puncture failure and requiring the puncture access technique to be changed; 2) anticoagulation agents were used in 93.33% patients before the procedure due to a high percentage of ACS (92.03%) in the present trial, which made the puncture more difficult in case of the first unsuccessful puncture resulting in possible subcutaneous hematomas; 3) a high percentage of STEMI (62.22%) patients were included in the study. The principle that time is heart muscle was followed in the setting of STEMI. The puncture access was immediately switched to avoid undue delay of D-to-B time after no more than three unsuccessful attempts, which can be proven by the fact that there was no significant difference in the puncture time [2 (1, 3) min vs. 2 (1, 3) min, p > 0.05] and D-to-B time [65 (65, 75) min vs. 65 (40,80) min, p > 0.05] between the two groups. 4) Tortuosity of the radial artery in the AS was observed in 16 patients in the dTRA group and in 1 patient in the cTRA group, which resulted in cannulation failure in 8 patients in the dTRA group and in 1 patient in the cTRA group, despite the assistance of a 0.014″ working guide wire. 5) The factors affecting the success rate of punctures are mainly associated with the personal experience and proficiency of the operator. In our study, 5 months of training on the dTRA was still relatively short. Considering the abovementioned, the puncture success rate of the dTRA (87.41%) in our study may be acceptable.
In the emergency context, the dTRA puncture time is a concern as it may delay the D-to-B time. A review about dTRA in STEMI indicated that the puncture time of the dTRA did not affect the timely opening of the culprit vessel [18]. Kim Y reported that the mean snuffbox puncture time was 2.7 ± 1.6 min in patients with STEMI, which did not delay the D-to-B time [16]. YJ Wang showed that puncture time in the dTRA group was longer than that in the cTRA group [2.4 (1.7–4.2) min vs. 1.7 (1.4–2.3) min; p < 0.001) whereas the door-to-wire time was not delayed in patients with STEMI [71 (54–88) min vs. 64 (56–82) min, p = 0.103] [19]. Similarly, our study showed that the dTRA puncture time was 2 (1, 3) min, and no significant differences were observed in D-to-B time [65 (65, 75) min vs. 65 (40, 80) min, p > 0.05] between the dTRA group and the cTRA group. Echo-guided puncture of dTRA may serve to reduce the time to successful vascular access. However, it is not clear whether ultrasound preparation and the radial artery exploration increase the D-to-B time in the emergency CAG or PCI, and further studies are needed. Thus, the dTRA puncture time seemed to be acceptable in the present study. Given the puncture success rate, the puncture time and the D-to-B time, the dTRA might be feasible as an alternative access in patients with acute chest pain.
Previous studies have reported that a shorter hemostasis duration was found in dTRA patients than in cTRA patients [20, 21]. Another study found that 2 h hemostasis may be sufficient in patients undergoing CAG by a 5-F sheath [22]. Ji Woong Roh et al. reported that 3 h hemostasis for PCI using the dTRA was feasible [12]. In this study, the hemostasis duration in the dTRA group was significantly shorter than that in the cTRA group [4 (4, 4) vs. 10 (8, 10), p < 0.001]. The short hemostasis duration greatly improved patient satisfaction and reduced the nursing workload postprocedure. However, the median hemostasis duration in our study was 4 h, longer than that of Ji Woong Roh et al. due to the use of potent dual antiplatelet agents and a large dose of heparin in the emergency context. A shorter hemostasis time (2-3 h) will be tried in our future practice in order to explore the major advantages of the distal radial approach in emergency intervention with a high bleeding risk. Most previous studies showed that fewer complications in the dTRA, such as hematoma, hemorrhage, pseudoaneurysm and arteriovenous fistula, were observed compared with those in the cTRA [21, 23]. Similar to previous studies, the incidence of minor bleeding in the dTRA group was significantly lower than that in the cTRA group in our study (0.85% vs. 5.48%, p = 0.045). Further analysis of the factors associated with puncture complications showed that the difference was not significant in the types and dosage of antithrombotic agents during the peri-procedure period. Moreover, despite the potent antithrombotic treatment, minor bleeding was found in only one patient with emergency CAG or PCI in the dTRA group, and no hematomas developed in the patients in the dTRA group, which affirmed the safety of dTRA in emergency intervention with a high bleeding risk.
RAO is the most common complication of conventional transradial interventional therapy. The proportion of multivessel lesions was high in patients undergoing PCI (34.67%) [21], but the proportion was higher in patients with ACS (58.6%) [16]. Emergency PCI was performed first in the culprit vessel to save the ischemic myocardium. Then, nonculprit vessels were then considered for the scheduled PCI via the same access. Thus, it is particularly important to minimize the RAO incidence in the emergency context. A prospective clinical trial performed by Andrea Pacchioni et al. reported that the RAO incidence in DRA was 0.5%, which was much lower than that in CRA (0.5% vs. 4.8%, p < 0.01) [24]. A dramatic reduction in RAO after dTRA was observed in the study of Eid-Lidt et al. [25] and YJ Wang et al. [19]. A recent meta- analysis showed that dTRA was associated with a significant lower risk of RAO (risk ratio [RR]: 0.36; 95% CI:0.23-0.56; p < 0.001) in comparison to cTRA [26]. In our study, a 1:1 PSM was performed to adjust for the potential confounders that might lead to increased RAO, including CS, MAP, and transient hypotension. The difference was not significant in these confounders after matching (p > 0.05). However, a downward trend in asymptomatic RAO was still detected by ultrasonography in the dTRA group compared with the cTRA group [1/88 (1.14%) vs. 6/103 (5.83%), p = 0.126]. The non-significant difference in RAO between the two groups may be related to the relatively low follow-up rate of ultrasonography and small sample size. The reason for the lower RAO incidence in dTRA was unclear. The puncture site of DRA is beyond the rise of several anastomotic branches, which may allow avoiding flow interruption in the forearm radial artery and reducing the risk of proximal extension thrombosis and forearm RAO [27]. Therefore, the dTRA showed a great advantage in reducing the RAO incidence in the emergency context, which makes it possible to reuse the same access in hemodialysis patients and to be able to schedule coronary intervention in patients and candidates for coronary bypass.
Certainly, there were some limitations in this study. First, this was a single-center study with a small sample size. The lack of a multicenter, large-sample, randomized design cannot completely exclude the influence of potential confounders even if PSM was performed to adjust for the between-group differences in the baseline data. Second, only patients with well-palpable DRA were considered suitable for emergency CAG or PCI via dTRA in this study, which might lead to selection bias. Third, the time range of follow-up, from 2 months to 2 years, was very wide in different individuals. Therefore, the incidence of RAO may have been overestimated by neglecting the reversibility of RAO after 1-3 months [28]. Fourth, the follow-up rate in vascular ultrasonography was low in our study (70.54% in the cTRA group and 74.58% in the dTRA group) due to the COVID-19 outbreak, which might have influenced the results of our study. Fifth, the lack of a preprocedural vascular ultrasound evaluation is an inherent weakness of this retrospective study. Vascular ultrasound is an effective tool to evaluate the feasibility and safety of dTRA. However, preprocedural ultrasound evaluation of the anatomy and caliber of the radial artery requires several minutes, which might lead to delays in D-to-B time. Thus, it remains controversial in the emergency context.
Conclusions
The dTRA for emergency CAG or PCI was feasible in terms of the cannulation success rate, puncture time and total procedure time in our study. The dTRA for emergency PCI did not increase D-to-B time in STEMI patients. Moreover, the dTRA showed a shorter hemostasis duration and a lower incidence of bleeding, hematoma and RAO in patients with acute chest pain, indicating that this puncture approach is safe in emergency CAG or PCI. A low incidence of RAO in emergency coronary interventions by the dTRA created an opportunity for future coronary interventions in non-culprit vessels in the same access.
Availability of data and materials
All authors had full access to the study data (including statistical reports and tables) and can take responsibility for the data integrity and the accuracy of the analysis. The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ACS:
-
Acute coronary syndrome
- APE:
-
Acute pulmonary embolism
- AD:
-
Aortic dissection
- ECG:
-
Electrocardiogram
- CAG:
-
Coronary angiography
- cTRA:
-
Conventional transradial access
- PCI:
-
Percutaneous coronary intervention
- RAO:
-
Radial artery occlusion
- TRA:
-
Transradial access
- AS:
-
Anatomical snuffbox
- DRA:
-
Distal radial access
- PSM:
-
Propensity score matching
- cTnI:
-
Cardiac troponin I
- BMI:
-
Body mass index
- MAP:
-
Mean artery pressure
- HR:
-
Heart rate
- HB:
-
Hemoglobin
- PLT:
-
Platelet
- Cr:
-
Creatinine
- ALT:
-
Alanine aminotransferase
- A:
-
Albumin
- TG:
-
Triglyceride
- TC:
-
Total cholesterol
- HDL-C:
-
High-density lipoprotein cholesterol
- LDL-C:
-
Low-density lipoprotein cholesterol
- CS:
-
Cardiogenic shock
- SD:
-
Standard deviations
- DM:
-
Diabetes mellitus
- STEMI:
-
ST-elevation myocardial infarction
- NSTEMI:
-
Non ST-elevation myocardial infarction
- UA:
-
Unstable angina
- LAD:
-
Left anterior descending artery
- LCX:
-
Left circumflex artery
- RCA:
-
Right coronary artery
- TFA:
-
Transfemoral access
References
Möckel M, Störk T. Acute chest pain. Internist (Berl). 2017;58(9):900–7. https://doi.org/10.1007/s00108-017-0299-8.
Valgimigli M, Gagnor A, Calabró P, Frigoli E, Leonardi S, Zaro T, MATRIX Investigators, et al. Radial versus femoral access in patients with acute coronary syndromes undergoing invasive management: a randomised multicentre trial. Lancet. 2015;385(9986):2465–76. https://doi.org/10.1016/S0140-6736(15)60292-6.
Sousa-Uva M, Neumann FJ, Ahlsson A, Alfonso F, Banning AP, Benedetto U, ESC Scientific Document Group, et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur J Cardiothorac Surg. 2019;55(1):4–90. https://doi.org/10.1093/ejcts/ezy289.
Hahalis G, Aznaouridis K, Tsigkas G, Davlouros P, Xanthopoulou I, Koutsogiannis N, et al. Radial artery and ulnar artery occlusions following coronary procedures and the impact of anticoagulation: ARTEMIS (radial and ulnar ARTEry occlusion meta-analysIS) systematic review and meta-analysis. J Am Heart Assoc. 2017;6(8):e005430. https://doi.org/10.1161/JAHA.116.005430.
Bernat I, Aminian A, Pancholy S, Mamas M, Gaudino M, Nolan J, et al. Best practices for the prevention of radial artery occlusion after transradial diagnostic angiography and intervention: an international consensus paper. JACC Cardiovasc Interv. 2019;12(22):2235–46. https://doi.org/10.1016/j.jcin.2019.07.043.
Kiemeneij F. Left distal transradial access in the anatomical snuffbox for coronary angiography (ldTRA) and interventions (ldTRI). Eur Interv. 2017;13(7):851–7. https://doi.org/10.4244/EIJ-D-17-00079.
Lee JW, Park SW, Son JW, Ahn SG, Lee SH. Real world experience of the left distal transradial approach for coronary angiography and percutaneous coronary intervention: a prospective observational study (LeDRA). Eur Interv. 2018;14(9):e995-1003. https://doi.org/10.4244/EIJ-D-18-00635.
Mehran R, Rao SV, Bhatt DL, Gibson CM, et al. Standardized bleeding defifinitions for cardiovascular clinical trials: a consensus report from the bleeding academic research consortium. Circulation. 2011;123(23):2736. https://doi.org/10.1161/CIRCULATIONAHA.110.009449.
Bertrand OF, Larochellière RD, Rodés-Cabau J, Proulx G, Gleeton O, Nguyen CM, et al. A randomized study comparing same-day home discharge and abciximab bolus only to overnight hospitalization and abciximab bolus and infusion after transradial coronary stent implantation. Circulation. 2006;114(24):2636–43. https://doi.org/10.1161/CIRCULATIONAHA.106.638627.
Valgimigli M, Frigoli E, Leonardi S, Vranckx P, Rothenbühler M, Tebaldi M, et al. Radial versus femoral access and bivalirudin versus unfractionated heparin in invasively managed patients with acute coronary syndrome (MATRIX): final 1-year results of a multicentre, randomised controlled trial. Lancet. 2018;392(10150):835–48. https://doi.org/10.1016/S0140-6736(18)31714-8.
Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, et al. 2017 ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the task force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2018;39(2):119–77. https://doi.org/10.1093/eurheartj/ehx393.
Roh JW, Kim Y, Takahata M, Shiono Y, Kim HY, Jeong MH, et al. Optimal hemostasis duration for percutaneous coronary intervention via the snuffbox approach: a prospective, multi-center, observational study (HEMOBOX). Int J Cardiol. 2021;338:79–82. https://doi.org/10.1016/j.ijcard.2021.06.035.
Coomes EA, Haghbayan H, Cheema AN. Distal transradial access for cardiac catheterization: a systematic scoping review. Catheter Cardiovasc Interv. 2020;96(7):1381–9. https://doi.org/10.1002/ccd.28623.
Hammami R, Zouari F, Abdessalem MAB, Sassi A, Ellouze T, Bahloul A, et al. Distal radial approach versus conventional radial approach: a comparative study of feasibility and safety. Libyan J Med. 2021;16(1):1830600. https://doi.org/10.1080/19932820.2020.1830600.
Koutouzis M, Kontopodis E, Tassopoulos A, Tsiafoutis I, Katsanou K, Rigatou A, et al. Distal versus traditional radial approach for coronary angiography. Cardiovasc Revasc Med. 2019;20(8):678–80. https://doi.org/10.1016/j.carrev.2018.09.018.
Kim Y, Lee JW, Lee SY, Bae JW, Lee SJ, Jeong MH, et al. Feasibility of primary percutaneous coronary intervention via the distal radial approach in patients with ST-elevation myocardial infarction. Korean J Intern Med. 2021;36(Suppl 1):S53–61. https://doi.org/10.3904/kjim.2019.420.
Cao J, Cai H, Liu W, Zhu H, Cao G. Safety and effectiveness of coronary angiography or intervention through the distal radial access: a meta-analysis. J Interv Cardiol. 2021;2021:4371744. https://doi.org/10.1155/2021/4371744.
Cao G, Cai HX, Cao J. Advancement in coronary angiography or percutaneous coronary intervention using the distal transradial artery access in acute coronary syndrome and complex coronary artery disease. Anatol J Cardiol. 2022;26(3):163–71. https://doi.org/10.5152/AnatolJCardiol.2021.933.
Wang YJ, Liu ZJ, Wu YX, Li ZX, Wang YT, Wang SH, et al. Early prevention of radial artery occlusion via distal transradial access for primary percutaneous coronary intervention. Front Cadiovasc Med. 2022;9:1071575. https://doi.org/10.3389/fcvm.2022.1071575.
Aoi S, Htun WW, Freeo S, Lee S, Kyaw H, Alfaro V, et al. Distal transradial artery access in the anatomical snufbox for coronary angiography as an alternative access site for faster hemostasis. Catheter Cardiovasc Interv. 2019;94(5):651–7. https://doi.org/10.1002/ccd.28155.
Li F, Shi GW, Yu XL, Song RX, Xiao JQ, Huang HM, et al. Safety and efficacy of coronary angiography and percutaneous coronary intervention via distal transradial artery access in the anatomical snuffbox: a single-centre prospective cohort study using a propensity score method. BMC Cardiovasc Disord. 2022;22(1):74. https://doi.org/10.1186/s12872-022-02518-8.
Roh JW, Kim Y, Kim HY, Ahn Y, Jeong MH. Comparison of 4-French versus 5-French sheaths for diagnostic coronary angiography via the snuffbox approach. Cardiol J. 2021;28(4):528–33. https://doi.org/10.5603/CJ.a2020.0003.
Wang H, Peng WJ, Liu YH, Ma GQ, Wang D, Bin SuB, et al. A comparison of the clinical effects and safety between the distal radial artery and the classic radial artery approaches in percutaneous coronary intervention. Ann Palliat Med. 2020;9(5):2568–74. https://doi.org/10.21037/apm-19-479.
Pacchioni A, Mugnolo A, Sanchez JS, Sgueglia GA, Pesarini G, Bellamoli M, et al. Radial artery occlusion after conventional and distal radial access: Impact of preserved flow and time-to-hemostasis in a propensity-score matching analysis of 1163 patients. Catheter Cardiovasc Interv. 2022;99(3):827–35. https://doi.org/10.1002/ccd.30005.
Eid-Lidt G, Rodríguez AR, Castellanos JJ, Farjat Pasos JI, Estrada Lopez KE, Gaspar J. Distal radial artery approach to prevent radial artery occlusion trial. JACC Cardiovasc Interv. 2021;14(4):378–85. https://doi.org/10.1016/j.jcin.2020.10.013.
Ferrante G, Condello F, Rao SV, et al. Distal vs conventional radial access for coronary angiography and/or intervention: a meta-analysis of randomized trials. JACC Cardiovasc Interv. 2022;15(22):2297–311. https://doi.org/10.1016/j.jcin.2022.09.006.
Sgueglia GA, Lee BK, Cho BR, et al. Distal radial access: consensus report of the first korea-europe transradial intervention meeting. JACC Cardiovasc Interv. 2021;14(8):892–906. https://doi.org/10.1016/j.jcin.2021.02.033.
Nagai S, Abe S, Sato T, Hozawa K, Yuki K, Hanashima K, et al. Ultrasonic assessment of vascular complications in coronary angiography and angioplasty after transradial approach. Am J Cardiol. 1999;83(2):180–6. https://doi.org/10.1016/s0002-9149(98)00821-2.
Acknowledgements
Not applicable.
Funding
Technology Support Project (social development) of Changzhou (CE20205004).
Author information
Authors and Affiliations
Contributions
WH Li and GH Cai conceived and supervised the study. WH Li, Q Wang, XF Liang and J Wang drafted the manuscript, T Chen, YB Song, J Wang, XF Liang, F Li, GW Shi, Y Li and JQ Xiao performed the data collection, follow up and statistical analysis. The author(s) read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study involving human participants was carried out in accordance with the 1975 Helsinki Declaration, as revised in 2000 (5), and was approved by the Ethics Committee of Affiliated Wujin Hospital of Jiangsu University (Ethics approval number:201938). Informed consent was obtained from all enrolled patients in the study.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
Supplementary Table. Comparisons of the clinical baseline databetween the two groups after crossover.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Li, W., Wang, J., Liang, X. et al. Comparison of the feasibility and safety between distal transradial access and conventional transradial access in patients with acute chest pain: a single-center cohort study using propensity score matching. BMC Geriatr 23, 348 (2023). https://doi.org/10.1186/s12877-023-04058-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12877-023-04058-y