Abstract
Background
Korea is expected to become a super-aged society in 2026, and improving nutritional status, which is directly related to health problems, is therefore important for increasing healthy life expectancy. Frailty is the most complex phenotype of aging, and leads to adverse health outcomes, disability, poor quality of life, hospitalization, and mortality. Malnutrition is a major risk factor for frailty syndrome. This study aimed to investigate the incidence of pre-frailty or frailty in the second wave (T2, 2018–2019) according to general characteristics and nutritional status in the first wave (T1, 2016–2017); and examine the longitudinal association of nutritional status in T1 and the incidence of pre-frailty or frailty in T2 among older adults living in a community.
Methods
A secondary data analysis was performed using the Korean Frailty and Aging Cohort Study (KFACS). Participants comprised 1125 community-dwelling older Korean adults aged 70–84 years (mean age: 75.03 ± 3.56 years; 53.8% males). Frailty was assessed using the Fried frailty index, and nutritional status was assessed using the Korean version of the Mini Nutritional Assessment Short-Form and blood nutritional biomarkers. Binary logistic regression was used to identify longitudinal associations between the nutritional status at T1 and pre-frailty or frailty at T2.
Results
Over the two-year follow-up period, 32.9% and 1.7% of the participants became pre-frail and frail, respectively. After the potential confounders were adjusted (sociodemographic, health behaviors, and health status characteristics), pre-frailty or frailty had a significant longitudinal association with severe anorexia (adjusted odds ratio [AOR], 4.17; 95% confidence interval [CI], 1.05–16.54), moderate anorexia (AOR, 2.31; 95% CI, 1.46–3.64), psychological stress or acute disease (AOR, 2.61; 95% CI, 1.26–5.39), and body mass index (BMI) less than 19 (AOR, 4.11; 95% CI, 1.20–14.04).
Conclusions
Anorexia, psychological stress, acute disease, and low BMI are the most significant longitudinal risk factors for pre-frailty or frailty in older adults. As nutritional risk factors may be preventable or modifiable, it is important to develop interventions targeting the same. Community-based health professionals in health-related fields should recognize and manage these indicators appropriately to prevent frailty among older adults living in the community.
Similar content being viewed by others
Background
According to the World Health Organization, maintaining proper nutritional status is one of the prerequisites for the well-being and “healthy aging” of older adults [1, 2]. Malnutrition, defined as undernourishment and deficient energy, frequently affects older adults [3, 4]. It is characterized by loss of muscle mass and weight [3, 4] and carries severe negative health outcomes [1]. Malnutrition accelerates age-associated changes, leading to loss of strength and muscle and increased sarcopenia [1, 3]. Malnutrition is a major risk factor associated with frailty syndrome [3] and is central to the phenotypic criteria for frailty [5].
Frailty is a multidimensional geriatric syndrome [5] that is related to adverse health outcomes, disability, poor quality of life, hospitalization, and mortality over time [6, 7]. It is recognized to be a reversible condition; researchers have proposed that early detection of frailty-related risk factors reduces its incidence [8]. Therefore, it is importance to assess nutrition and frailty among older adults, and develop consequent interventions [8, 9]. Exploratory research is needed to better understand the association between nutritional status and frailty, and to contribute to the successful management of these two geriatric characteristics.
Globally, malnutrition and frailty among community-dwelling older adults are becoming increasingly prevalent [9,10,11,12]. A systematic literature review exploring malnutrition and frailty among older adults living in the community showed that two out of three malnourished older adults were physically frail [12]. However, previous studies confirming the association between nutrition and frailty are controversial and have limitations in explaining causal relationships because of their cross-sectional design [13, 14]. Very few longitudinal studies have confirmed the causal association between nutritional status and frailty [15, 16]. Specific longitudinal risk factors for nutritional status should be identified in order to develop frailty preventative measures including nutritional assessments and interventions. Additionally, most studies have focused on older adults with disabilities in institutions and hospitals rather than those living in communities with larger populations and where early prevention should be given greater attention [14, 17]. Therefore, this study investigated whether specific nutritional status factors were longitudinally associated with frailty in community-dwelling older adults.
The aims of this longitudinal study were 1) to investigate the incidence of pre-frailty or frailty at follow-up according to general characteristics and nutritional status at the baseline and 2) to identify specific longitudinal factors associated with nutritional status at the baseline and the incidence of pre-frailty or frailty at follow-up in community-dwelling older adults enrolled in the nationwide Korean Frailty and Aging Cohort Study (KFACS).
Methods
Design and study population
This study was based on data from the first (T1, baseline period in 2016–2017) and second (T2, two-year follow-up period in 2018–2019) waves of the KFACS, a multicenter longitudinal study covering 10 centers across rural, suburban, and urban regions in Korea [18, 19]. This cohort study has been followed every two years [18, 19]. Recently, the third wave (T3, 2020–2021) was conducted. However, the data has not been released. KFACS participants were recruited by each center and included older adults aged 70–84 years, stratified according to age (70–74, 75–79, and 80–84 years, 6:5:4 ratio) and sex [18, 19]. The age range reflects the frailty consensus’s proposal that all older adults aged ≥ 70 years be screened for frailty [7, 19]. T1 of the KFACS included 3014 older adults [18, 19]. To identify new cases of pre-frail or frail at T2 among robust older adults at T1, this study included only robust older adults at T1. Exclusion criteria were older adults who were pre-frail or frail at T1 (n = 1249), those with missing data related to frailty (n = 106) or nutritional status (n = 532), and those who withdrew informed consent later (n = 2). The final sample size selected for this study was 1125 older adults who were robust at T1 (Fig. 1).
Measurements
Frailty
Frailty was assessed at T2 using the Fried Frailty Phenotype with a modified cutoff point [5, 19, 20]. The index consists of five domains [19]: 1) Unintentional weight loss was defined as a loss of 4.5 kg, or more than 5% of the total body weight in the last year. 2) Weakness was measured by the maximal grip strength in kg after measuring twice for each hand using a hand grip dynamometer (T.K.K.5401, Takei Scientific Inc. Co., Ltd., Japan). Weakness was defined as the 20th percentile of grip strength stratified by gender and body mass index quartile. 3) Exhaustion was measured using two self-reported questions from the Center for Epidemiological Studies-Depression scale: “I felt that everything I did was an effort” and “I could not get going.” If the answer was yes to either of these questions for three or more days in a week, it was categorized as exhaustion. 4) Walking speed was measured by a 4-m gait speed test using an automatic timer (Gaitspeedometer, Dyphi, Daejeon, Korea) with an acceleration and deceleration phase of 1.5 m. The mean values were selected after measuring the variables twice and the lowest 20% of gait speed stratified by gender and height was suggested as a cut-off. 5) Low physical activity was measured using the International Physical Activity Questionnaire and was calculated using a metabolic equivalent task. Low physical activity was defined as a person with less than 20% of total energy consumed for a week based on the 2008 Korea Elderly Survey [19,20,21,22]. Each domain was scored by 0 or 1, and the summed scores range from 0 to 5. A score of 0 was considered robust, 1–2 indicated pre-frailty, and 3–5 indicated frailty [5, 19]. Based on the scores, we designated two groups, robust and non-robust (i.e., pre-frail or frail), for this study.
Nutritional status
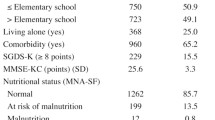
Nutritional status was assessed at T1 using blood nutritional biomarkers and the Korean version of the Mini Nutritional Assessment Short-Form (MNA-SF) [23], a widely used, validated, and established nutrition screening scale. The MNA-SF consists of six items [23]: 1) Anorexia was assessed using the self-reported question about decreased food intake due to loss of appetite, digestive problems, and chewing or swallowing difficulties in the past three months. It was categorized into three groups: no decrease, moderate, and severe. 2) Weight loss in the past three months was categorized into four groups: no change, 1‒3 kg loss, does not know, and > 3 kg loss. 3) Mobility was measured using a question about activity and was categorized into three groups: goes out, able to get out of bed/chair but does not go out, and bed or chair bound. 4) Experience of psychological stress or acute disease in the past three months was categorized into two groups: yes and no. 5) Presence of neuropsychological problems was categorized into three groups: no problems, mild dementia, and severe dementia or depression. 6) BMI was categorized into four groups: BMI ≥ 23, 21 ≤ BMI < 23, 19 ≤ BMI < 21, and BMI < 19. Each item was weighted according to the response. The summed weighted scores range from 0 to 14. A score of 12‒14 indicates normal nutritional status, 8‒11 indicates a risk of malnutrition, and 0‒7 indicates malnutrition. Blood nutritional biomarkers included hemoglobin, albumin, total protein, and total cholesterol levels.
Confounders
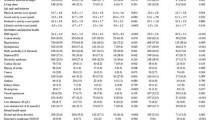
Based on a literature review, this study included three confounding factors (sociodemographic, health behaviors, and health status characteristics) previously self-reported as general characteristics from T1 [19, 22, 24]. Sociodemographic characteristics included age, gender, educational level, marital status, living area, and living arrangement. Health behavioral characteristics included smoking, drinking, and sleep. Health status characteristics included activities of daily living (ADL) dependency, instrumental activities of daily living (IADL) dependency, cognitive function, oral health, and comorbidity. ADL/IADL dependency was assessed using the Korean ADL/IADL scale [24]. Cognitive function was assessed using the Korean version of the Mini-Mental State Examination, and was defined as either low (≤ 23) or normal (> 24) [19]. Oral health was categorized into three groups—poor, fair, and good—based on a question regarding chewing difficulties [19, 22]: “Are you currently experiencing discomfort when chewing food due to problems in your mouth, such as teeth, dentures, or gums?” The number of comorbidities was estimated from the total number of self-reports and the current medical history diagnosed by a physician [19, 22].
Statistical analysis
Descriptive statistics were presented for all variables, and the results were presented as numbers (%) for categorical variables. Chi-squared tests or Fisher’s exact tests were performed to examine differences in the general characteristics of participants, their nutritional status, and frailty. The cumulative incidence of pre-frailty or frailty was determined from follow-up visits in the longitudinal analysis. A binary logistic regression was performed to calculate the odds ratios (ORs) with 95% confidence intervals (CI) of longitudinal associations between nutritional status at T1 and frailty at T2, adjusting for potential confounders. All analyses were performed using SPSS 28.0 software (IBM, Inc., Chicago, IL), and p-values < 0.05 were considered significant.
Results
Baseline participants’ general characteristics and nutritional status
Tables 1 and 2 summarize the participants’ general characteristics and nutritional status at T1, grouped by their frailty status at T2. At T1, the participants’ average age was 75.03 ± 3.56 years, and 53.8% were male. At T1, based on participants’ nutritional status, 90.4% had normal nutrition, 9.4% were at risk of malnutrition, and 0.2% were malnourished.
Incidence of pre-frailty or frailty at follow-up according to general characteristics and nutritional status at baseline
Over the two-year follow-up period, 32.9% and 1.7% of participants became pre-frail and frail, respectively. The incidence of pre-frailty or frailty was higher in older females, those with lower education levels, single marital status (bereaved, divorced, separated, and not married), living in rural living areas, never or past drinkers, having poor or fair oral health, and two or more comorbidities (all p < 0.01, Table 1). Malnutrition or risk of malnutrition as per the MNA was not associated with incidence of pre-frailty or frailty two years later. However, the incidence of pre-frailty or frailty was higher in older adults with severe or moderate anorexia, psychological stress, or acute disease (all p < 0.05, Table 2). Supplementary Material Figure S1 shows the incidence of pre-frailty or frailty at T2 according to the nutritional status screening items at T1. Over the two-year follow-up period, the incidence of pre-frailty or frailty was 50.4% and 58.3% among older adults at T1 with moderate and severe anorexia, respectively. In addition, the incidence of pre-frailty or frailty was 33.8% and 47.6% in older adults without and with psychological stress or acute disease at T1, respectively.
Longitudinal association between nutritional status and frailty
Table 3 shows the results of binary logistic regression between nutritional status at T1 and frailty at T2, with the robust group as the reference (Cox and Snell R2, 0.10; Nagelkerke R2, 0.14). After adjusting for potential confounding factors of general characteristics, severe (adjusted odds ratio [AOR], 4.17; 95% confidence interval [CI], 1.05–16.54) and moderate (AOR, 2.31; 95% CI, 1.46–3.64) anorexia were found to be significant predictors of pre-frailty or frailty, as were psychological stress or acute disease (AOR, 2.61; 95% CI, 1.26–5.39), and a BMI less than 19 (AOR, 4.11; 95% CI, 1.20–14.04).
Discussion
This study examined the incidence of pre-frailty or frailty over a two-year follow-up period and confirmed the longitudinal association between specific nutritional status factors and frailty in community-dwelling older Korean adults. Our findings highlight the aspects to be considered during screening, educating, and in counseling programs that aim to reduce incidence of nutrition-related frailty in community-dwelling older adults.
Regarding our first aim, according to the results, 32.9% and 1.7% of the older adults who were robust became pre-frail and frail, respectively, after two years. Similar to our study, according to a systematic review of cohort studies among community-dwelling older adults ≥ 60 years using any frailty diagnostic criteria for identification [11], 30.9% of previously robust older adults became pre-frail after a median 2.5 years of follow-up. In particular, considering that only 3% of frail older adults ≥ 60 years living in the community became robust based on the frailty phenotype criteria [25], the incidence of frailty is very high among robust older adults living in the community. Therefore, it is important to design interventions to increase awareness of factors that increase the risk of frailty and of ways to minimize that risk.
According to the results, regarding our second aim, this study found a significant longitudinal association between specific nutritional status factors such as anorexia, psychological stress, acute disease, and BMI at T1, and the incidence of pre-frailty or frailty at T2, even after adjusting for general characteristics. However, this study did not find a significant longitudinal association between malnutrition, or the risk of malnutrition, and frailty compared to normal nutrition, which is contrary to previous cross-sectional studies where nutritional status was associated with frailty [12]. This could be because of time considerations. Wei et al. [26] showed that robust malnourished older adults developed frailty after a five-year follow-up (AOR, 3.45; 95% CI, 1.00–11.9). According to the cycle of malnutrition and frailty phenotype proposed by Fried et al. [5], malnutrition causes negative energy balance and weight loss, resulting in sarcopenia, decreased physical activity, and increased disability and dependence. Therefore, malnutrition takes a long time to affect frailty. However, because our study was only conducted over two years, nutritional status could not affect frailty. This result indicates the need to identify longitudinal associations over a longer period for all community-dwelling older adults.
The observed association between anorexia and frailty is consistent with the findings of previous studies [27,28,29]. Evidence indicates that anorexia is a predictor of malnutrition, frailty, disability, and mortality in older adults [27,28,29]. Proper nutritional evaluation is essential for the integrated evaluation of older adults; in particular, it is necessary to identify the causes or consequences of anorexia [28]. Anorexia in older adults was described in two categories in a scoping review: physiological dysfunctions related to pathologies and polypharmacy, and non-physiopathological dysfunctions related to psychological, sociocultural, and environmental problems [30]. As non-physiopathological problems are potentially modifiable, they may be an appropriate avenue for interventions to prevent and manage anorexia [30]. The prevention and management of anorexia, involves food manipulation, modification of environmental risk factors, and treatment of pharmacological and medical problems [31]. A randomized controlled trial that provided older adults with solid nutritional supplements in consideration of appetite, edentulous status, and appropriate texture confirmed positive effects on weight gain and appetite increase [32]. Therefore, early detection of older adults at risk for anorexia, and the provision of subsequent interventions that have been confirmed to be effective in previous studies, can help prevent frailty.
The MNA-SF assesses psychological stress and acute diseases and can identify psychological frailty, including cognitive functioning, mood, and motivational components, which are important in determining the state of frailty [33, 34]. Frail older adults tend to suffer from anxiety, depression, and acute diseases [35,36,37]. An increase in psychological distress, such as anxiety and depression, in older adults has negative consequences for nutrition, frailty, disability, and death [36,37,38]. Psychological distress factors are associated with hyposalivation, which has been confirmed to be associated with anorexia [39, 40]. Acute diseases experienced by older adults have also been shown to affect nutrition and frailty [41, 42]. These findings require further exploration related to the effect of psychological distress and acute diseases on the nutritional status and frailty in older adults.
Weight loss is a major component of other phenotypic definitions of frailty [43]. In this study, older adults with a low BMI (< 19) had a higher risk of pre-frailty or frailty than those with a BMI (≥ 23) (AOR, 4.11; 95% CI, 1.20–14.04). A recent systematic review and meta-analysis showed that being underweight (BMI < 18.5) was associated with an increased risk of frailty (relative risk [RR], 1.45; 95% CI, 1.10–1.90) [44]. However, obesity is also a risk factor for frailty; BMI (≥ 30) was associated with a higher risk (RR, 1.40; 95% CI, 1.17–1.67) and confirmed the U-shaped risk relationship between BMI and frailty [44]. This study confirmed that the risk of pre-frailty or frailty was higher among those who were underweight than among those who were obese. However, considering that BMI and frailty have U-shaped patterns, future studies are needed to subdivide the degree of BMI and to confirm the risk relationship between frailty and BMI.
For the prevention and management of frailty among community-dwelling older adults, it is necessary to comprehensively consider not only individual general and health risk factors [45] but also nutrition-specific risk factors. Japan, which has rapidly changed into a super-aged society, has already prepared detailed food standards and guidelines [46]. It successfully conducted a lunch box project for community-dwelling older adults by identifying nutritional risk factors, such as frailty status, number of teeth, chewing, swallowing function, and the presence or absence of a caregiver [46]. Similarly, since July 2020, Korea has been conducting a pilot project for customized meal support and nutrition management services for community-dwelling low-income older adults as part of the community-integrated health promotion project [47]. This project considers physical function, oral function such as chewing and swallowing, and nutritional status through health screening for older adults [47]. These projects [46, 47], which comprehensively evaluate individual risk factors, are customized and practical nutritional evaluations and frailty intervention strategies. Considering the close association between malnutrition, frailty, and adverse health outcomes, early identification and prevention of nutritionally associated risk factors plays a crucial role in health policymaking. However, as anorexia diagnosis in older adults focuses on weight loss, BMI, and oral intake rather than appetite assessment [48], a standardized assessment that includes appetite, food taste, digestion, and oral function is needed in the future.
This study has several limitations. First, the sample of this study included individuals living independently in the community who could ambulate to visit the center, and excluded home-bound disabled or institutionalized older adults and dementia patients with communication problems. Therefore, it is possible that participants with a relatively low probability of frailty were recruited. In addition, according to the MNA-SF, malnutrition, the risk of malnutrition, and normal nutritional status were 0.2%, 9.4%, and 90.4% for all participants, respectively. The distribution of nutritional status indicated a significant difference between groups, which may have weakened the effect size between nutritional status and frailty. Future studies should focus on extensive primary data integration that consider residents of nursing homes or facilities in the community to compare and predict the results. Second, our results were derived from a secondary data analysis. The KFACS only recruited older adults aged 70–84 years due to the relatively small frailty prevalence rate of Korean adults aged 65–69. In addition, it was also relatively difficult for older adults aged 85 or older to visit the center and complete follow-up surveys [7, 49]. Since the prevalence of frailty can be different worldwide, comparative studies that included adults aged 65–69 years are required. In contrast, considering that frailty deteriorates as age increases, a future study is required to predict the longitudinal association of nutritional status and frailty through re-analysis that includes older adults aged 85 years and older. Nevertheless, this study was a large, population-based study of older adults to investigate the longitudinal association between nutritional status and frailty in Korea. Third, nutritional status was assessed using the self-report questionnaire of the MNA-SF; therefore, there may be problems with reporting and/or recall bias. Specifically, it should be noted that weight loss and immobility of the MNA-SF are closely associated domains of the frailty phenotype [50]. In the future, validation through an additional objective investigation method will be required. Nevertheless, the MNA-SF is the most validated nutritional screening tool for older adults, and this study included blood nutritional biomarkers as objective data. Finally, this study did not consider the cross-sectional effects of nutritional status and covariates at T2 on the incidence of frailty at T2. Hence, further studies are required with repeated measures analysis that include gradual changes in nutritional status and covariates to confirm the potential causal relationship as well as longitudinal association between nutritional status and frailty. These studies are expected to be possible when data above the third wave is released.
Conclusions
This study investigated the incidence of pre-frailty or frailty in 34.6% of the participants in a longitudinal study of older adults living in a community over a two-year period. Specifically, this study found that anorexia, psychological stress, acute disease, and BMI were significant longitudinal factors associated with frailty. The specific risk factors of MNA-SF identified in this study can be used to develop screening, customized nutrition education, and counseling programs to reduce the incidence of nutrition-related frailty in community-dwelling older adults.
Availability of data and materials
The data that support the findings of this study are available from the KFACS but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the corresponding authors (NK) upon reasonable request and with permission of KFACS.
Abbreviations
- ADL:
-
Activities of daily living
- AOR:
-
Adjusted odds ratio
- BMI:
-
Body mass index
- CI:
-
Confidence interval
- KFACS:
-
Korean Frailty and Aging Cohort Study
- IADL:
-
Instrumental activities of daily living
- IRB:
-
Institutional Review Board
- MNA-SF:
-
Mini Nutritional Assessment Short-Form
- OR:
-
Odds ratio
- RR:
-
Relative risk
References
Norman K, Haß U, Pirlich M. Malnutrition in older adults-recent advances and remaining challenges. Nutrients. 2021;13(8):2764. https://doi.org/10.3390/nu13082764.
World Health Organization. Keep fit for life: meeting the nutritional needs of older persons. 2002. https://www.who.int/publications-detail-redirect/keep-fit-for-life-meeting-the-nutritional-needs-of-older-persons. Accessed 22 Sept 2021.
Bales CW, Ritchie CS. Sarcopenia, weight loss, and nutritional frailty in the elderly. Annu Rev Nutr. 2002;22:309–23. https://doi.org/10.1146/annurev.nutr.22.010402.102715.
Ferrari Bravo M, Gallo F, Marchello C, Boicelli R, Lupi S, Atzei M, et al. Assessment of malnutrition in community-dwelling elderly people: cooperation among general practitioners and public health. Iran J Public Health. 2018;47(5):633–40.
Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–56. https://doi.org/10.1093/gerona/56.3.m146.
Kojima G, Iliffe S, Jivraj S, Walters K. Association between frailty and quality of life among community-dwelling older people: a systematic review and meta-analysis. J Epidemiol Community Health. 2016;70(7):716–21. https://doi.org/10.1136/jech-2015-206717.
Morley JE, Vellas B, van Kan GA, Anker SD, Bauer JM, Bernabei R, et al. Frailty consensus: a call to action. J Am Med Dir Assoc. 2013;14(6):392–7. https://doi.org/10.1016/j.jamda.2013.03.022.
Marcucci M, Damanti S, Germini F, Apostolo J, Bobrowicz-Campos E, Gwyther H, et al. Interventions to prevent, delay or reverse frailty in older people: a journey towards clinical guidelines. BMC Med. 2019;17(1):193. https://doi.org/10.1186/s12916-019-1434-2.
van der Pols-Vijlbrief R, Wijnhoven HA, Schaap LA, Terwee CB, Visser M. Determinants of protein-energy malnutrition in community-dwelling older adults: a systematic review of observational studies. Ageing Res Rev. 2014;18:112–31. https://doi.org/10.1016/j.arr.2014.09.001.
Collard RM, Boter H, Schoevers RA, Oude Voshaar RC. Prevalence of frailty in community-dwelling older persons: a systematic review. J Am Geriatr Soc. 2012;60(8):1487–92. https://doi.org/10.1111/j.1532-5415.2012.04054.x.
Ofori-Asenso R, Chin KL, Mazidi M, Zomer E, Ilomaki J, Zullo AR, et al. Global incidence of frailty and prefrailty among community-dwelling older adults: a systematic review and meta-analysis. JAMA Netw Open. 2019;2(8):e198398. https://doi.org/10.1001/jamanetworkopen.2019.8398.
Verlaan S, Ligthart-Melis GC, Wijers SLJ, Cederholm T, Maier AB, de van der Schueren MAE. High prevalence of physical frailty among community-dwelling malnourished older adults: a systematic review and meta-analysis. J Am Med Dir Assoc. 2017;18(5):374–82. https://doi.org/10.1016/j.jamda.2016.12.074
Bakhtiari A, Pourali M, Omidvar S. Nutrition assessment and geriatric associated conditions among community dwelling Iranian elderly people. BMC Geriatr. 2020;20(1):278. https://doi.org/10.1186/s12877-020-01668-8.
Hong X, Yan J, Xu L, Shen S, Zeng X, Chen L. Relationship between nutritional status and frailty in hospitalized older patients. Clin Interv Aging. 2019;14:105–11. https://doi.org/10.2147/CIA.S189040.
Rodríguez-Mañas L, Rodríguez-Sánchez B, Carnicero JA, Rueda R, García-Garcia FJ, Pereira SL, et al. Impact of nutritional status according to GLIM criteria on the risk of incident frailty and mortality in community-dwelling older adults. Clin Nutr. 2021;40(3):1192–8. https://doi.org/10.1016/j.clnu.2020.07.032.
Lorenzo-López L, Maseda A, de Labra C, Regueiro-Folgueira L, Rodríguez-Villamil JL, Millán-Calenti JC. Nutritional determinants of frailty in older adults: a systematic review. BMC Geriatr. 2017;17(1):108. https://doi.org/10.1186/s12877-017-0496-2.
Salminen KS, Suominen MH, Kautiainen H, Pitkälä KH. Associations between nutritional status, frailty and health-related quality of life among older long-term care residents in Helsinki. J Nutr Health Aging. 2020;24(3):319–24. https://doi.org/10.1007/s12603-019-1320-9.
Korean Frailty and Aging Cohort Study. Korean Frailty and Aging Cohort Study. 2016. https://www.kfacs.kr/html/. Accessed 14 Aug 2022.
Won CW, Lee S, Kim J, Chon D, Kim S, Kim CO, et al. Korean frailty and aging cohort study (KFACS): cohort profile. BMJ Open. 2020;10(4):e035573. https://doi.org/10.1136/bmjopen-2019-035573.
Kim S, Kim M, Jung HW, Won CW. Development of a frailty phenotype questionnaire for use in screening community-dwelling older adults. J Am Med Dir Assoc. 2020;21(5):660–4. https://doi.org/10.1016/j.jamda.2019.08.028.
Jeon SY, Won CW, Choi HR, Kim BS, Kim SY, Hur JH. Physical frailty predicts cognitive decline in elderly people: prospective findings from the living profiles of older people survey in Korea. Korean J Fam Pract. 2015;5:702–7.
Ministry of Health and Welfare. 2008 Korea Elderly Survey. 2009. http://www.mohw.go.kr/react/jb/sjb030301vw.jsp?PAR_MENU_ID=03&MENU_ID=0321&CONT_SEQ=264469&page=1. Accessed 14 Aug 2022.
Nestlé Nutrition Institute. Mini Nutritional Assessment (MNA). 2022. https://www.mna-elderly.com/. Accessed 14 Aug 2022.
Won CW, Yang KY, Rho YG, Kim SY, Lee EJ, Yoon JL, et al. The development of Korean Activities of Daily Living (K-ADL) and Korean Instrumental Activities of Daily Living (K-IADL) scale. J Korean Geriatr Soc. 2002;6:107–20.
Kojima G, Taniguchi Y, Iliffe S, Jivraj S, Walters K. Transitions between frailty states among community-dwelling older people: a systematic review and meta-analysis. Ageing Res Rev. 2019;50:81–8. https://doi.org/10.1016/j.arr.2019.01.010.
Wei K, Thein FS, Nyunt MSZ, Gao Q, Wee SL, Ng TP. Nutritional and frailty state transitions in the Singapore longitudinal aging study. J Nutr Health Aging. 2018;22(10):1221–7. https://doi.org/10.1007/s12603-018-1096-3.
Martone AM, Onder G, Vetrano DL, Ortolani E, Tosato M, Marzetti E, et al. Anorexia of aging: a modifiable risk factor for frailty. Nutrients. 2013;5(10):4126–33. https://doi.org/10.3390/nu5104126.
Malafarina V, Uriz-Otano F, Gil-Guerrero L, Iniesta R. The anorexia of ageing: physiopathology, prevalence, associated comorbidity and mortality. A systematic review. Maturitas. 2013;74(4):293–302. https://doi.org/10.1016/j.maturitas.2013.01.016.
Tsutsumimoto K, Doi T, Makizako H, Hotta R, Nakakubo S, Makino K, et al. Aging-related anorexia and its association with disability and frailty. J Cachexia Sarcopenia Muscle. 2018;9(5):834–43. https://doi.org/10.1002/jcsm.12330.
Roy M, Gaudreau P, Payette H. A scoping review of anorexia of aging correlates and their relevance to population health interventions. Appetite. 2016;105:688–99. https://doi.org/10.1016/j.appet.2016.06.037.
Landi F, Calvani R, Tosato M, Martone AM, Ortolani E, Savera G, et al. Anorexia of aging: risk factors, consequences, and potential treatments. Nutrients. 2016;8(2):69. https://doi.org/10.3390/nu8020069.
Pouyssegur V, Brocker P, Schneider SM, Philip JL, Barat P, Reichert E, et al. An innovative solid oral nutritional supplement to fight weight loss and anorexia: open, randomised controlled trial of efficacy in institutionalised, malnourished older adults. Age Ageing. 2015;44(2):245–51. https://doi.org/10.1093/ageing/afu150.
Fitten LJ. Psychological frailty in the aging patient. Nestle Nutr Inst Workshop Ser. 2015;83:45–53. https://doi.org/10.1159/000382060.
Soysal P, Veronese N, Arik F, Kalan U, Smith L, Isik AT. Mini nutritional assessment scale-short form can be useful for frailty screening in older adults. Clin Interv Aging. 2019;14:693–9. https://doi.org/10.2147/CIA.S196770.
Chang SF, Lin HC, Cheng CL. The relationship of frailty and hospitalization among older people: evidence from a meta-analysis. J Nurs Scholarsh. 2018;50(4):383–91. https://doi.org/10.1111/jnu.12397.
Soysal P, Veronese N, Thompson T, Kahl KG, Fernandes BS, Prina AM, et al. Relationship between depression and frailty in older adults: a systematic review and meta-analysis. Ageing Res Rev. 2017;36:78–87. https://doi.org/10.1016/j.arr.2017.03.005.
Zhao W, Zhang Y, Liu X, Yue J, Hou L, Xia X, et al. Comorbid depressive and anxiety symptoms and frailty among older adults: findings from the West China health and aging trend study. J Affect Disord. 2020;277:970–6. https://doi.org/10.1016/j.jad.2020.08.070.
Rodda J, Walker Z, Carter J. Depression in older adults. BMJ. 2011;343:d5219. https://doi.org/10.1136/bmj.d5219.
Bergdahl M, Bergdahl J. Low unstimulated salivary flow and subjective oral dryness: association with medication, anxiety, depression, and stress. J Dent Res. 2000;79(9):1652–8. https://doi.org/10.1177/00220345000790090301.
Ohara Y, Kawai H, Shirobe M, Motokawa K, Fujiwara Y, Kim H, et al. Association between anorexia and hyposalivation in community-dwelling older adults in Japan: a 6-year longitudinal study. BMC Geriatr. 2020;20(1):504. https://doi.org/10.1186/s12877-020-01905-0.
Jensen GL, Cederholm T, Correia MITD, Gonzalez MC, Fukushima R, Higashiguchi T, et al. GLIM criteria for the diagnosis of malnutrition: a consensus report from the global clinical nutrition community. JPEN J Parenter Enteral Nutr. 2019;43(1):32–40. https://doi.org/10.1002/jpen.1440.
Mudge AM, Hubbard RE. Management of frail older people with acute illness. Intern Med J. 2019;49(1):28–33. https://doi.org/10.1111/imj.14182.
Abate M, Di Iorio A, Di Renzo D, Paganelli R, Saggini R, Abate G. Frailty in the elderly: the physical dimension. Eura Medicophys. 2007;43(3):407–15.
Yuan L, Chang M, Wang J. Abdominal obesity, body mass index and the risk of frailty in community-dwelling older adults: a systematic review and meta-analysis. Age Ageing. 2021;50(4):1118–28. https://doi.org/10.1093/ageing/afab039.
Feng Z, Lugtenberg M, Franse C, Fang X, Hu S, Jin C, et al. Risk factors and protective factors associated with incident or increase of frailty among community-dwelling older adults: a systematic review of longitudinal studies. PLoS One. 2017;12(6):e0178383. https://doi.org/10.1371/journal.pone.0178383.
Ministry of Health, Labor and Welfare. Nutrition policy in Japan to leave no one behind. 2022. https://www.mhlw.go.jp/nutrition_policy/en/. Accessed 12 Nov 2022.
Ministry of Health and Welfare. 2021 Community-integrated health promotion project: nutrition guide. 2021. http://www.mohw.go.kr/react/jb/sjb030301vw.jsp?PAR_MENU_ID=03&MENU_ID=032901&CONT_SEQ=365181. Accessed 12 Nov 2022.
Cox NJ, Ibrahim K, Sayer AA, Robinson SM, Roberts HC. Assessment and treatment of the anorexia of aging: a systematic review. Nutrients. 2019;11(1):144. https://doi.org/10.3390/nu11010144.
Ministry of Health and Welfare. Living profiles of older people survey in Korea. 2008. http://www.mohw.go.kr/react/jb/sjb030301vw.jsp?PAR_MENU_ID=03&MENU_ID=032901&CONT_SEQ=336610. Accessed 20 Feb 2023.
Bollwein J, Volkert D, Diekmann R, Kaiser MJ, Uter W, Vidal K, et al. Nutritional status according to the mini nutritional assessment (MNA®) and frailty in community dwelling older persons: a close relationship. J Nutr Health Aging. 2013;17(4):351–6. https://doi.org/10.1007/s12603-013-0034-7.
Acknowledgements
None.
Funding
This work was supported by Basic Science Research Program through the National Research Foundation of Korea and funded by the Ministry of Education (Grant 2020R1A6A1A03041989); and the Korea Health Technology R&D Project through the Korea Health Industry Development Institute, which is funded by the Ministry of Health & Welfare, Republic of Korea (Grant HI15C3153). Namhee Kim received a scholarship from Brain Korea 21 FOUR Project funded by National Research Foundation of Korea, Yonsei University College of Nursing.
Author information
Authors and Affiliations
Contributions
NK, GSK, CWW, and MK had full access to all the data in the study and had primary responsibility for the final content. NK, GSK, JJL, MKP, and JS reviewed the literature, designed the study and drafted and revised the manuscript. NK and JJL analyzed the data. NK, GSK, JJL, and MKP contributed to study design and data collection. All authors reviewed the results, drafted the manuscript, and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The Korean Frailty and Aging Cohort Study (KFACS) protocol and this study were approved by the Institutional Review Board (IRB) of the Clinical Research Ethics Committee of the Kyung Hee University Hospital, Seoul, Republic of Korea (IRB number: 2015‒12‒103; 2022‒07‒074, respectively). All participants and/or their legal guardians provided written informed consent and all methods were carried out in accordance with relevant guidelines and regulations.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Kim, N., Kim, G.S., Won, C. et al. Two-year longitudinal associations between nutritional status and frailty in community-dwelling older adults: Korean Frailty and Aging Cohort Study. BMC Geriatr 23, 216 (2023). https://doi.org/10.1186/s12877-023-03903-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12877-023-03903-4