Abstract
Background
In the aging population of Western societies, an increasing number of older adults have multiple chronic diseases. As multifaceted health problems imply the involvement of several healthcare professionals, multimorbid older people frequently face a fragmentation of health care. Addressing these challenges, we developed a local, collaborative, stepped, and personalized care management approach (LoChro-Care) and evaluated its effectiveness.
Methods
A two-group, parallel randomized controlled trial was conducted comparing LoChro-Care recipients (IG) to participants with usual care (CG). Patients aged 65 + with chronic conditions were recruited at inpatient and outpatient departments of the Medical Center, University of Freiburg. Participants were allocated using block randomization (nIG = 261, nCG = 263). LoChro-Care comprised individualized care provided by chronic care managers with 7 to 13 contacts over 12 months. Questionnaires were given at 3 time points (T0: baseline, T1: after 12 months, T2: after 18 months). The primary outcome was the physical, psychological, and social health status represented by a composite score of functional health and depressive symptoms. Secondary outcomes were the participants’ evaluation of their health care situation, health-related quality of life (HRQL), and life-satisfaction (LS). The data were analyzed using linear mixed modelling.
Results
We analyzed N = 491 participants (nIG = 244, nCG = 247), aged M = 76.78 years (SD = 6.35). For the composite endpoint, neither a significant difference between IG and CG (p = .88) nor a group-time interaction (p = .52; p = .88) could be observed. Participants in both groups showed a significant decline on the primary outcome between T0 and T2 (p < .001). Post hoc analyses revealed a decline in both functional health (p < .001) and depressive symptoms (p = .02). Both groups did not differ in their evaluation of their health care situation (p = .93), HRQL (p = .44) or LS (p = .32). Relevant confounding variables were female gender and multimorbidity.
Conclusion
Supporting patients’ self-management in coordinating their individual care network through LoChro-Care did not result in any significant effect on the primary and secondary outcomes. A decline of functional health and depressive symptoms was observed among all participants. Potential future intervention adaptations are discussed, such as a more active case management through direct referral to (in-)formal support, an earlier treatment initiation, and the consideration of specific sociodemographic factors in care management planning.
Trial registration
German Clinical Trials Register (DRKS): DRKS00013904 (02.02.2018), https://drks.de/search/de/trial/DRKS00013904
Similar content being viewed by others
Background
Enhancements in, for example, living conditions, nutrition, and medical treatments have led to increased life expectancy and, thus, to an aging population in Western societies. In Germany, the number of people aged 67 years and above increased by 54% between 1990 and 2018 – a trend that continues to rise [1]. With increasing age, the probability for the coexistence of several chronic health conditions, called multimorbidity, becomes more likely. A representative study in Germany showed that 75.8% of women and 68.0% of men aged 65–74 years have two or more coexisting chronic diseases, such as cardiovascular diseases, cancer, chronic pulmonary diseases, musculoskeletal disorders, diabetes mellitus, dementia, and depression [2]. Furthermore, the co-occurrence of multiple health conditions is associated with reduced functional capacity, loss of autonomy, poor self-reported health status, quality of life, need of help or even institutionalization, and mortality [2,3,4,5,6]. Considerably impaired functional health and poor perceived quality of life, in turn, were reportedly related with female gender, older age, being single or widowed, and low socioeconomic status [4, 5, 7]. In addition, research indicates a reciprocal association between impaired functional health, particularly physical impairments, and the onset and course of depressive symptoms [8, 9]. Hence, the patients’ functional health and depressive symptoms are considered to be highly relevant in this population.
Since multimorbidity is also associated with greater health care utilization [2, 3], this trend represents not only a major challenge for the health care system in general but also for individual health care provision. The presence of multimorbidity can thus lead to fragmented health care due to the involvement of numerous health professionals [10]. In this context, the World Health Organization (WHO) explicitly recommends a continuum of health care provision in terms of, i.e., coordinated, cross-sectoral care management [11]. Such interventions should aim at supporting preventive actions, improving functional ability, and averting or delaying adverse developments, rather than managing a single health condition in isolation [11].
However, most interventions have been developed and tested primarily with the focus on single diseases, such as depression, diabetes mellitus, and dementia [12,13,14,15,16], while studies evaluating complex care interventions for older adults with multiple chronic diseases are scarce. As shown by systematic reviews, only a few randomized controlled trials (RCT) have been carried out, none of which were conducted in Germany [17, 18]. Moreover, these studies showed mixed findings regarding the interventions’ effectiveness [17, 18]. In particular, studies either observed no improvements after the given treatment or results in favor of the control group on relevant clinical outcomes like functioning, cognition, quality of life, and depression [17].
In addition, literature reveals that information on the specific components of such care interventions is scarce, and limited knowledge about beneficial elements of complex care approaches for older multimorbid people exists [17, 19, 20]. Frequently identified components with potential impact are multidisciplinary teams, a comprehensive assessment, case management, care pathways/care plans, support for self-management, and education [11, 19,20,21,22]. As recommended in the German S3-treatment guideline for multimorbidity [6], the patient’s preferences, values, and needs should be prioritized. Therefore, elements such as shared decision-making and goal-setting also appear to be relevant components [11].
Given the literature described above, the development and evaluation of new approaches addressing the multiple needs and the resulting involvement of several health care providers in multimorbid older people is of great importance. In accordance with some of the aforementioned care elements and based on the “Ariadne principles” for patient-centered management of multimorbidity in primary care settings [23], we developed the LoChro-Care intervention – a new local, collaborative, stepped, and personalized care management approach for older people with chronic diseases (c.f. study protocol) [24]. It focuses on the enhancement of patients’ self-management in coordinating their individual care network in accordance with their health problems and subjective preferences. The objective of this study is to evaluate the effectiveness of LoChro-Care in terms of improvements in the physical, psychological, and social health status among older people with chronic diseases receiving LoChro-Care in comparison with usual care. We put forward the following hypotheses: (1) Older people receiving LoChro-Care will report an enhanced physical, psychological, and social health status as indicated by better functional health and reduced depressive symptoms. Moreover, LoChro-Care recipients will rate their (2) health care situation, as well as their (3) health-related quality of life and life-satisfaction, better than non-recipients.
Methods
Study design
In a two-group parallel RCT, LoChro-Care (intervention group, IG) was compared with usual care (control group, CG). The study was conducted at the Medical Center, University of Freiburg, Germany. Recruitment took place between January 2018 and March 2020. From the beginning of 2020, several procedures were adjusted due to the COVID-19 pandemic. From then on, we conducted the follow-up assessments, as well as the intervention contacts, exclusively by telephone. Ethical approval was granted by the ethics council of the University of Freiburg, Germany (495/17). The study follows the CONSORT guideline for reporting results from randomized trials [25] (see CONSORT Checklist – LoChro-study, additional file 1).
Study recruitment
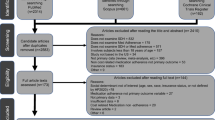
The a priori calculated sample size aimed for n = 606 study participants, assuming a dropout rate of 20% at the last follow-up time point (T2), a significance level of 0.05, 95% power, and a standardized mean difference between IG and CG of 0.30. Recruitment took place at the emergency center, selective wards, and at the geriatric, diabetes, and memory outpatient clinics of the Medical Center, University of Freiburg. Thus, we recruited both inpatients and outpatients, who were enrolled by research associates. Eligible patients were older adults aged 65 years or above with one or multiple chronical illnesses or geriatric symptoms who lived in Freiburg and nearby surrounding areas. In total, n = 2,721 potential participants took part in a short screening using the German version of the “Identification of Seniors at Risk” (ISAR) screening tool [26] (Fig. 1, flow diagram), which assesses the participants’ risk of unplanned readmission and need for nursing care. The inclusion criterion for study participation was an ISAR total score of two or more. Exclusion criteria were an ISAR score of less than two, a terminal medical condition, and insufficient German language skills. From the eligible patients, n = 1,477 did not meet the inclusion criteria and n = 720 refused to participate because they were satisfied with their current care situation or due to a lack of interest or time, for example (Fig. 1). Thus, the study contained n = 524 participants in total, from which nIG = 261 were randomly allocated to the IG receiving the LoChro-Care, and nCG = 263 CG-participants remained under usual care. We applied a block randomization without stratification. The allocation to the two groups was done by a research associate who was not involved in the recruitment, data collection procedure, or intervention provision using a computer-generated randomization schedule.
Flow diagram of the LoChro-study
Note: 1Received intervention = participant received at least one session. 2Did not receive intervention = participant received no intervention due to the following reasons: death 27.78%, unable to be reached 12.96%, too much time expenditure 12.96%, decline of health condition 11.11%, no perceived need 11.11%, no longer interested 9.26%, relocation 5.56%, other reasons 9.26%. 3Discontinued intervention = participant discontinued intervention due to the following reasons: death 24.32%, unable to be reached 24.32%, decline of health condition 16.22%, no perceived need 8.11%, too much time expenditure 2.70%, no longer interested 2.70%, dissatisfaction with intervention 2.70%, relocation 2.70%, other reasons 16.22%. 4Changed respondent = in comparison to T0, the respondent changed from proxy to patient (while proxy assessments were generally excluded in the analysis reported here). Lost to follow up = reasons for lost to follow-up assessments were: death, unable to be reached, too much time expenditure, decline of health condition, no perceived need for intervention, no longer interested, relocation, dissatisfaction with intervention, changed respondent (proxy-assessment), or other reasons. ISAR = Identification of Seniors at Risk screening measurement (German Version; [26]). Pat. = Patient; Pat. Assessment = patients’ self-reported questionnaires
Procedure
Patients, or the legal representative in the case of severe dementia or comparable conditions, granted written informed consent for study participation. Afterwards, the participants took part in the first questionnaire survey (baseline, T0). The follow-up questionnaire surveys took place 12 (T1) and 18 (T2) months later at the participants’ home (13.64% at T1, 5.86% at T2), if possible, or by telephone (86.36% at T1, 94.14% at T2). Responses were collected by research associates during individual interviews. In the case of medical conditions like dementia with limiting cognitive abilities for answering, the legal representative or relative responded to the questionnaire surveys (proxy-assessment). As our outcome variables primarily aimed at the subjective perception of health and psychosocial well-being, which can hardly be judged by third parties, these were not assessed via the proxy assessments. Thus, only the patients’ self-reported data are considered in the analysis reported here (patient assessment, see flow diagram Fig. 1).
LoChro-Care intervention
LoChro-Care was designed as a local, collaborative, stepped, and personalized care intervention. It focuses on enhancing the patients’ self-management in coordinating their individual care network by providing assistance to maintain or establish contact to formal and informal support (e.g., general practitioner, family, regional geriatric outpatient services). It comprised 7–16 contacts with trained chronic care managers (CCM) and lasted 12 months. A total of four CCM with extensive experience in the sectors of nursing, health education, and social work provided the intervention (qualifications: CCM 1: Bachelor of Science (BSc) Nursing Science, nurse; CCM 2: Master of Arts (MA) Health Education, nurse; CCM 3: MA Social Work; CCM 4: BSc Health Education, MA Social Work student). At least the first three contacts were home visits. Depending on the constitution of the patient, the monitoring contacts could also take place by telephone. From the beginning of 2020, the intervention contacts were conducted exclusively by telephone due to the COVID-19 pandemic. Figure 2 shows the elements of the LoChro-Care intervention. Following the “Ariadne principles” for the management of multimorbidity in primary care [23], LoChro-Care included: a) a comprehensive assessment of the individuals’ health, psychosocial, and care conditions; b) the development of an individualized care plan in accordance with the patients’ prioritized health problems and preferences; c) the implementation, adaptation, and monitoring of the care plan; and d) a closing session. Extra modules were provided for patients with mild depression or diabetes, which were administered by the CCM responsible for the respective patient. In the case of mild depression, six additional contacts were implemented, including a short problem-solving therapy. In the case of diabetes, three extra contacts were included that aimed at the improvement of the patients’ self-management skills concerning diabetes. Additionally, there was an option to involve trained volunteers for support if patients could not implement the care plan on their own and no primary caregiver was available. For the implementation of the intervention, standardized action plans for each intervention contact between the CCM and the patient were developed. In addition, standardized care plans for recurring patient issues were created (incl. informational materials) and adapted to the individual patients’ care situation, considering formal and informal support. Given the case of sufficient contact with the general practitioner and family caregivers, the CCM informed the patient about self-management and local geriatric outpatient services suitable for the patient’s needs. In the case of insufficient support, the CCM assisted with establishing contact with formal and informal care providers, e.g., the general practitioner, primary caregivers, trained volunteers, or geriatric services. In the monitoring sessions, the CCM and the patient collaboratively evaluated the implementation of planned actions, the patient’s current health problems, needs, and preferences, and adapted the care plan accordingly. The CCM themselves neither implemented any specific treatment measures nor connected the patients directly to health or psychosocial services. Instead, they identified appropriate care providers and assisted the patients with contacting them. In order to ensure treatment fidelity, the CCM had regular supervision sessions by an interdisciplinary geriatric team and research associates responsible for the process evaluation, as well as team intervision.
Measurements
The same measurements were collected in each group at each time point. The primary outcome was the patients’ physical, psychological, and social health status. This was represented by a composite score consisting of the participants’ functional health and depressive symptoms, as these two impairments are assumed to be highly prevalent in the target population and reciprocally associated [8, 9]. The first component, functional health, was assessed using the “WHO Disability Assessment Schedule 2.0” (WHODAS 2.0) [27]; the latter, depressive symptoms, was assessed with the “Patient Health Questionnaire” (PHQ-9) [28]. The WHODAS assesses aspects of functional health during the last 30 days via six domains: cognition, mobility, self-care, interactions with other people, activities of daily living, and social participation. For an overall WHODAS-score, responses of the 32 items were counted, and the resulting score was transformed to a scale range of 0–100. Higher scores thereby indicate lower levels of functional health. The PHQ-9 measures depressive symptoms according to the DSM-IV criteria (Diagnostic and Statistical Manual of Mental Disorders) and consists of nine items (reference period: the last two weeks). The summary score ranges from 0–27, with a higher score indicating more severe depressive symptoms. In order to bring these differently measured outcomes to the same metric and, thus, to calculate the composite score, the Proportion-of-Maximum-Scaling (POMS) method was used [29]. Therein, each scale was transformed into a scale ranging from 0 (minimum) to 100 (maximum) using the following formula:
One secondary outcome was the patient’s evaluation of their health care situation. This was assessed by the “Patient Assessment of Chronic Illness Care” (PACIC) [30] (German version), which asks for the patients’ experiences with the described care elements within the last 6 months (11 items) and their overall satisfaction with health care (one item). For the analyses presented here, we used the one item of interest, namely: “In what percentage of cases was I satisfied with the organization of my medical care”. The two other secondary outcomes were health-related quality of life (HRQL) (using a self-developed item) and life-satisfaction (LS) [31]. Each of these was assessed with a single-item scale ranging from 0–10, with higher values indicating a higher HRQL or LS, respectively.
Multimorbidity was assessed using a weighted multimorbidity index, which can have values from zero to 37 [32]. Information on multimorbidity stemmed from the hospital’s documentation system and additional patient-reported diagnoses.
Data analysis
All statistical analyses were executed using SPSS (version 28). Both intention-to-treat (ITT) and per-protocol (PP) analysis were performed. The statistician was blinded to the group allocation. An alpha-level of 0.05 (two-sided) indicated statistical significance.
The analysis compared change over time within the outcome variables between both groups. Linear mixed modelling (LMM) analysis was applied due to its robustness in handling missing data, as well as being able to handle both within- and between-subjects effects [33]. Intra-individual change over time was modelled on the first LMM level, while the subject-related inter-individual comparison was modelled on the second level [34]. For each primary and secondary outcome, a LMM was built following three consecutive steps: first, a random-intercept model (M1) for inter-class correlation (ICC) estimations was constructed including a random intercept for each participant. Second, a loaded model (M2), including all of the fixed effects with a respective choice of covariance matrix, was modelled. In a third step, the full model (M3) included the repeated measurement effect and defined covariance matrix [33]. The model fit was compared using likelihood ratio tests (LRT), based on the respective -2 log-likelihood indices and degrees of freedom per model [35].
Control variables were included since adjusted analyses are also recommend as sensible for RCTs when certain variables are expected to be prognostic [25]. Hence, the control variables comprised individual characteristics, namely gender, age, degree of multimorbidity, family status, residential status, health insurance status, and educational level, as well as self-reported depression and diabetes at baseline, as these two groups received specific LoChro-Care components. Additionally, differences in recruitment path (e.g., inpatient and outpatient setting) and interview setting (face-to-face vs. telephone) were included. The categorical control variables were dummy coded. The missing data and sample-dropouts were handled through the application of maximum likelihood estimation (restricted). As a long-format of the data set was used, the baseline values of each variable, as well as the values at T1 and T2, are represented through the combination with the measurement time point variable [33].
Systematic differences between dropouts and non-dropouts were examined with regards to baseline characteristics using t-tests for independent samples and contingency tables.
Results
Sample
At baseline, a total number of N = 501 participants were assessed (nIG = 249 and nCG = 252; flow diagram Fig. 1 “patient assessment”). The dropout rate was 32.34% (n = 162) after 12 months (T1) and 7.96% (n = 27) after 18 months (T2). Dropouts showed more impairments and depressive symptoms, and higher levels of multimorbidity. Unplanned inpatient hospitalizations (e.g., because of falls or stroke) were documented as Serious Adverse Events (SAE) in n = 28 cases, but we did not exclude these cases from our study. Exclusion criteria for the analysis were: a) pilot phase participation, b) more than 50% missing values, c) cases where the respondent changed from proxy to patient in comparison to baseline, and d) proxy assessment. The ITT sample consisted of nITT = 491 participants. IG-participants who did not finish the intervention with a closing session were excluded in the PP analysis. Among these excluded IG-participants, n = 54 (20.69%) did not receive LoChro-Care (e.g., because of death [n = 15, 27.78%] or decline in the health condition [n = 6, 11.11%]; Fig. 1). Additionally, n = 37 (14.18%) IG-participants discontinued the intervention. Thus, the PP sample consisted of nPP = 408 cases.
At baseline, the mean age in the ITT sample was M = 76.78 (SD = 6.35) years, ranging from 65 to 94 years. In total, n = 275 (56.01%) participants were female, n = 229 (47.64%) were married, and n = 448 (91.75%) lived in their private homes. The multimorbidity-index mean was M = 5.46 (SD = 3.44), ranging from zero to 20. Further demographic characteristics of the ITT, as well as the PP sample, can be found in Table 1. The baseline means and standard deviations for all outcomes can be found in the supplementary Table S1 (additional file 2).
Preliminary analysis
For the composite score (ITT), the ICC with r = 0.54 was relatively high due to the nature of the repeated measurement design. The model specifications (loaded model and full model) significantly enhanced the model-fit (Χ1(21) = 438.61, p < 0.001; Χ2(2) = 6.36, p = 0.02) [36]. Further information on the model fit and likelihood-ratio tests for the LMM of each outcome can be found in Table S2 (additional file 3).
Primary analysis
Primary outcome
In regards to the participants’ physical, psychological, and social health status, the final model (M3; ITT) indicates that the composite score was significantly affected by time (bT0-T2 = 7.88, p < 0.001; bT1-T2 = 4.53, p = 0.01), gender (b = 6.14, p < 0.001), depression (b = 6.99, p < 0.01), multimorbidity (b = 1.26, p < 0.001), private health insurance (b = 5.59, p = 0.02), and General Certificate of Secondary Education (GCSE) for the highest school degree (b = 4.09, p = 0.03). The effects of group (b = -0.24, p = 0.88) and group*time (bT0-T2 = -1.20, p = 0.52; bT1-T2 = -0.29, p = 0.88) were not significant predictors (see Table 2).
Over time, the participants in both the IG and CG showed higher composite-score levels (MT0 = 28.44 [SD = 4.82]; MT1 = 32.83 [SD = 4.70]; MT2 = 35.72 [SD = 4.71]), indicating a general drop in their physical, psychological, and social health status (see Table 3). With regards to this decrease, the IG and CG did not differ significantly at any given time point (FT0[1, 727.81] = 0.02, p = 0.88; FT1[1, 914.36] = 0.08, p = 0.77; FT2[1, 932.58] = 0.60, p = 0.40).
Furthermore, the results in Table 2 indicate that female participants had significantly lower levels of physical, psychological, and social health status (b = 6.14, p < 0.001; female M = 34.45 [SD = 17.10]), male M = 28.33 [SD = 18.22]). Participants with lower degrees of multimorbidity displayed a significantly better health status (b = 1.26, p < 0.001; r[1071] = 0.30, p < 0.001) than those with higher levels of multimorbidity. Participants who entered this study with self-reported depression showed significantly lower levels of health status (b = 6.99, p < 0.01; MDepYes = 42.09 [SD = 18.21]; MDepNo = 29.98 [SD = 17.14]). This also applies to participants whose highest school degree was not GCSE-level (b = 4.09, p = 0.03; MGCSEYES = 32.78 [SD = 18.08]; MGCSENO = 29.63 [SD = 17.12]). Those who were not privately health insured indicated higher levels in the composite score (b = 5.59, p = 0.02; MPrivateNo = 32.42 [SD = 17.86], MPrivateYes = 21.92 [SD = 14.40]). These differences did not change over time and did not substantially differ between the ITT and PP samples (see Table 2).
Post-hoc analysis
Post-hoc LMM analysis with the WHODAS and PHQ-9, respectively, were executed to take a closer look at the decline in the participants’ health status in both groups over time. Time mainly affected a difference in the WHODAS scores (bT0-T2 = 11.04, p < 0.001; bT1-T2 = 7.05, p < 0.001), while the change in PHQ-9 scoring was much smaller (bT0-T2 = 4.82, p = 0.02; bT1-T2 = 2.49, p = 0.18). In each analysis, there was no significant difference between the IG and CG (bWHODAS = -1.18, p = 0.52; bPHQ-9 = 0.70, p = 0.65), as well as no significant interaction between group and time (bWHODAS_T0T2 = 1.67, p = 0.42, bWHODAS_T1T2 = -0.79, p = 0.71; bPHQ-9_T0T2 = -2.39, p = 0.21, bPHQ-9_T1T2 = -1.38, p = 0.48; see Table 2). Furthermore, both outcomes were similarly and positively affected by gender (bWHODAS = 6.54, p < 0.001; bPHQ-9 = 5.58, p < 0.001) and multimorbidity (bWHODAS = 1.59, p < 0.001; bPHQ-9 = 0.91, p < 0.001), while only the PHQ-9 was significantly and positively affected by a self-reported diagnosis of depression at baseline (bWHODAS = 3.83, p = 0.14; bPHQ-9 = 9.98, p < 0.001). Again, these results are comparable for ITT and PP.
Secondary outcomes
In regards to satisfaction with care, HRQL, and LS, none of these secondary outcomes were significantly affected by the intervention variables of group, time, or the interaction between group and time (see Table 2). In general, women showed significantly lower levels of satisfaction with care (b = -5.16, p < 0.01), HRQL (b = -0.58, p < 0.01), and LS (b = -0.54, p < 0.05). Levels of multimorbidity, a self-reported diagnosis of depression, family status, health insurance, and the recruitment path were significant predictors of only a few of these secondary outcomes.
Discussion
In this RCT, we analyzed the effectiveness of a newly developed local, collaborative, stepped, and personalized care management approach for older people with chronic diseases, LoChro-Care. The results revealed no significant differences between participants receiving the LoChro-Care intervention and participants with usual care on any of the primary or secondary outcome variables. Thus, no improvements in the participants’ physical, psychological, and social health status, as indicated by functional health and depressive symptoms, were observed. In addition, participants who received LoChro-Care did not rate their health care situation, HRQL, or LS better than participants with usual care did. In sum, LoChro-Care yielded no effect over and above usual care, although individualized support by a CCM was provided.
In contrast to previous interventions which only focused on single diseases [12,13,14,15,16], LoChro-Care explicitly aimed to address the multiple health problems older people can experience and the resulting involvement of several health care providers. On the part of the participants, we could infer that we have reached the target group. For example, as indicated by the mean WHODAS score that represents the degree of functional health impairments, our sample lay on the 90th percentile of the general population [27]. Thus, the sample can be classified as relatively highly burdened, although a great variance has been found that covers the two extremes of no impairments to great impairments. With regards to the intervention, LoChro-Care included several recommended care elements, such as a comprehensive assessment, individualized care plans, support for self-management, and education [11, 19, 21, 22]. More precisely, it focused on the enhancement of the patients’ self-management in coordinating their individual care network of formal and informal support. Even though LoChro-Care comprised the prioritization of the patients’ preference, collaborative decisions on the treatment plan and the focus on the patients’ care situation in accordance with the German treatment guideline for multimorbidity [6], we could not detect an intervention effect. One explanation might be that the functional impairments were too severe to be compensated by patient self-management support alone. Although the CCM developed individualized care plans, taking into account the patients’ constitution and context, and additional informal support by trained volunteers was offered, the implementation of the care plan may not have been actionable for some patients. Hence, the assistance of highly-burdened older people by a CCM might not only address patients’ self-management but also a more active case management through direct referral to formal and informal support, as well as treatments for specific health conditions. The CCM provided extra modules for depression and diabetes, but the extent and intensity of these modules may have been too small. The plausibility of these hypotheses could be explored by deeper analyses of our process evaluation data. Future research may evaluate the effectiveness of a modified LoChro-Care approach.
In addition, another potential explanation for the absence of an intervention effect might be that our study participants may have already started with a considerably high level of health problems, whose progression could no longer be delayed. Hence, LoChro-Care probably could not reveal an effect at all. In this regard, we observed a decline in functional health and increasing depressive symptoms across the study period in all participants. A period of 18 months can be a long time for older people, making degeneration and reduced functional capacity more likely. In that sense, a longitudinal study in older adults revealed that the decline in activities of daily living and gait speed – aspects of functional health – took place most rapidly [37]. Thus, it can be questioned whether care interventions that primarily aim at patients’ self-management have the potential to counteract functional decline, and if so, at what point in time an effective change in progression would still be possible. Hence, future interventions aiming at averting or delaying functional decline and disease progression should start early.
In line with the results reported here, previous RCTs that examined the effectiveness of interventions for older adults with multiple health complaints showed no clear superiority of the intervention group participants [17, 18]. Although the heterogeneity of the target population in these investigations and in our study was intentional, this may have also made it more difficult to demonstrate an effect. In this debate, it is criticized that previous RCTs used numerous different outcome measurements with partly unclear psychometric properties, making it difficult to interpret and compare the results [17]. In contrast and especially with regards to the primary outcome, we applied well-established and validated instruments in our study (WHODAS; [27], PHQ-9; [28]). Moreover, the questionnaires used could be considered as suitable for the assessment of relevant outcome variables commonly experienced in older people. For example, the WHODAS questionnaire asks for existing functional impairments in different areas, like restrictions in activities of daily living, self-care, and social participation, which may be reciprocally associated with depressive symptoms [8, 9]. In this context, future research could aim at the development of a core outcome set, which describes a consensus about central outcome variables relevant to the target population, integrating the experts’ and the patients’ perspectives. This could facilitate the evaluation of the effectiveness of an intervention and the comparability of the studies’ results.
Limitations
Although we reached a comprehensive sample size in the context of geriatric research, which formed a sound basis for the statistical analyses, some limitations should be mentioned. Potential bias could result from the regional specificity and the exclusion criteria applied. The study area was restricted to Freiburg and surrounding areas. Specific characteristics of this area, like the relatively high socioeconomic performance, might have influenced the implementation of the intervention and study results. Therefore, future studies should investigate similar health care approaches for older people with multiple chronic diseases in other German areas for comparison. In addition, we did not include patients with terminal conditions or insufficient German language skills. These factors could likely be conditions occurring in the population of older multimorbid people and in ethnically diverse societies, which pose specific demands on the care management; therefore, they should be explored in future research.
Finally, the effect of the COVID-19 pandemic cannot be ruled out. From the beginning of 2020, several study procedures were adapted to the pandemic situation. The monitoring and closing sessions of the intervention were then primarily provided by telephone. In principle, this was not an issue of particular concern because the intervention design and manual had already included telephone contacts between the CCM and patients. Moreover, the telephone contacts were feasible in most cases. Nevertheless, the general negative effects of the COVID-19 pandemic, such as restricted contacts, reduced doctor visits, or impaired mood, could have interfered with the intervention and influenced the evaluation of its effectiveness.
Practical implications
In sum, we can infer several practical implications for future research and practice as indicated above. Attempts to modify LoChro-Care or to develop new interventions for older multimorbid people could include more active assistance in establishing formal and informal supports. In addition, it could comprise comprehensive case management that goes beyond self-management support. In this regard, the patients’ degree of multimorbidity, severity of the already existing health conditions, and prognostic progression should always be considered, and the optimal time-point for treatment initiation needs to be determined. In addition, it would be worthwhile to give more attention to both specific diagnoses with potential impact on the health status (e.g., depression) and sociodemographic factors. In particular, gender-specific needs could be addressed in the provision of care. In this context, it could be beneficial to gain more insight into the patients’ individual needs and perceived helpful intervention components, as well as into the care managers’ perspective on feasible care elements. Therefore, future research could first use qualitative methods (e.g., interviews) to explore the demanded intervention elements. Such results, in turn, might inform the development of care interventions and facilitate the identification of potential effective care management elements in respect to the target group and intervention goal.
Conclusion
In this study, we developed a new, local, collaborative, stepped, and personalized care management approach for older people with chronic diseases, LoChro-Care, which addressed the patients’ self-management in coordinating their individual care network. Notwithstanding, our results indicated no significant effect of LoChro-Care on any of the primary or secondary outcomes. In addition, the results revealed a decline in functional health and depressive symptoms over time in all participants. In view of the ongoing aging society, it could be worthwhile to adapt and evaluate supportive care interventions like LoChro-Care. These should target close patient support and specific sociodemographic and contextual factors in the population of interest, as well as an early implementation of the intervention to avert or delay the progression of health complaints and functional impairments.
Availability of data and materials
The datasets generated and analyzed in the current study are not publicly available due to data protection reasons but are available from the corresponding author upon reasonable request.
Abbreviations
- BSc:
-
Bachelor of Science
- CCM:
-
Chronic Care Manager
- CG:
-
Control Group
- CONSORT:
-
Consolidated Standards of Reporting Trials
- DSM:
-
Diagnostic and Statistical Manual of Mental Disorders
- GCSE:
-
General Certificate of Secondary Education
- HRQL:
-
Health-Related Quality of Life
- ICC:
-
Inter-Class-Correlation
- IG:
-
Intervention Group
- ISAR:
-
Identification of Seniors at Risk
- ITT:
-
Intention-To-Treat
- LMM:
-
Linear Mixed Modelling
- LRT:
-
Likelihood Ratio Tests
- LS:
-
Life-Satisfaction
- MA:
-
Master of Arts
- PACIC:
-
Patient Assessment of Chronic Illness Care
- PHQ-9:
-
Patient Health Questionnaire
- POMS:
-
Proportion of Maximum Scaling
- PP:
-
Per-Protocol
- RCT:
-
Randomized Controlled Trial
- SAE:
-
Serious Adverse Events
- WHO:
-
World Health Organization
- WHODAS:
-
World Health Organization Disability Assessment Schedule
References
Statistisches Bundesamt (Destatis). Bevölkerung im Wandel: Annahmen und Ergebnisse der 14. koordinierten Bevölkerungsvorausberechnung. Wiesbaden: Statistisches Bundesamt; 2019.
Robert Koch-Institut. Gesundheit in Deutschland. Gesundheitsberichterstattung des Bundes. Gemeinsam getragen von RKI und Destatis.: Kapitel 08 Wie gesund sind die älteren Menschen? Berlin: Robert Koch-Institut; 2015.
Palladino R, Tayu Lee J, Ashworth M, Triassi M, Millett C. Associations between multimorbidity, healthcare utilisation and health status: evidence from 16 European countries. Age Ageing. 2016;45:431–5. https://doi.org/10.1093/ageing/afw044.
Borglin G, Jakobsson U, Edberg A-K, Hallberg IR. Self-reported health complaints and their prediction of overall and health-related quality of life among elderly people. Int J Nurs Stud. 2005;42:147–58. https://doi.org/10.1016/j.ijnurstu.2004.06.003.
Stenzelius K, Westergren A, Thorneman G, Hallberg IR. Patterns of health complaints among people 75+ in relation to quality of life and need of help. Arch Gerontol Geriatr. 2005;40:85–102. https://doi.org/10.1016/j.archger.2004.06.001.
Scherer M, Wagner HO, Lühmann D, Muche-Borowski C, Schäfer I, Dubben HH, et al. S3-Leitlinie Multimorbidität: Kurzversion. 2017. https://www.degam.de/files/Inhalte/Leitlinien-Inhalte/Dokumente/DEGAM-S3-Leitlinien/053-047_Multimorbiditaet/053-047k_Multimorbiditaet_13-11-2017.pdf. Accessed 20 Dec 2022.
Alcañiz M, Solé-Auró A. Feeling good in old age: factors explaining health-related quality of life. Health Qual Life Outcomes. 2018;16:48. https://doi.org/10.1186/s12955-018-0877-z.
Barnett K, Mercer SW, Norbury M, Watt G, Wyke S, Guthrie B. Epidemiology of multimorbidity and implications for health care, research, and medical education: a cross-sectional study. Lancet. 2012;380:37–43. https://doi.org/10.1016/S0140-6736(12)60240-2.
Collard RM, Comijs HC, Naarding P, Penninx BW, Milaneschi Y, Ferrucci L, Oude Voshaar RC. Frailty as a predictor of the incidence and course of depressed mood. J Am Med Dir Assoc. 2015;16:509–14. https://doi.org/10.1016/j.jamda.2015.01.088.
Dunlay SM, Chamberlain AM. Multimorbidity in Older Patients with Cardiovascular Disease. Curr Cardiovasc Risk Rep. 2016;10:1–9. https://doi.org/10.1007/s12170-016-0491-8.
Araujo de Carvalho I, Epping-Jordan J, Pot AM, Kelley E, Toro N, Thiyagarajan JA, Beard JR. Organizing integrated health-care services to meet older people’s needs. Bull World Health Organ. 2017;95:756–63. https://doi.org/10.2471/BLT.16.187617.
Coventry PA, Hudson JL, Kontopantelis E, Archer J, Richards DA, Gilbody S, et al. Characteristics of effective collaborative care for treatment of depression: a systematic review and meta-regression of 74 randomised controlled trials. PLoS One. 2014;9:e108114https://doi.org/10.1371/journal.pone.0108114.
Ekers D, Murphy R, Archer J, Ebenezer C, Kemp D, Gilbody S. Nurse-delivered collaborative care for depression and long-term physical conditions: A systematic review and meta-analysis. J Affect Disord. 2013;149:14–22. https://doi.org/10.1016/j.jad.2013.02.032.
Renders CM, Valk GD, Griffin S, Wagner EH, Eijk JT, Assendelft WJ. Interventions to improve the management of diabetes mellitus in primary care, outpatient and community settings. Cochrane Database Syst Rev. 2001;(4):CD001481. https://doi.org/10.1002/14651858.CD001481.
Woods B, Aguirre E, Spector AE, Orrell M. Cognitive stimulation to improve cognitive functioning in people with dementia. Cochrane Database Syst Rev. 2012;(2):CD005562. https://doi.org/10.1002/14651858.CD005562.pub2.
Hölzel LP, Bjerregaard F, Bleich C, Boczor S, Härter M, König H-H, et al. Coordinated Treatment of Depression in Elderly People in Primary Care. Dtsch Arztebl Int. 2018;115:741–7. https://doi.org/10.3238/arztebl.2018.0741.
Eklund K, Wilhelmson K. Outcomes of coordinated and integrated interventions targeting frail elderly people: a systematic review of randomised controlled trials. Health Soc Care Community. 2009;17:447–58. https://doi.org/10.1111/j.1365-2524.2009.00844.x.
Smith SM, Wallace E, O’Dowd T, Fortin M. Interventions for improving outcomes in patients with multimorbidity in primary care and community settings. Cochrane Database Syst Rev. 2016;3:CD006560. https://doi.org/10.1002/14651858.CD006560.pub3.
Briggs AM, Valentijn PP, Thiyagarajan JA, Araujo de Carvalho I. Elements of integrated care approaches for older people: a review of reviews. BMJ Open. 2018;8:0221196. https://doi.org/10.1136/bmjopen-2017-021194.
Frich LMH. Nursing interventions for patients with chronic conditions. J Adv Nurs. 2003;44:137–53. https://doi.org/10.1046/j.1365-2648.2003.02779.x.
Kastner M, Cardoso R, Lai Y, Treister V, Hamid JS, Hayden L, et al. Effectiveness of interventions for managing multiple high-burden chronic diseases in older adults: a systematic review and meta-analysis. CMAJ. 2018;190:E1004–12. https://doi.org/10.1503/cmaj.171391.
Göhner A, Bitzer EM, Dreher E, Farin-Glattacker E, Heimbach B, Kohler K, et al. Integriertes Versorgungsmanagement für chronisch erkrankte ältere Menschen in der eigenen Häuslichkeit: Evidenz aus Cochrane-Reviews. [Integrated care management for older people with chronic diseases in domesticity: evidence from Cochrane reviews]. Z Gerontol Geriatr. 2021;54:54–60. https://doi.org/10.1007/s00391-020-01796-1.
Muth C, van den Akker M, Blom JW, Mallen CD, Rochon J, Schellevis FG, et al. The Ariadne principles: how to handle multimorbidity in primary care consultations. BMC Med. 2014;12:223. https://doi.org/10.1186/s12916-014-0223-1.
Frank F, Bjerregaard F, Bengel J, Bitzer EM, Heimbach B, Kaier K, et al. Local, collaborative, stepped and personalised care management for older people with chronic diseases (LoChro): study protocol of a randomised comparative effectiveness trial. BMC Geriatr. 2019;19:64. https://doi.org/10.1186/s12877-019-1088-0.
Moher D, Hopewell S, Schulz KF, Montori V, Gøtzsche PC, Devereaux PJ, et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340:c869. https://doi.org/10.1136/bmj.c869.
Singler K, Heppner HJ, Skutetzky A, Sieber C, Christ M, Thiem U. Predictive validity of the identification of seniors at risk screening tool in a German emergency department setting. Gerontology. 2014;60:413–9. https://doi.org/10.1159/000358825.
Üstün TB, Kostanjesek N, Chatterji S, Rehm J. World Health Organization. Measuring health and disability: manual for WHO Disability Assessment Schedule (WHODAS 2.0). Geneva: World Health Organization; 2010.
Löwe B, Spitzer RL, Zipfel S, Herzog W. PHQ-D Gesundheitsfragebogen für Patienten: Manual. 2nd ed. Karlsruhe: Pfizer; 2002.
Little TD. Longitudinal structural equation modeling. New York, N.Y.: Guilford Press; 2013.
Gugiu PC, Coryn C, Clark R, Kuehn A. Development and evaluation of the short version of the Patient Assessment of Chronic Illness Care instrument. Chronic Illn. 2009;5:268–76. https://doi.org/10.1177/1742395309348072.
Beierlein C, Kovaleva A, László Z, Kemper CJ, Rammstedt B. Eine Single-Item-Skala zur Erfassung der Allgemeinen Lebenszufriedenheit: Die Kurzskala Lebenszufriedenheit-1 (L-1). Mannheim: GESIS-Working Papers 2014/33; 2014.
Tooth L, Hockey R, Byles J, Dobson A. Weighted multimorbidity indexes predicted mortality, health service use, and health-related quality of life in older women. J Clin Epidemiol. 2008;61:151–9. https://doi.org/10.1016/j.jclinepi.2007.05.015.
West BT, Welch KB, Gałecki AT. Linear mixed models: A practical guide using statistical software. Boca Raton, London, New York: CRC Press; 2015.
Singer JD, Willett JB. Applied longitudinal data analysis: Modeling change and event occurrence. Oxford: Oxford University Press; 2003.
Verbeke G, Molenberghs G. Linear mixed models for longitudinal data. New York: Springer; 2000.
Heck RH, Thomas SL, Tabata LN. Multilevel and longitudinal modeling with IBM SPSS. New York, London: Routledge; 2014.
Diehr PH, Thielke SM, Newman AB, Hirsch C, Tracy R. Decline in health for older adults: five-year change in 13 key measures of standardized health. J Gerontol A Biol Sci Med Sci. 2013;68:1059–67. https://doi.org/10.1093/gerona/glt038.
Acknowledgements
We would like to thank the Department of General Practice and Health Services Research, University Hospital Heidelberg, Heidelberg, Germany for providing the German Version of the Patient Assessment of Chronic Illness Care – Short Form (Gugiu et al., 2009). In addition, we would like to thank Rebekka Allen for proofreading the manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL. The study was funded by the German Federal Ministry of Education and Research (grant number: 01GL1703 A-C). The funding body had no role in the collection, analysis, and interpretation of study data, nor did they play a role in the writing or the submission of the manuscript for publication.
Author information
Authors and Affiliations
Contributions
GM and LMH were responsible for the data management, data analysis, and manuscript writing. EFG and SVR supervised the first author. SVR, EFG, FF, EMB, KK, AM, JB, KL, and JS developed the concept and design of the study. JK and ED conducted recruitment and assessment, and SVR, BH, CM, and IH supported the recruitment and assessment. CS and AM were responsible for the data on diagnoses, medication, and multimorbidity. FF drafted the first version of the intervention and accompanied its further development. FF, EMB, and LL supervised the intervention, and EMB and LL were responsible for the formative evaluation. SVR, KKo, ED, and AG were responsible for the project management and coordination of the study. All authors contributed to interpretation of the data and read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethical approval for this study was granted by the ethics council of the University of Freiburg, Germany (reference number: 495/17). Informed consent of all participants was obtained prior to participation. Either the patients themselves or a legal representative/relative granted the informed consent in writing. All methods were performed in accordance with the relevant guidelines and regulations.
Consent for publication
Not applicable.
Competing interests
All authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
CONSORT Checklist.
Additional file 2:
Table S1. Descriptives of the LoChro primary and secondary outcomes in the intention-to-treat (ITT) and per-protocol (PP) sample at the first measurement time point (t0).
Additional file 3: Table S2.
The inter-class correlation (ICC) values and likelihood ratio tests (LRT) of the differently specified linear mixed models (LMM) predicting the change in participants‘ composite score values over the period of three consecutive time points for both the intention-to-treat (ITT) and per-protocol (PP) versions of the data set (REML).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Metzner, G., Horstmeier, L.M., Bengel, J. et al. Local, collaborative, stepped, and personalized care management for older people with chronic diseases – results from the randomized controlled LoChro-trial. BMC Geriatr 23, 92 (2023). https://doi.org/10.1186/s12877-023-03797-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12877-023-03797-2