Abstract
Background
Low levels of physical activity (PA) and high sedentary time (ST) are common in older adults and lack of PA is a risk factor for cardiovascular disease (CVD). Knowledge about associations with accelerometer-measured PA, ST and CVD risk in older adults is insufficient. This study examines the associations of accelerometer-measured PA and ST with cardiovascular risk measured using the Framingham risk score (FRS) and all-cause mortality in older adults.
Methods
A population-based sample of 660 (277 men, 383 women) older people (mean age 68.9) participated in the Oulu45 cohort study from 2013‒2015. PA and ST were measured with wrist-worn accelerometers at baseline for two weeks. Ten-year CVD risk (%) was estimated with FRS. The data for all-cause mortality were identified from the Digital and Population Data Services Agency, Finland after an average of 6.2 years follow-up. The associations between moderate to vigorous physical activity (MVPA), light physical activity (LPA), ST and FRS were analyzed using the multivariable linear regression analysis. Associations between LPA, ST and mortality were analyzed using the Cox proportional-hazard regression models.
Results
Each 10 min increase in MVPA (β = -0.779, 95% CI -1.186 to -0.371, p < 0.001) and LPA (β = -0.293, 95% CI -0.448 to -0.138, p < 0.001) was negatively associated with FRS while a 10 min increase in ST (β = 0.290, 95% CI 0.158 to 0.421, p < 0.001) was positively associated with FRS. After adjustment for waist circumference, only ST was significantly associated with FRS. Each 10 min increase in LPA was associated with 6.5% lower all-cause mortality risk (HR = 0.935, 95% CI 0.884 to 0.990, p = 0.020) and each 10 min increase in ST with 5.6% increased mortality risk (HR = 1.056, 95% CI 1.007 to 1.108, p = 0.025).
Conclusion
A higher amount of daily physical activity, at any intensity level, and avoidance of sedentary time are associated with reduced cardiovascular disease risk in older people. Higher time spent in light physical activity and lower sedentary time are associated with lower all-cause mortality.
Similar content being viewed by others
Background
Worldwide, almost 30% of the population does not meet physical activity (PA) recommendations [1]. Lack of physical activity is more common in older adults than in younger adults [1, 2]. Physical inactivity has been estimated to cause 6% of coronary heart diseases (CHD) and over five million deaths could be averted annually if all inactive persons become active [3].
More time spent in moderate-to-vigorous physical activity (MVPA) [4, 5] as well as in light [6] and total PA [4, 5] has been associated with lower incidence of cardiovascular disease (CVD) in middle-aged and older adults. In older adults, higher levels of MVPA [7] and light physical activity (LPA) [6, 7] as well as lower levels of sedentary time (ST) [7] have also been associated with lower incidence of CHD events. However, recommended levels of MVPA could attenuate increased CHD risk associated with ST [7].
The Framingham risk score (FRS) has been shown to predict coronary heart diseases (CHD) more accurately than the presence of metabolic syndrome [8]. In middle-aged adults, more time spent in MVPA has been associated with lower FRS regardless of length of time spent [9]. Accelerometer-measured ST is demonstrated to be positively associated with FRS for men [10]. A recent study suggests that adults with CVD or high CVD risk measured by FRS spend more time in ST and have lower MVPA levels than individuals with low CVD risk [11]. Another study using cluster analysis showed that inactive (highest ST, lowest LPA and MVPA) middle-aged women had higher CVD risk measured with FRS than women with at least moderate PA, while inactive men had higher CVD risk compared to very active men [12].
Higher levels of accelerometer-measured total PA, at any intensity level [7, 13,14,15] and lower ST [7, 14,15,16] have been associated with reduced risk of premature mortality in adults and older adults. However, in some studies, the association between LPA or ST and mortality has not been found after adjustment of MVPA [17]. High ST has been associated with higher mortality rates, especially for individuals with low MVPA [7, 18]. A recent study indicated that LPA could increase life expectancy, particularly for adults with low MVPA [19].
Accelerometers have been found to be reliable for measuring the amount and intensity of PA and ST [2, 20, 21]. Knowledge about dose–response associations with accelerometer-measured PA, especially LPA and ST, and health outcomes in older adults is insufficient [2, 21, 22]. However, associations between accelerometer-measured PA and incidents of CVD have been shown to be much stronger than reported in questionnaire-based studies [4, 17]. Wrist-worn accelerometers are well accepted and their use has been encouraged in population-based studies of older adults [2, 23]. There are no population-based studies on associations between accelerometer-measured PA, ST and FRS among older adults.
This study evaluates how accelerometer-measured PA and ST are associated with cardiovascular diseases risk, measured with the FRS, in a population-based sample of older adults. The study also aimed to reveal the associations between accelerometer-measured PA, ST and mortality in older adults.
Methods
Participants
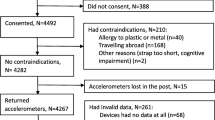
This study is part of the Oulu45 cohort described in detail in our previous study [24]. Those participants born in 1945 in Oulu, Finland, who were still alive and whose addresses were known (n = 887) were invited to the follow-up study at the age of 67‒70 years (in 2013‒2015) and 714 (80%) participated. The final study population (n = 660, 92.4% of the total study population) of this prospective population-based study consisted of men (n = 277) and women (n = 383) whose PA and ST were successfully measured with accelerometer at baseline of the follow-up.
The baseline and follow-up study were approved by the Ethical Committee of the Northern Ostrobothnia Hospital District in Oulu, Finland (EETTMK 33/2001 and EETTMK 12/2013), and were undertaken in accordance with the Declaration of Helsinki. The participants provided their informed written consent for the study.
Questionnaires
Cholesterol and hypertension medications were classified (no or yes) based on self-reported medications in use. Previously diagnosed coronary artery disease (CAD) and diabetes (type 1 or 2) were self-reported (no or yes). Smoking status was categorized as current smoker or non-smoker. An AUDIT questionnaire [25] was used to examine alcohol consumption and the total scores were calculated for those participants who answered all questions. Risky alcohol use was classified as ≥ 8 points.
Questions about perceived health (“How would you describe your health at the moment?”) and functional ability (“How would you describe your functional ability?”) were asked and answers dichotomized as satisfied (very good or pretty good) and non-satisfied (moderate, pretty poor, or very poor).
Clinical examination and blood samples
The participants’ height (to nearest 0.5 cm) and weight (to nearest 0.1 kg) were measured. The waist circumference (WC) (to nearest 0.5 cm) was measured three times on the midpoint of the lowest rib and the iliac crest [26], and the mean of measurements was used in analyses. Blood pressure was measured twice (Omron M3, Omron Healthcare Europe BV, Netherlands) after 5 min’ rest in a seated position and the mean values of those measurements were used in the analyses. Visceral fat area was measured via bioelectrical impedance (InBody 720, InBody, Seoul, Korea).
Blood samples were taken after overnight fasting. The plasma cholesterol and triglycerides were analyzed immediately after the blood samples were taken. A standardized oral glucose tolerance test (OGTT) was performed after overnight fasting for participants without previously diagnosed diabetes or diabetes medication and whose plasma glucose levels were < 8.0 mmol/l measured using a blood glucose meter (Bauer Contour, Bayer Consumer Care AG). Diabetes was defined as plasma fasting glucose ≥ 7.0 mmol/l or 2-h glucose ≥ 11.1 mmol/l [27].
Cardiovascular risk and register data
The FRS [28] was used to estimate the ten-year risk for CVD as a percentage. The FRS defines CVD risk including coronary death, myocardial infarction, coronary insufficiency, angina pectoris, heart failure, claudication, and ischemic and hemorrhagic stroke or transient ischemic attack. The FRS were calculated separately for all men and women from whom all the data needed were available. Age, HDL cholesterol, total cholesterol, systolic blood pressure (treated or not treated), smoking (yes/no), and diabetes mellitus (previously known diabetes or screen- detected type 2 diabetes on OGTT) (yes/no) were included in the FRS. Those participants whose FRS was > 20% were defined as high-risk individuals for CVD [28]. The FRS data was valid for 565 participants (21 missing values) without previously diagnosed CAD.
The date of deaths were requested from the Digital and Population Data Services Agency, Finland (until 14.10.2020) and participants were categorized as alive or dead. Follow-up time was calculated until death or until 14.10.2020 if the participant was alive.
Physical activity
PA and ST were measured with a wrist-worn uniaxial accelerometer (Polar Active, Polar Electro Ltd., Kempele, Finland) at baseline in 2013–2015. Polar Active provides MET (metabolic equivalent) values (1 MET = 3.5 mL/kg/min) every 30 s to estimate energy expenditure of daily PA [29]. A strong correlation between energy expenditure measured by indirect calorimetry and Polar Active (r = 0.987) [30] has been demonstrated, and also with double-labeled water (r = 0.86) [31]. The participants were asked to wear the activity monitor for two weeks (24 h/day) on the wrist of their non-dominant hand. The activity monitor did not give feedback to the participants.
Daily averages (min/day) of accelerometer-measured PA were calculated for each participant and classified into five activity levels (very light 1.00–1.99 MET, light 2.00–3.49 MET, moderate 3.50–4.99 MET, vigorous 5.00–7.99 MET, and very vigorous ≥ 8.00 MET) using the limits set by the manufacturer. For the analysis, all activity with intensities between 1.00 and 1.99 MET was classified as ST, all activity with intensities between 2.00 and 3.49 MET as LPA, and all activity ≥ 3.50 MET was classified as MVPA. Previously, under free-living environment and in the most of the intensity levels, it has been shown that Polar Active is more comparable with hip-worn Actigraph GT3X monitor when using the standard Polar Active thresholds instead of using traditional thresholds (ST ≤ 1.5 MET, LPA 1.51–2.99 MET, MPA 3.00–5.99 MET and ≥ 6.00 MET) [32]. The cut-off values of PA intensity measured with Polar Active were developed for younger adults, which could affect the measurement reliability for older adults. All activity with intensities 1 MET or higher was calculated on wear time. Participants with at least four valid days (monitor wear time of ≥ 600 min/day) were included in the analyses. The first and last measurement days were excluded as incomplete. The mean of the valid measurement days was 13.
Statistics
The results were analyzed using IBM SPSS Statistics software (SPSS 27 for Windows, SPSS Inc., Chicago, Illinois), and the statistical significance was set to p < 0.05. Differences among sexes were analyzed for categorical variables with the chi-square test and with independent samples t-test for continuous variables. Participants who reported diagnosed CAD were excluded when analyzing FRS.
The associations between MVPA, LPA, ST, and FRS were analyzed using the multivariable linear regression model. The models were built separately for MVPA, LPA and ST. Unstandardized regression coefficients (β) and 95% confidence intervals (CI) were estimated per 10 min and per 30 min increases in MVPA, LPA and ST. Based on the results of the univariate analysis, the models were adjusted for AUDIT score, living status (alone or with someone else) and monitor wear time (Model 1). Model 2 was adjusted for the same variables as Model 1 and also with WC. Furthermore, Model 1 for both ST and LPA were also adjusted with MVPA. Participants with previously diagnosed CAD were excluded in multivariable linear regression models for FRS. VIF < 5 was used in models for value of non-collinearity.
The Cox proportional hazards regression model was used to reveal the associations between LPA, ST, and all-cause mortality for the whole study population. In the Cox regression models, all participants who were alive when the follow-up ended (14.10.2020) and who had no missing values were considered as censored (n = 606). For the deaths, 28 events were included in the models. Hazard ratios (HR) and 95% CI were estimated per 10 min and per 30 min increases in LPA and ST. AUDIT score, blood pressure, blood pressure or cholesterol medication, smoking and living alone were not associated with mortality in univariate analysis. Based on univariate analysis FRS was included in the Cox proportional hazards regression model to describe the overall CVD risk. The models were also controlled for PA monitor wear time. MVPA was not included in the Cox proportional hazards regression models because it was not associated with mortality in univariate analysis.
Results
The characteristics of study participants are presented in Table 1. Over 11% of study participants had self-reported previously diagnosed CAD and almost 17% had self-reported or screen-detected diabetes. Almost a half the participants had hypertension medication and almost every third used cholesterol medication. After the follow-up visit, 29 (4.4%) participants had died (see Table 1).
Physical activity levels and risk factors for coronary artery disease are presented in Table 2. Men spent 42 min more sedentary time than women (p < 0.001) and amount of LPA was 13 min higher for woman (p = 0.034). There was no significant difference in MVPA among the sexes. CVD risk was high for 192 (83.8%) men and for 80 (23.8%) women (p < 0.001) (see Table 2).
The associations between MVPA, LPA, ST, and FRS according to the multivariable linear regression models are presented in Figs. 1 and 2. Every 10 min increase in MVPA (β = -0.779, 95% CI -1.186 to -0.371, p < 0.001) and LPA (β = -0.293, 95% CI -0.448 to -0.138, p < 0.001) was negatively associated with FRS and every 10 min increase in ST (β = 0.290, 95% CI 0.158 to 0.421, p < 0.001) was positively associated with FRS when monitor wear time, living status, and AUDIT scores were controlled for. Every 30 min increase in MVPA (β = -2.336, 95% CI -3.559 to -1.113, p < 0.001) or LPA (β = -0.879, 95% CI -1.345 to -0.413, p < 0.001) or 30 min increase in ST (β = 0.869, 95% CI 0.475 to 1.264, p < 0.001) having an equal but major association for FRS (see Fig. 1). When the Model 1 was adjusted also with sex, the association between LPA, MVPA, ST and Framingham risk score remained the same (data not shown) (Table 3).
Framingham risk scores (FRS) for the participants without diagnosed coronary artery disease (n = 565). The lines represent the % change in FRS (the unstandardized regression coefficient, 95% CI) per each 10 or 30 min’ increase in sedentary time (ST), light physical activity (LPA), and moderate to vigorous physical activity (MVPA). Models were controlled for monitor wear time, living alone, and AUDIT scores
Framingham risk scores (FRS) for the participants without diagnosed coronary artery disease (n = 565). The lines represent the % change in FRS (the unstandardized regression coefficient, 95% CI) score per each 10 or 30 min’ increase in sedentary time (ST), light physical activity (LPA), and moderate to vigorous physical activity (MVPA). Models were controlled for waist circumference, monitor wear time, living alone, and AUDIT scores
When the model was controlled also with WC (see Fig. 2), the positive association between every 10 min (β = 0.131, 95% CI 0.003 to 0.260, p = 0.046) and every 30 min increase in ST (β = 0.394, 95% CI 0.008 to 0.781, p = 0.046) and FRS remained the same. The association between LPA and FRS were of borderline significance (p = 0.052). There was no significant association between MVPA (p = 0.241) and FRS when WC was adjusted (see Fig. 2). When the Model 2 was adjusted also with sex, the association between ST, LPA and Framingham risk score remained the same. MVPA was negatively associated with Framingham risk score after adjustment sex (data not shown).
Furthermore, monitor wear time, living status, AUDIT scores, and MVPA were controlled for analyzing associations between ST, LPA and FRS. When the model was adjusted with ST and MVPA simultaneously (per 10 min increase for both), associations between ST and FRS remained similar (β = 0.216, 95% CI 0.054 to 0.378, p = 0.009) while there was no statistically significant association between MVPA and FRS. When the model was adjusted for both LPA and MVPA (per 10 min increases for both), associations between LPA and FRS (β = -0.233, 95% CI -0.385 to -0.061, p = 0.007) as well as MVPA and FRS (β = -0.598, 95% CI -1.024 to -0.172, p = 0.006) remained similar (data not shown on the figures).
The associations between LPA, ST, and all-cause mortality after 6.2 (min 0.6; max 7.7) years follow-up are presented in Fig. 3. Based on the results of the Cox regression model, every 10 min increase in LPA was associated with 6.5% lower mortality risk (HR = 0.935, 95% CI 0.884 to 0.990, p = 0.020) and every 10 min increase in ST with 5.6% increased mortality risk (HR = 1.056, 95% CI 1.007 to 1.108, p = 0.025). Every 30 min increase in LPA was associated with 18.1% lower mortality risk (HR = 0.819, 95% CI 0.691 to 0.969, p = 0.020), whereas every 30 min increase in ST was associated with 17.8% higher mortality risk (HR = 1.178, 95% CI 1.021 to 1.360, p = 0.025) (see Fig. 3). FRS was not significantly associated with mortality in Cox regression models. When the Cow regression models were adjusted also with sex, the association between LPA, ST and Framingham risk score remained the same (data not shown).
Discussion
For the first time, this population-based study analyzed the association between accelerometer-measured physical activity and sedentary time and the risk of cardiovascular diseases measured by Framingham Risk Score in older adults. We found that more time spent in MVPA and LPA and less time spent sedentary were associated with lower CVD risk. Associations between LPA and ST with FRS were also found after adjustment of MVPA. However, after adjustment of WC only ST was associated with FRS. After an average of 6.2 years follow-up, more time spent in LPA and lower ST were also associated with all-cause mortality independently after adjusting CVD risk measured with FRS.
In this study, more time spent in MVPA or LPA and less ST were all associated with lower CVD risk measured with FRS. For MVPA and ST, the results are similar to previous findings with a younger population [9,10,11]. Previously, both LPA and MVPA were associated with lower CVD risk, measured with Reynold risk score in older women [33] which is also consistent with the findings of our study. When MVPA and LPA were both entered in the same model to describe the total PA level, the association between MVPA and FRS was over twice as strong as the association between LPA and FRS.
Based on our results, ST is an important and independent risk factor for CVD measured by FRS. When ST and MVPA were in the same model, only ST remained a statistically significant determinant of FRS there was no association between MVPA and FRS. Previously, high ST was shown to be associated with metabolic syndrome also in individuals with high cardiorespiratory fitness [34]. When WC was adjusted, only ST, not PA, was associated with lower FRS. Previously, low MVPA, but not ST were associated with high WC [35].
The results of this study are consistent with previous findings in that higher levels of accelerometer-measured LPA [7, 13,14,15] and lower levels of ST [7, 14,15,16] were associated with lower mortality rates. The associations between LPA, ST, and mortality were found regardless of CVD risk measured by FRS. However, in this study we found no association between MVPA and mortality, which is contradictory to previous studies with a larger sample size [7, 14, 15, 17]. In this study, there were only 29 deaths during the follow-up, which might explain the lack of association between MVPA and all-cause mortality. With such a small sample size, lack of association between MVPA and mortality may also be simply by chance. Associations between LPA, ST, and mortality could come out with small sample size because of higher levels of LPA and ST than MVPA.
Based on PA guidelines, older adults should spend 150 min per week in MVPA or should be at least as active as they can be if they have functional limitations [36, 37]. Adherence to PA recommendations even in later life has been shown to be associated with lower all-cause and cardiovascular mortality risk despite a previously inactive lifestyle [38]. However, the PA guidelines regarding MVPA for older adults could be too hard to achieve so our results about associations between accelerometer-measured LPA and ST to lower CVD risk and mortality is supportive. For public health, it might be the most important that older adults with high ST could at least increase LPA to promote their cardiovascular health and decrease the risk of mortality. A recently published review suggested that “sitting less and moving more” is practical and acceptable advice to decrease cardiovascular risk [39].
The strength of this study is its population-based, prospective nature. The target population was a homogenous, stable, and representative sample of aged men and women, obtained from the National Population Register of Finland, which has 100% coverage. Another strength of this study is accelerometer-measured PA and ST. The measurement period for PA in this study was approximately 13 days, instead of the usual one week. Thus, the PA measurement data could represent real-life conditions more accurately than shorter measurement periods. Wrist-worn accelerometers are well accepted in older adults and data is usually high quality [2].
There are some limitations in this study. In this study we used wrist-worn accelerometer. Previously, wrist-worn accelerometers were shown to be less precise to estimate ST in young adults compared to hip-worn monitors [40]. Another limitation of this study is that the most unwell participants may have dropped out of the study, which may introduce some bias to the results. On average, the participants attending the follow-up visit may be more active and healthier than the total 1945 birth cohort members, which may reduce the representativeness of the sample and the results of this study cannot be generalized to very ill or institutionalized older people.
A longer follow-up period might clarify the associations between MVPA, LPA, ST, and mortality, which were partly contradictory in this study compared to previous studies. In this study, we examined the associations between PA, ST, and all-cause mortality, not CVD mortality.
For a future studies, it is important to study associations between PA and ST, and CVD risk with larger study samples of older adults. It is also important to study how the length of PA and ST bouts are associated with risk of CVD and mortality in older people. After a longer follow-up, it might also be interesting to study associations with PA, ST, and CVD mortality.
Conclusions
In conclusion, this prospective population-based study indicated that more time spent in PA and less time spent in ST were associated with lower CVD risk and also lower mortality rates in older people. Based on these results, a greater amount of daily physical activity, at any intensity level and avoiding sedentary time is recommended to reduce the risk of cardiovascular disease and all-cause mortality in old age.
Availability of data and materials
Oulu45 data is available from the University of Oulu, Infrastructure for Population Studies. Permission to use the data can be applied for research purposes via electronic material request portal. In the use of data, we follow the EU general data protection regulation (679/2016) and Finnish Data Protection Act. The use of personal data is based on cohort participant’s written informed consent at his/her latest follow-up study, which may cause limitations to its use. Please, contact NFBC project center (NFBCprojectcenter@oulu.fi) and visit the cohort website (www.oulu.fi/nfbc) for more information.
Abbreviations
- AUDIT:
-
Alcohol use disorders identification test
- BMI:
-
Body mass index
- CAD:
-
Coronary artery disease
- CHD:
-
Coronary heart disease
- CI:
-
Confidence intervals
- CVD:
-
Cardiovascular disease
- FRS:
-
Framingham risk score
- HR:
-
Hazard ratios
- LPA:
-
Light physical activity
- MET :
-
Metabolic equivalent
- MVPA:
-
Moderate to vigorous physical activity
- OGTT:
-
Oral glucose tolerance test
- PA:
-
Physical activity
- ST:
-
Sedentary time
- T2DM:
-
Type 2 diabetes
- VFA:
-
Visceral fat area
- WC:
-
Waist circumference
References
Guthold R, Stevens GA, Riley LM, Bull FC. Worldwide trends in insufficient physical activity from 2001 to 2016: a pooled analysis of 358 population-based surveys with 19 million participants. Lancet Global Health. 2018;6(10):e1077-86.
Doherty A, Jackson D, Hammerla N, Plötz T, Olivier P, Granat MH, et al. Large Scale Population Assessment of Physical Activity Using Wrist Worn Accelerometers: The UK Biobank Study. PLoS ONE. 2017;12(2):e0169649.
Lee IM, Shiroma EJ, Lobelo F, Puska P, Blair SN, Katzmarzyk PT, et al. Effect of physical inactivity on major non-communicable diseases worldwide: an analysis of burden of disease and life expectancy. Lancet. 2012;380(9838):219–29.
Ramakrishnan R, Doherty A, Smith-Byrne K, Rahimi K, Bennett D, Woodward M, et al. Accelerometer measured physical activity and the incidence of cardiovascular disease: Evidence from the UK Biobank Cohort study. PLoS Med. 2021;18(1):e1003487.
Dempsey P, Strain T, Khaw KT, Wareham N, Brage S, Wijndaele K. Prospective Associations of Accelerometer-Measured Physical Activity and Sedentary Time With Incident Cardiovascular Disease, Cancer, and All-Cause Mortality. Circulation. 2020;141(13):1113–5.
LaCroix AZ, Bellettiere J, Rillamas-Sun E, Di C, Evenson KR, Lewis CE, et al. Association of Light Physical Activity Measured by Accelerometry and Incidence of Coronary Heart Disease and Cardiovascular Disease in Older Women. JAMA Netw Open. 2019;2(3):e190419.
Ballin M, Nordström P, Niklasson J, Nordström A. Associations of Objectively Measured Physical Activity and Sedentary Time with the Risk of Stroke, Myocardial Infarction or All-Cause Mortality in 70-Year-Old Men and Women: A Prospective Cohort Study. Sports Med. 2021;51(2):339–49.
Wannamethee SG, Shaper AG, Lennon L, Morris RW. Metabolic Syndrome vs Framingham Risk Score for Prediction of Coronary Heart Disease, Stroke, and Type 2 Diabetes Mellitus. Arch Intern Med. 2005;165(22):2644–50 (https://jamanetwork.com/ Available from).
Glazer NL, Lyass A, Esliger DW, Blease SJ, Freedson PS, Massaro JM, et al. Sustained and shorter bouts of physical activity are related to cardiovascular health. Med Sci Sports Exerc. 2013;45(1):109–15.
Wennman H, Vasankari T, Borodulin K. Where to Sit? Type of Sitting Matters for the Framingham Cardiovascular Risk Score. AIMS Public Health. 2016;3(3):577–91. https://doi.org/10.3934/publichealth.2016.3.577.
Vasankari V, Husu P, Vähä-Ypyä H, Suni JH, Tokola K, Borodulin K, et al. Subjects with cardiovascular disease or high disease risk are more sedentary and less active than their healthy peers. BMJ Open Sport Exer Med 2018;4(1). http://bmjopensem.bmj.com/ Available from
Niemelä M, Kangas M, Farrahi V, Kiviniemi A, Leinonen AM, Ahola R, et al. Intensity and temporal patterns of physical activity and cardiovascular disease risk in midlife. Prev Med. 2019;1(124):33–41.
LaMonte MJ, Buchner DM, Rillamas-Sun E, Di C, Evenson KR, Bellettiere J, et al. Accelerometer-Measured Physical Activity and Mortality in Women Aged 63 to 99. J Am Geriatr Soc. 2018;66(5):886–94.
Ekelund U, Tarp J, Steene-Johannessen J, Hansen BH, Jefferis B, Fagerland MW, et al. Dose-response associations between accelerometry measured physical activity and sedentary time and all cause mortality: Systematic review and harmonised meta-analysis. BMJ. 2019;366:l4570.
Jefferis BJ, Parsons TJ, Sartini C, Ash S, Lennon LT, Papacosta O, et al. Objectively measured physical activity, sedentary behaviour and all-cause mortality in older men: Does volume of activity matter more than pattern of accumulation? Br J Sports Med. 2019;53(16):1013–20.
Diaz KM, Howard VJ, Hutto B, Colabianchi N, Vena JE, Safford MM, et al. Patterns of sedentary behavior and mortality in U.S. middle-aged and older adults a national cohort study. Ann Intern Med. 2017;167(7):465–75.
Lee IM, Shiroma EJ, Evenson KR, Kamada M, LaCroix AZ, Buring JE. Accelerometer-measured physical activity and sedentary behavior in relation to all-cause mortality: The women’s health study. Circulation. 2018;137(2):203–5.
Ekelund U, Tarp J, Fagerland MW, Johannessen JS, Hansen BH, Jefferis BJ, et al. Joint associations of accelero-meter measured physical activity and sedentary time with all-cause mortality: A harmonised meta-analysis in more than 44 000 middle-aged and older individuals. Br J Sports Med. 2020;54(24):1499–506.
del Pozo CB, Biddle SJH, Gardiner PA, Ding D. Light-Intensity Physical Activity and Life Expectancy: National Health and Nutrition Survey. Am J Prev Med. 2021;61(3):428–33.
Falck RS, McDonald SM, Beets MW, Brazendale K, Liu-Ambrose T. Measurement of physical activity in older adult interventions: a systematic review. Br J Sports Med. 2016;50(8):464–70.
Lee IM, Shiroma EJ. Using accelerometers to measure physical activity in large-scale epidemiological studies: issues and challenges. Br J Sports Med. 2014;48(3):197–201.
DiPietro L, Al-Ansari SS, Biddle SJH, Borodulin K, Bull FC, Buman MP, et al. Advancing the global physical activity agenda: recommendations for future research by the 2020 WHO physical activity and sedentary behavior guidelines development group. Int J Behav Nutr Phys Act. 2020;17(1):143.
Keogh A, Dorn JF, Walsh L, Calvo F, Caulfield B. Comparing the Usability and Acceptability of Wearable Sensors Among Older Irish Adults in a Real-World Context: Observational Study. JMIR Mhealth Uhealth. 2020;8(4):e15704.
Länsitie M, Niemelä M, Kangas M, Venojärvi M, Härkönen P, Keinänen-Kiukaanniemi S, et al. Physical activity profiles and glucose metabolism — A population-based cross-sectional study in older adults. Transl Sports Med. 2021;4(4):439–46.
Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption–II. Addiction. 1993;88(6):791–804.
Marti B, Tuomilehto J, Salomaa V, Kartovaara L, Korhonen HJ, Pietinen P. Body fat distribution in the Finnish population: environmental determinants and predictive power for cardiovascular risk factor levels. J Epidemiol Community Health (1978). 1991;45(2):131–7.
World Health Organization, International Diabetes Federation. Definition and diagnosis of diabetes mellitus and intermediate hyperglycaemia : report of a WHO/IDF consultation. 2006th ed. 2006.
D’Agostino RB, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117(6):743–53.
Hautala A, Martinmaki K, Kiviniemi A, Kinnunen H, Virtanen P, Jaatinen J, et al. Effects of habitual physical activity on response to endurance training. J Sports Sci. 2012;30(6):563–9.
Brugniaux J, Niva A, Pulkkinen I, Laukkanen RM, Richalet JP, Pichon AP. Polar Activity Watch 200: a new device to accurately assess energy expenditure. Br J Sports Med. 2010;44(4):245–9.
Kinnunen H, Tanskanen M, Kyrolainen H, Westerterp KR. Wrist-worn accelerometers in assessment of energy expenditure during intensive training. Physiol Meas. 2012;33(11):1841–54.
Leinonen AM, Ahola R, Kulmala J, Hakonen H, Vaha-Ypya H, Herzig KH, et al. Measuring Physical Activity in Free-Living Conditions-Comparison of Three Accelerometry-Based Methods. Front Physiol. 2017;10(7):681.
LaMonte MJ, Lewis CE, Buchner DM, Evenson KR, Rillamas-Sun E, Di C, et al. Both Light Intensity and Moderate-to-Vigorous Physical Activity Measured by Accelerometry Are Favorably Associated With Cardiometabolic Risk Factors in Older Women: The Objective Physical Activity and Cardiovascular Health (OPACH) Study. J Am Heart Assoc. 2017;6(10):e007064.
van der Velde JHPM, Schaper NC, Stehouwer CDA, van der Kallen CJH, Sep SJS, Schram MT, et al. Which is more important for cardiometabolic health: sedentary time, higher intensity physical activity or cardiorespiratory fitness? The Maastricht Study. Diabetologia. 2018;61(12):2561–9.
Wanner M, Richard A, Martin B, Faeh D, Rohrmann S. Associations between self-reported and objectively measured physical activity, sedentary behavior and overweight/obesity in NHANES 2003–2006. Int J Obes. 2017;41(1):186–93.
Piercy KL, Troiano RP, Ballard RM, Carlson SA, Fulton JE, Galuska DA, et al. The Physical Activity Guidelines for Americans. JAMA. 2018;320(19):2020–8.
Bull FC, Al-Ansari SS, Biddle S, Borodulin K, Buman MP, Cardon G, et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br J Sports Med. 2020;54(24):1451–62.
Moholdt T, Skarpsno ES, Moe B, Nilsen TIL. It is never too late to start: Adherence to physical activity recommendations for 11–22 years and risk of all-cause and cardiovascular disease mortality The HUNT Study. Br J Sports Med. 2021;55(13):743–50.
Dunstan DW, Dogra S, Carter SE, Owen N. Sit less and move more for cardiovascular health: emerging insights and opportunities. Nat Rev Cardiol. 2021;18(9):637–48.
Marcotte RT, Petrucci GJ Jr, Cox MF, Freedson PS, Staudenmayer JW, Sirard JR. Estimating Sedentary Time from a Hip- and Wrist-Worn Accelerometer. Med Sci Sports Exerc. 2020;52(1):225–32.
Acknowledgements
We thank all cohort members who have participated in this study. We gratefully acknowledge the Northern Finland Birth Cohort (NFBC) Project Center for data collection and management.
Funding
This study was supported by the Ministry of Education, Finland; the University of Oulu, Finland; the Oulu Deaconess Institute Foundation sr. Finland; the City of Oulu, Finland; the University Hospital of Oulu, Finland.
Author information
Authors and Affiliations
Contributions
ML and JJ participated in the data collection, analyzing and interpreting the data, and writing the manuscript. MK, MV and MT assisted in interpreting the data and writing the manuscript. SK and RK were responsible for planning the study design, and participated in interpreting the data and writing the manuscript. All the authors revised preliminary versions of the manuscript and read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The baseline and follow-up study were approved by the Ethical Committee of the Northern Ostrobothnia Hospital District in Oulu, Finland (EETTMK 33/2001 and EETTMK 12/2013). The participants provided their informed written consent for the study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Länsitie, M., Kangas, M., Jokelainen, J. et al. Cardiovascular disease risk and all-cause mortality associated with accelerometer-measured physical activity and sedentary time ‒ a prospective population-based study in older adults. BMC Geriatr 22, 729 (2022). https://doi.org/10.1186/s12877-022-03414-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12877-022-03414-8