Abstract
Aims
To investigate the short-term effect of implementing a modified comprehensive geriatric assessment and regularly case conferencing in nursing homes on neuropsychiatric symptoms.
Background
Neuropsychiatric symptoms are common and may persist over time in nursing home residents. Evidence of effective interventions is scarce.
Design
A parallel cluster-randomised controlled trial.
Methods
The intervention was monthly standardised case conferencing in combination with a modified comprehensive geriatric assessment. The control group received care as usual.
Main outcome measure.
The total score on the short version of the Neuropsychiatric Inventory (NPI-Q, 12-items).
Results
A total of 309 residents at 34 long-term care wards in 17 nursing homes (unit of randomisation) were included. The intervention care units conducted on average two case conference-meetings (range 1–3), discussing a mean of 4.8 (range 1–8) residents. After 3 months, there were no difference of NPI-Q total score between the intervention (-0.4) and the control group (0.5) (estimated mean difference = -1.0, 95% CI -2.4 to 0.5, p = 0.19). There was a difference in favour of the intervention group on one of the secondary outcome measures, the apathy symptoms (-0.5 95% CI: -0.9 to -0.1, p = 0.03).
Conclusion
In this study there were no short-term effect of case conferencing and modified comprehensive geriatric assessments after three months on the total score on neuropsychiatric symptoms. The intervention group had less apathy at 3 months follow-up compared to those receiving care as usual. The findings suggest that a more comprehensive intervention is needed to improve the total Neuropsychiatric symptoms burden and complex symptoms.
Trial registration
Due to delays in the organisation, the study was registered after study start, i.e. retrospectively in Clinicaltrials.gov # NCT02790372 at https://clinicaltrials.gov/; Date of clinical trial registration: 03/06/2016.
Similar content being viewed by others
Background
Nursing home (NH) residents are often frail older adults with complex needs due to several concurrent chronic conditions, including dementia [1]. However, the diversity of residents’ needs, ranging from social care needs to palliative care needs, adds to the complexity of NH care [2]. The typical NH residents in Norway, are 85 years on average and approximately 80% have dementia [3].
About 90% of those living with dementia develop at least one neuropsychiatric symptom (NPS) such as agitation, aggression, apathy and depression [4] during the course of their disease [5, 6]. Several reports have defined specific subsyndromes, aggregated into groups of NPS consisting of agitation (agitation/aggression, disinhibition and irritability), affective (depression and anxiety), psychosis (delusions and hallucination), and apathy [6]. Many NPS persist over time [6]. The agitation sub-syndrome and apathy are among the very prevalent and persistent NPS [7]. The severity of affective symptoms, apathy and psychosis can remain relatively stable over time [8].
The aetiology of NPS is mostly unknown, but factors such as neuropathological changes in the brain, unmet psychosocial needs and physical health problems are of importance [9, 10]. Infections, pain and dehydration exemplify common health problems associated with inclining NPS [11,12,13]; these health problems possibly relate to nursing care quality and are therefore modifiable [14, 15]. For people with dementia, non-pharmacological interventions, i.e. psychosocial interventions should be used as the first-line treatment to manage the NPS [16]. Furthermore, to modify NPS, person-centred care and individualised interventions are recommended [16, 17] along with an evaluation of all possible root causes of NPS [18, 19].
Comprehensive geriatric assessment and case conferencing
The term ‘geriatric assessment’ most commonly refers to a clinician’s (primary care clinician or a geriatrician) evaluation of an older adult’s health conditions [20]. However, ‘geriatric assessment’ is also used when referring to a more intensive multidisciplinary program, known as a Comprehensive Geriatric Assessment (CGA) [20] which is an established method in clinical practice to assess older adult’s health [21]. CGA is performed at varying levels of intensity in different settings; accordingly, its content may vary with the healthcare setting [22]. To identify health problems that can be treated for better health outcomes, CGA starts with a systematic evaluation of the adult [20, 21], followed by an individual care plan that explicitly states the individual’s goals of care, who is responsible for achieving these goals, and a timeline for review of progress. Some studies show that the use of CGA contribute to less hospitalizations, higher care quality and lower mortality [20].
Case Conferencing represents one way to implement CGA and follow-up individual care plans [23]. In the following text, the abbreviation CC refers to the case-conferencing working process, while the term CC-meeting refers to the arranged meetings for case-conferencing. The CC-approach promotes a person-centred care perspective since it involves individualised interventions aiming at assessing unmet needs and health problems, thereby the potential to modifying NPS [24]. CC is shown to facilitate communication and coordination between nursing home staff [25]. Regular CC-meetings enable the NH staff to communicate in a structured, systematic and goal-oriented way, and thereby establish a common thought-through understanding of each case [26]. This approach ensure that the resident’s individual needs can be identified and individualised care interventions developed [26].
Even so, research investigating the effect of CC regarding NPS in dementia care is scarce [24]. A systematic review from 2012 reported positive effects of CC on NPS in four out of seven studies [24]. However, none of included studies used CGA as a part of the intervention nor did they use the Neuropsychiatric Inventory-Questionnaire [27] but used other measurements. A recent cluster-randomised controlled trial (c-RCT) study from 2016 reporting short term effects found that a multicomponent intervention including similar approach and comprehensive assessment significantly reduced agitation in NH residents at 8 and 12 weeks [28]. Summarized, large high-quality studies on the effect of CC combined with CGA on NPS in NH are needed [24].
The primary aim of the present study was to investigate the short-term effects of implementing modified CGA (m-CGA) and regularly CC compared to standard practice on total neuropsychiatric symptoms load in nursing home residents. The secondary aim was to investigate the effect on important NPS including the subsyndromes agitation (agitation/aggression, disinhibition and irritability), affective (depression and anxiety), psychosis (delusions and hallucination) and apathy (one item).
Methods
Study design
This was a parallel group c-RCT. The cluster design was chosen to avoid contamination as the intervention was targeted towards the entire staff and potentially all residents in the nursing homes. The study was conducted between April 2015 and May 2016. The CONSORT checklists with extension for cluster-randomised trials and non-pharmacologic trials were used to guide the reporting [29, 30]. There were no changes to the methods after trial commencement. This paper reports the outcome after three months on primary outcome, total NPS, and secondary outcomes on important NPS subsyndromes and apathy. The trial was registered in ClinicalTrial.gov 03/06/2016 (NCT 02,790,372) and approved by the Regional Ethical committee for Medical Research in Western Norway (2014/1642).
Setting
In Norway, the municipalities are responsible for the NH service which are mostly public, financed by taxes and up to 85% of the residents’ retirement income [31]. These NH are typically organised with a director for the whole NH, a varying number of physically separated wards with ward managers, and a number of care units within each ward. Usually, the ward managers are registered nurses (RN). A care unit typically has six to eight beds with one RN and one (at night) to four (during daytime) Licensed Practical Nurses (LPN) along with varying numbers of unskilled nursing assistants. A general practitioner has the medical responsibility and visits the nursing home 1–2 times per week. In Norway there are no established guidelines for admitting persons with dementia and NPS to a special care unit or to geriatric psychiatry. Residents with dementia can be admitted to a special care unit if the nursing home resident demonstrates a challenging behavior for the staff or other residents and if there are available beds. In many cases the nursing home staff will consult geriatric psychiatry ahead of admission.
Participants
Inclusion criterion for the NH was acceptance to partake in the trial. Only regular care units were invited. Special dementia care units with enhanced staffing were excluded. For each unit, at least one RN with at least 3 years work experience and employed in minimum 75% position during the study period was in charge of conducting the intervention.
Inclusion criteria for the residents were: (1) long-term stay, (2) residential time ≥ 60 days, (3) life expectancy ≥ 6 months (evaluated by the RN), and (4) informed consent to participate signed by the resident. In cases where the resident was unable to provide informed consent, the RN informed the next-of kin about the study and asked for consent on behalf of the resident.
Procedure
The recruitment was done in three steps: (1) the NH, (2) the RN in the care units and (3) the residents. NH managers received an e-mail informing them about the study and inviting the NH to participate. The NH managers who accepted to participate recruited the RN based on the inclusion criteria. Participating RN recruited the residents based on the specified inclusion criteria. Before study start, all participating RNs were trained in using the assessment tools used for measuring outcome (see below). These RNs also arranged CC-meetings and were not blinded after allocation to intervention. All NH received payment to compensate for time used on data collection. NH allocated to intervention got additional training in how to conduct the intervention. The participating staff members (RN, LPN and assistants) received the training and performed the m-CGA and CC-meetings during their ordinary working hours. The intervention NH received payment to compensate for training, The NH did not receive additional resources during the study period, rendering their work processes comparable to normal operation. The NH were given facilitation on when to perform the CC-meeting through the study period, but they were in charge on how they would implement them.
Intervention
The intervention comprised a monthly m-CGA of the residents included in the study using validated instruments assessing common physical and psychological health problems associated with NPS, followed by a monthly structured CC-meeting discussing and developing an individual care plan. The participating RN performed m-CGA to facilitate clinical decision-making, and then arranged a CC-meeting with the care team in the NH (Table 1). Two experienced university lecturers in nursing, including the first author, provided a standardized four-hour intervention training course for the NH management and RN separately for each NH (Table 1). The number of participating RN per unit in the training sessions and time from training to inclusion were recorded in a protocol.
Case conference meetings
A RN in each of the care units who participated in the intervention-training course arranged the CC-meetings together with at least two LPN in line with the structure and recommendations shown in Table 1. During the CC-meetings, the participants had specific roles, a chairperson or responsible for the minutes and care plan documentation, in accordance with detailed written information provided by the researchers. The RN arranged at least one monthly CC-meeting for all included residents. About 20 min were scheduled per resident. To avoid meetings exceeding 90 min, care units with more than six residents arranged two monthly CC-meetings. Further details about the CC-meetings are published previously [32].
Modified Comprehensive Geriatric Assessment (m-CGA)
The RN in each unit assessed the NH residents by a set of recommended geriatric assessment instruments (see Table 1 and procedures above). The results of the assessment were then used for support in the clinical decision process during the CC-meetings.
-
The Physical Self Maintenance Scale (PSMS) [33] assessed performance of activities of daily living. This scale consists of six items scaled from total independence [1] to total dependence [5], with a total score ranging from 6–30. Higher score indicates greater dependency.
-
The Cornell Scale for Depression in Dementia (CSDD) [34], Norwegian version [35], assessed depression symptoms. The score ranges between 0 and 38; higher scores indicate more depressive symptoms. The CSDD has recently been validated in Norwegian NH residents and the psychometric properties are acceptable [36].
-
The Clinical Dementia Rating (CDR) scale assessed severity of dementia. The CDR covers six domains (memory, orientation, judgment and problem solving, community affairs, home and hobbies, and personal care). Each domain has five response categories (0, 0.5, 1, 2, 3) [37, 38]. The CDR standard global score is calculated by means of an algorithm giving priority to memory (https://www.alz.washington.edu/cdrnacc.html).
-
Quality of Life in Late-Stage Dementia (QUALID) scale [39], Norwegian version [40]. For each resident, the frequency of 11 observable behaviours during the previous week was registered (total score range 11–55). A higher score indicates poorer QoL.
-
The Brief agitation rating scale (BARS) [41] is a subscale of the Cohen-Mansfield Agitation Inventory (CMAI) [42]. If any NPI-Q aggression/agitation items score was ≥ 1, the BARS was included in the monthly m-CGA. The Norwegian version of BARS [43] consists of 10 items: hitting, pushing, grabbing, pacing, restlessness, repetitive sentences, and repetitive mannerisms, complaining and making strange noises. The frequencies of these symptoms are rated from 1 (never) to 7 (several times per hour), reporting the frequency of the agitated behaviour during the preceding 2 weeks retrospectively. The sum score ranges from 10 to 70, with a higher score indicating more agitation [41].
-
The 24-h registration of behaviour form includes a table showing hours in the horizontal rows and days in the columns. By using different colour codes for the various observed behaviours, this form visualizes when the observed behaviour occurs. The observation period could be a week or more. This symptom profile identifies and helps to understand environmental triggers of behaviour. Furthermore, this form portrays the frequency of behavioural events throughout the day (https://www.aldringoghelse.no/skalaer-og-tester/#dognregistreringsskjema-registreringsskjema-for-atferd). It was utilized as an additional measure in the m-CGA to help the staff getting a clearer picture of NPS.
Measures
All participants' age, gender and clinical background (PSMS, CSDD and CDR were assessed at baseline (Table 2).
Outcomes
This paper reports on the outcomes measured with the short version Neuropsychiatric Inventory-Questionnaire (NPI-Q, 12-items) [44]. The NPI-Q is adapted from the NPI, a validated informant-based interview that assesses NPS during the previous month [27]. The NPI-Q includes the following 12 NPS: delusion, hallucination, agitation/aggression, depression/dysphoria, anxiety, apathy/indifference, irritability/lability, euphoria, disinhibition, aberrant motor behaviour, night-time behaviour, and appetite and eating disorders, one item each. These symptoms are registered as present or not. If present, the severity of the symptom is scaled from 1 (Mild), 2 (Moderate) to 3 (Severe), which gives a range in total score from 1 to 36 (higher score indicates more severe symptoms). The NPI-Q was assessed at baseline and after three months in both groups.
Primary outcome
The primary outcome was the change from baseline to three months in total score of NPI-Q 12 items.
Secondary outcomes
The secondary outcomes consisted of changes from baseline to three months in the score of the three sub-syndrome sum-scores of the NPI-Q 12 items: 1) agitation constructed by three items (agitation/aggression, irritability, and disinhibition giving a score range of 0–9), 2) affective symptoms (depression and anxiety, score range 0–6), 3) psychosis (delusions and hallucination, score range 0–6) and 4) apathy symptoms (a single item, score range 0–3) [45].
Intervention compliance
The RN chairing the CC-meeting recorded in a logbook the date for the meeting, who participated, and the initials of the resident in focus. The logbook was collected at the end of the trial. Based on these logs, the number of CC-meetings per resident and care unit as well as number of residents per CC-meeting and the attending staff were calculated.
Sample size
The sample size was calculated to detect an effect size of 0.4 (small to moderate) on NPI-Q [46]. Based on previous research, we assumed an intra-cluster correlation coefficient (ICC) within the NH of p = 0.04 [46]. A power of 80% with a significance level of 5% required 380 residents in 10 intervention clusters and 10 control clusters, with on average 19 residents in each cluster. No information about the actual cluster-sizes were available when performing the power calculation, thus an equal cluster-size was assumed. The calculations were performed with IBM SPSS Sample Power 3.0 (IBM Corp., Armonk, N.Y., USA).
Randomisation and allocation
The NH was defined as a single cluster and was randomised. We assumed that the NH size could influence on how the intervention was delivered; therefore, the randomisation included stratification for NH size into three blocks: small (9–25 residents; 4 NH), medium (26–59 residents; 11 NH), and large (> 60 residents; 2 NH). The trial service at Norwegian University of Science and Technology conducted a computer-generated randomisation and kept the allocation concealed until the baseline data were completed. Information about allocation was then given to the first author (GTS) who informed the NH managers about the allocation by e-mail.
Statistical methods
A statistician with no knowledge about the intervention contributed to the analyses. There was some skewness in most variables, however, overall, the baseline characteristics and outcome variables seemed approximately normally distributed. The baseline characteristics of the groups were compared using independent sample t-test for continuous variables and a Chi-squared test for categorical variables. The outcomes were analysed according to intention-to-treat (ITT). Mixed linear models assessed the effects of the intervention, with change in scores from baseline to 3 months follow-up as the dependent outcome variable and treatment specified as a fixed effect. To allow for a potential cluster effect on change scores, the ID of the nursing homes was included as a random effect and the intra-cluster correlation coefficient (ICC) on change scores is reported [47]. Statistical calculations were performed using the software R (The R Project for Statistical Computing), version 2.131 and the IBM SPSS Statistics for Windows, version 22 (IBM Corp., Armonk, N.Y., USA).
In addition, a per-protocol analysis was conducted. The criterion for per-protocol was that the care units completed at least 50% of the CC-meetings with at least one RN and two LPNs present.
Results
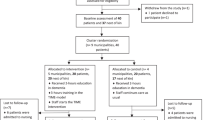
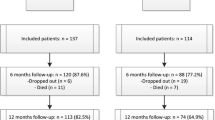
A total of 118 NH was approached, out of which 17 NH participated (Fig. 1). The participating NH had totally 35 wards, 61 care units and 594 residents. Of these, 309 residents (55.6% of those eligible) in 59 care units organised into 34 wards participated.
Eight NH (159 residents, 19 wards organised in 28 care units) were randomised to the intervention group and nine NH (150 residents in 15 wards organised in 29 care units) to the control group. The groups were similar at baseline (Table 2) with 68% being female, and the mean number of residents per care unit in the interventions group was 5.7 (range 1–13) and 5.2 (range 1 to 11) in the control group.
Drop-outs
Compared to those alive after three months, the 38 residents who died had a significant higher baseline score on PSMS and CDR. The 271 remaining residents had no missing data.
Implementation of the intervention
In total 36 RNs from the 28 intervention care units participated in the CC training course. The time between baseline assessment and intervention initiation varied between 5 to 9 days. During the three months intervention period, the intervention care units conducted on average two CC-meetings (range 1–3), with a mean of 4.8 (range 1–8) residents discussed at each CC-meeting. On average, each resident was discussed on 2.1 (range 1- 3) CC-meetings. Usually, one RN (range 1–2) and two LPNs (range 1–4) were present at the CC-meetings. Nineteen (68%) of the 28 care units performed at least two CC-meetings with one RN and two LPNs present. The nursing staff agreed upon the most urgent health problems or unmet needs which they wanted to target without use of pharmacological intervention (Table 1).
Between-group differences
The ITT analyses revealed no difference of change in NPI-Q 12 items total score between the intervention (-0.4) and the control group (0.5) (estimated mean difference = -1.0, 95% CI -2.4 to 0.5, p = 0.19) (Table 3).
For the secondary outcomes, the NPI-sub-syndromes agitation, affection, and psychosis, there was no effects of the intervention (Table 3). For the single item on apathy, there was a difference in favour of the intervention group (estimated mean difference = -0.5, 95% CI -0.9 to -0.05, p = 0.03). The per-protocol analysis gave the similar results as the intention to treat analysis and is thus not reported.
Within-group changes
There were no within-group changes for any of the outcomes from baseline to 3 months (Table 3).
Discussion
The present c-RCT study aimed to investigate if m-CGA in combination with the CC-intervention for 3 months could reduce NPS in general. There were no short-term effects of the m-CGA and CC-intervention on changes in neuropsychiatric total symptom score (NPI-Q 12 items), but there was an effect on the secondary outcome apathy.
The NPS total score was chosen as the primary outcome since the resident’s limitations, needs and difficulties probably contribute to NPS [11,12,13, 15]. However, no between-group or within-group effect of the intervention was found on the primary outcome. The nursing intervention on a specific NPS such as agitation will vary from interventions of other types of symptoms. This was supported in another c-RCT aiming to reduce NPS in Norwegian NH residents that was ongoing simultaneously with our study [28]. This study used a multi-component and multi-disciplinary intervention program for three months, and the primary aim for their intervention was to create a mutual understanding of NPS and to tailor a detailed treatment including both pharmacological and non-pharmacological actions to reduce agitation [28]. The secondary outcomes concerning the total NPS score, apathy or any sub-syndrome in this study was not significant [28].
High age, frailty and infirmities characterize the Norwegian NH population [48]. This was also the case in this study, making the findings generalizable as all residents in the participating care units were included regardless of level and type of NPS. This is a likely an explanation why their NPS load at baseline was quite mild, with a mean NPI-Q score of 4.8 (range 0–36). The effect of the CC-intervention, if implemented in residents with higher intensity or directed toward a specific NPS like aggression remains unknown. Further studies focusing on NPS can thus be recommended to have an inclusion criteria for NPS load to ensure that the residents in most need of interventions targeting NPS symptoms are included. It may also indicate that interventions in studies similar to our study should be more specific and targeted toward specific symptoms. All residents in the intervention NH were included and no specific type of NPS were prioritised, rather the care plans comprised a multimodal and holistic set of individualised nursing interventions.
The present study showed a between-group difference after three months in favour of the intervention group on apathy. We do not have a firm explanation for why a between-group difference was found for apathy and not for affective or psychotic field of NPS. However, as reported previously, apathy is one of the most prevalent and persistent NPS among NH residents [7]. Some evidence suggests that apathy among residents with dementia could benefit from individualized treatment using non-pharmacologic interventions [16]. Accordingly, when developing an individual care plan including m-CGA the staff takes into account the residents’ past preference for activities, functional resources and environmental factors [49]. These areas were emphasised in the execution of CC-meetings in the present study and could be a plausible explanation for the reduction of apathy. Research has shown that reducing apathy might increase quality of life [50] and higher level of apathy is associated with carer distress [51]. Furthermore, CC may have increased the awareness of the residents’ psychosocial situation, accompanied with an adapted way of interacting with these residents. However, the present study did not record the specific initiated nursing interventions following the CC, neither observed if the care interactions became more health promoting.
This study implemented a complex intervention that was designed to facilitate the nursing home staff to improve the planning and coordination of the nursing interventions to meet residents’ needs and well-being possibly related to NPS. This was supported by a connected qualitative study in four of the included NH disclosing that nursing interventions and their evaluations developed to be more effective, timely, better planned and coordinated [32]. Thus, the residents’ needs, and problems were better handled after partaking in this trial.
Strength and limitations
The strengths of our study are the cluster-randomised controlled design, the use of internationally recommended assessment tools, the large number of residents included, and the local anchoring with the NH RNs implementing the intervention. The real-world or pragmatic context for the study allowed nursing home staff to conduct their work without being observed and influenced, eliminating any potential occurrence of the Hawthorne or Observer effect. The NH did not receive additional resources, rendering work processes comparable to normal operation. In addition, it was adjusted for the effect of clustering.
Nevertheless, some limitations must be considered. It has a short follow up time. Special care units were excluded as they are not comparable to regular units regarding number of beds and staffing levels. The study was designed with limited blinding; a statistician did the analyses blinded for allocation to intervention and control groups. However, since this intervention involved the whole staff, blinding was not possible. Despite the large sample in our study, we did not achieve the number of NH (17 vs. 20) and residents (309 vs. 380) required by the power calculation. By randomizing the nursing homes and not the units within the nursing homes, differences between the nursing homes could potentially influence the results. However, by doing a stratified randomization on the size of the nursing homes, this limitation is considered to be small. The use of proxies (RN) to conduct the outcome assessments are always based on their knowledge and observations of the residents and may not fully be in accordance with the resident’s situation. On the other hand, reliability and validity of proxy data are found to be high for tasks of daily living and health conditions which may be easily observed [52]. In the Norwegian NH setting, most residents have cognitive impairment [3], and the proportion of residents with severe degree of dementia is considerable [3]. Thus, use of self-report questionnaires may not be trustworthy. We ensured that NPS was measured by a proxy who know the resident best [27]. Those assessing the residents knew the residents well and had got training/education in the assessment tools and the scoring, minimalizing the risk of unreliable assessment of residents. The baseline subsyndrome scores of NPI-Q in the present study were in line with another Norwegian study of NH residents [53]. However, the NPI-Q sum score and the subsyndrome scores were low and we cannot rule out the possibility of having a floor effect.
Conclusions
The present cluster-randomised controlled trial found no change in total NPS after three months; however, the intervention group had less apathy at 3 months follow-up compared to those receiving care as usual. This finding should be further investigated in studies targeting one or a few NPS including only NH residents with dementia.
Relevance for clinical practice
This study did not give conclusive findings for short-term effect of CC and m-CGA on NPS, and longer follow up is needed to suggest conclusions on clinical relevance. However, this type of interventions might have an impact on the quality of care by contributing to a systematic and regular assessment of NH residents’ individual needs and focusing on clinical decision-making. This could contribute to strengthening person-centered care by focusing on the resident’s health problems and needs. This study also demonstrates that implementing CC in combination with m-CGA is a feasible way to develop and follow-up individual care plans, as shown previous research [23]. Based on the experiences from this study, implementation strategies should take into account time for training of the staff and time for the nursing home as organisation to adapt to changes in routines. Hence, there is a need for more research on how nursing interventions are planned and implemented in NH. There is also a need for more research focusing on the other dimensions of quality of care, i.e. how do CC and m-CGA contribute to better care planning and quality care for the residents with NPS.
Availability of data and materials
The datasets generated and/or analyzed during the current study will be available from the corresponding author on reasonable request. The reason is that it is not possible to share all data unless it is de-identified.
References
Lane NE, Wodchis WP, Boyd CM, Stukel TA. Disability in long-term care residents explained by prevalent geriatric syndromes, not long-term care home characteristics: a cross-sectional study. BMC Geriatr. 2017;17(1):49.
Nakrem S, Vinsnes AG, Seim A. Residents’ experiences of interpersonal factors in nursing home care: A qualitative study. Int J Nurs Stud. 2011;48(11):1357–66.
Helvik AS, Engedal K, Benth JS, Selbaek G. Prevalence and Severity of Dementia in Nursing Home Residents. Dement Geriatr Cogn Disord. 2015;40(3–4):166–77.
Edberg AK, Bird M, Richards DA, Woods R, Keeley P, Davis-Quarrell V. Strain in nursing care of people with dementia: nurses’ experience in Australia, Sweden and United Kingdom. Aging Ment Health. 2008;12(2):236–43.
Zuidema S, Koopmans R, Verhey F. Prevalence and predictors of neuropsychiatric symptoms in cognitively impaired nursing home patients. J Geriatr Psychiatry Neurol. 2007;20(1):41–9.
Helvik A, Selbæk G, SaltyteBenth J, Røen I, Bergh S. Correction: The course of neuropsychiatric symptoms in nursing home residents from admission to 30-month follow-up. Plos One. 2018;13:e0209875.
van der Linde RM, Matthews FE, Dening T, Brayne C. Patterns and persistence of behavioural and psychological symptoms in those with cognitive impairment: the importance of apathy. Int J Geriatr Psychiatry. 2017;32(3):306–15.
Helvik AS, Engedal K, Wu B, Benth J, Corazzini K, Røen I, et al. Severity of Neuropsychiatric Symptoms in Nursing Home Residents. Dementia and geriatric cognitive disorders extra. 2016;6(1):28–42.
Cohen-Mansfield J. Theoretical frameworks for behavioral problems in dementia. Alzheimer’s Care Today. 2000;1(4):8.
Kovach CR, Noonan PE, Schlidt AM, Wells T. A Model of Consequences of Need-Driven, Dementia-Compromised Behavior. J Nurs Scholarsh. 2005;37(2):134–40.
Hodgson NA, Gitlin LN, Winter L, Czekanski K. Undiagnosed Illness and Neuropsychiatric Behaviors in Community Residing Older Adults With Dementia. Alzheimer Dis Assoc Disord. 2011;25(2):109–15.
Léger JM, Moulias R, Robert P, Vellas B, Chapuy PH, Monfort JC, et al. Agitation and aggressiveness among the elderly population living in nursing or retirement homes in France. International psychogeriatrics / IPA. 2002;14(4):405–16.
Rostad HM, Grov EK, Utne I, Halvorsrud L. Characteristics of nursing home residents with late-stage dementia and pain. Sykepleien Forskning 2019;14(79184):e-79184.
Nelson ST, Flynn L. Relationship between missed care and urinary tract infections in nursing homes. Geriatr Nurs. 2015;36(2):126–30.
Tible OP, Riese F, Savaskan E, von Gunten A. Best practice in the management of behavioural and psychological symptoms of dementia. Ther Adv Neurol Disord. 2017;10(8):297–309.
National Institute for Health and Care Excellence (NICE). Dementia: Supporting People with Dementia and their Carerers in Health and Social Care. London: NICE; 2006.
Ford AH. Neuropsychiatric aspects of dementia. Maturitas. 2014;79(2):209–15.
Whall AL, Kolanowski AM. The need-driven dementia-compromised behavior model–a framework for understanding the behavioral symptoms of dementia. Aging Ment Health. 2004;8(2):106.
Vernooij-Dassen M, Vasse E, Zuidema S, Cohen-Mansfield J, Moyle W. Psychosocial interventions for dementia patients in long-term care. Int Psychogeriatr. 2010;22(7):1121–8.
Ward KT, Reuben DB. Comprehensive Geriatric Assessment https://www.uptodate.com/contents/comprehensive-geriatric-assessment. UpToDate: Alphen aan den Rijn: Wolters Kluwer; 2018.
Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. The Lancet. 2013;381(9868):752–62.
Pilotto A, Cella A, Pilotto A, Daragjati J, Veronese N, Musacchio C, et al. Three decades of comprehensive geriatric assessment: evidence coming from different healthcare settings and specific clinical conditions. J Am Med Dir Assoc. 2017;18(2):192.e1-192.e11.
Bird M, Llewellyn-Jones RH, Korten A. An evaluation of the effectiveness of a case-specific approach to challenging behaviour associated with dementia. Aging Ment Health. 2009;13(1):73–83.
Reuther S, Dichter MN, Buscher I, Vollmar HC, Holle D, Bartholomeyczik S, et al. Case conferences as interventions dealing with the challenging behavior of people with dementia in nursing homes: a systematic review. International psychogeriatrics / IPA. 2012;24(12):1891–903.
Phillips JL, West PA, Davidson PM, Agar M. Does case conferencing for people with advanced dementia living in nursing homes improve care outcomes? Evidence from an integrative review. Int J Nurs Stud. 2013;50(8):1122–35.
Lichtwarck B, Myhre J, Goyal AR, Rokstad A, Selbaek G, Kirkevold Ø, Bergh S. Experiences of nursing home staff using the targeted interdisciplinary model for evaluation and treatment of neuropsychiatric symptoms (TIME) - a qualitative study. Aging & mental health. 2019;23(8):966–75. https://doi.org/10.1080/13607863.2018.1464116.
Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology. 1994;44(12):2308–14.
Lichtwarck B, Selbaek G, Kirkevold Ø, Rokstad AMM, Benth JŠ, Lindstrøm JC, et al. Targeted Interdisciplinary Model for Evaluation and Treatment of Neuropsychiatric Symptoms: A Cluster Randomized Controlled Trial. Am J Geriatr Psychiatry. 2018;26(1):25–38.
Campbell M, Elbourne D, Altman D, group ftC. The CONSORT statement: extension to cluster randomised trials. BMJ. 2004;328:702–8.
Boutron I, Altman DG, Moher D, Schulz KF, Ravaud P, for the CNPTG. Consort statement for randomized trials of nonpharmacologic treatments: A 2017 update and a consort extension for nonpharmacologic trial abstracts. Ann Intern Med. 2017;167(1):40–7.
Eurofound. Care homes for older Europeans: Public, for-profit and non-profit providers. 2017. Available from: https://www.alzheimer-europe.org/var/plain_site/storage/original/application/f5253c2c572b6e0c28b45922149d2289.pdf.
Nakrem S, Stensvik GT, Skjong RJ, Ostaszkiewicz J. Staff experiences with implementing a case conferencing care model in nursing homes: a focus group study. BMC Health Serv Res. 2019;19(1):191.
Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9(3):179–86.
Alexopoulos GS, Abrams RC, Young RC, Shamoian CA. Cornell Scale for Depression in Dementia. Biol Psychiat. 1988;23(3):271–84.
Barca ML, Engedal K, Selbæk G. A Reliability and Validity Study of the Cornell Scale among Elderly Inpatients, Using Various Clinical Criteria. Dement Geriatr Cogn Disord. 2010;29(5):438–47.
Stensvik GT, Helvik AS, Nakrem S, Haugan G. Cornell’s Depression for Dementia Scale: A psychometric study among Norwegian nursing home residents. Arch Gerontol Geriatr. 2021;93:104325.
Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL. A new clinical scale for the staging of dementia. Br J Psychiatry. 1982;140(6):566–72.
Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43(11):2412–4.
Weiner MF, Martin-Cook K, Svetlik DA, Saine K, Foster B, Fontaine CS. The quality of life in late-stage dementia (QUALID) scale. J Am Med Dir Assoc. 2000;1(3):114–6.
Roen I, Selbaek G, Kirkevold O, Engedal K, Lerdal A, Bergh S. The Reliability and Validity of the Norwegian Version of the Quality of Life in Late-Stage Dementia Scale. Dement Geriatr Cogn Disord. 2015;40(3–4):233–42.
Finkel SI, Lyons JS, Anderson RL. A brief agitation rating scale (BARS) for nursing home elderly. J Am Geriatr Soc. 1993;41(1):50–2.
Cohen-Mansfield J. Conceptualization of agitation: results based on the Cohen-Mansfield Agitation Inventory and the Agitation Behavior Mapping Instrument. International psychogeriatrics. 1996;8(Suppl 3):309–54. https://doi.org/10.1017/s1041610297003530.
Sommer OH, Engedal K. Reliability and validity of the Norwegian version of the Brief Agitation Rating Scale (BARS) in dementia. Aging Ment Health. 2011;15(2):252–8.
Kaufer DI, Cummings JL, Ketchel P, Smith V, MacMillan A, Shelley T, et al. Validation of the NPI-Q, a brief clinical form of the Neuropsychiatric Inventory. J Neuropsychiatry Clin Neurosci. 2000;12(2):233–9.
Selbæk G, Engedal K, Benth JŠ, Bergh S. The course of neuropsychiatric symptoms in nursing-home patients with dementia over a 53-month follow-up period. Int Psychogeriatr. 2014;26(01):81–91.
Rapp MA, Mell T, Majic T, Treusch Y, Nordheim J, Niemann-Mirmehdi M, et al. Agitation in nursing home residents with dementia (VIDEANT trial): Effects of a cluster-randomized, controlled, guideline implementation trial. J Am Med Dir Assoc. 2013;14(9):690–5.
Campbell M, Grimshaw JM, Elbourne DR. Intracluster correlation coefficients in cluster randomized trials: empirical insights into how should they be reported. BMC Med Res Methodol. 2004;4:9.
Haugan G. Meaning-in-life in nursing-home patients: a correlate with physical and emotional symptoms. J Clin Nurs. 2014;23(7–8):1030–43.
Theleritis C, Siarkos K, Politis AA, Katirtzoglou E, Politis A. A systematic review of non-pharmacological treatments for apathy in dementia. Int J Geriatr Psychiatry. 2018;33(2):e177–92.
Helvik AS, Bergh S, Šaltytė Benth J, Selbaek G, Husebo BS, Tevik K. Pain in nursing home residents with dementia and its association to quality of life. Aging & mental health. 2021;1–11. Advance online publication. https://doi.org/10.1080/13607863.2021.1947968.
Terum TM, Testad I, Rongve A, Aarsland D, Svendsboe E, Andersen JR. The association between aspects of carer distress and time until nursing home admission in persons with Alzheimer’s disease and dementia with Lewy bodies. Int Psychogeriatr / IPA. 2021;33(4):337–45.
Lynn Snow A, Cook KF, Lin PS, Morgan RO, Magaziner J. Proxies and other external raters: methodological considerations. Health Serv Res. 2005;40(5 Pt 2):1676–93.
Goyal AR, Bergh S, Engedal K, Kirkevold M, Kirkevold Ø. The course of anxiety in persons with dementia in Norwegian nursing homes: A 12-month follow-up study. J Affect Disord. 2018;235:117–23.
Acknowledgements
The authors would like to thank the nurses and patients who voluntarily participated in the study.
Funding
The study was funded by Sør-Trøndelag University College/NTNU Department of Public Health and Nursing, and The Norwegian Nurses Organisation.
Author information
Authors and Affiliations
Contributions
GTS: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Resources, Data Curation, Writing—original draft, Writing—review & editing. ASH: Methodology, Validation, Writing—original draft, Writing—review & editing. GH: Conceptualization, Methodology, Validation, Writing—original draft, Writing—review & editing. AS: Conceptualization, Methodology, Writing—original draft, Writing—review & editing. ØS: Formal analysis. SN: Conceptualization, Methodology, Formal analysis, Writing—original draft, Writing—review & editing. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All examination protocols were approved by the Regional Ethical committee for Medical Research in Western Norway (2014/1642) and were conducted in accordance with the ethical principles stated in the Declaration of Helsinki. We obtained written informed consent prior to the study from all participants.
Consent for publication
Not Applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Stensvik, GT., Helvik, AS., Haugan, G. et al. The short-term effect of a modified comprehensive geriatric assessment and regularly case conferencing on neuropsychiatric symptoms in nursing homes: a cluster randomized trial. BMC Geriatr 22, 316 (2022). https://doi.org/10.1186/s12877-022-02976-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12877-022-02976-x