Abstract
Background
To study the relationship between objective daily physical activity (PA), as measured by implantable cardioverter defibrillators (ICDs)/cardiac resynchronization therapy defibrillators (CRTDs), and long-term prognoses in patients with age > 75 years at high risk of sudden cardiac death (SCD).
Methods
In total, 133 patients with age > 75 years old (age 79.52 ± 3.68 years) in the SUMMIT study were retrospectively analysed. The major endpoint was all-cause mortality, and the minor endpoint was cardiac death.
Results
The mean follow-up time was 57.1 ± 24.2 months (range: from 4 to 96 months). In total, 46 all-cause mortality and 23 cardiac death events occurred. The receiver operating characteristic curve indicated a baseline PA cut-off value of 6.47% (93 min/day) can predict all-cause mortality in patients with age > 75 years, with an area under the curve of 0.670 (95% confidence interval (CI): 0.573–0.767, P = 0.001). The sensitivity was 67.4%, and the specificity was 66.7%. Patients with baseline PA ≤ 6.47% had higher rates of all-cause mortality (51.7% vs 20.5%, P < 0.001) and cardiac death (25.0% vs 11.0%, P = 0.040). The estimated Kaplan-Meier survival curves showed that patients with PA ≤ 6.47% had an increased cumulative incidence of all-cause mortality (Log-rank P < 0.0001) and cardiac death (Log-rank P = 0.0067). Multivariate Cox regression analysis showed that PA ≤ 6.47% was an independent predictor of all-cause mortality (hazard ratio (HR) 3.137, 95% CI: 1.667–5.904, P < 0.001) and cardiac death (HR value 3.345, 95% CI: 1.394–8.028, P = 0.007).
Conclusions
Daily PA of about 1.5 h was associated with lower all-cause mortality and cardiac death risk in patients with age > 75 years and high risk of SCD with ICDs/CRTDs. PA monitoring may aid in long-term management of older patients at high risk of SCD.
Similar content being viewed by others
Background
The global population is ageing rapidly, leading to an increased burden on the health and economic systems [1]. Cardiovascular disease has been the major cause of mortality in older people [2]. Sudden cardiac death (SCD), as a serious public health problem worldwide, accounts for virtually half of all cardiovascular deaths [3]. An implantable cardioverter defibrillator (ICD) can effectively terminate malignant tachyarrhythmia, prevent SCD and improve survival rate [4]. A number of studies indicate physical activity (PA) is related to many chronic diseases, premature mortality, and poor cardiovascular prognoses [5, 6]. However, PA is a safe, low-cost, environmentally friendly, easily accessible treatment strategy that is often not implemented in clinical practice. For older people in particular, physical activities are often limited. Survey results have shown that a low proportion of patients, especially older patients, perform the recommended amount of PA [7, 8].
Most of the previous studies on PA and cardiovascular diseases used self-assessment questionnaires, which have a certain level of bias and error, such as recall biases, especially for older people due to their levels of education and cognitive function [9, 10]. In addition, most of the populations in previous studies generally comprised middle-aged adults. Studies have shown that PA recorded by remote cardiovascular implantable electronic devices (CIEDs) is related to cardiovascular prognoses [11]. With the extension of life expectancy and continuous advancements in CIED implantation technology, an increasing number of old people undergo CIED implantation, and the proportion of old people implanted with CIEDs is also increasing. The relationship between PA and cardiovascular prognoses patients with age > 75 years and implantable cardioverter defibrillators (ICDs)/cardiac resynchronization therapy defibrillators (CRTDs) is not well established.
In the present study, continuous PA was recorded by ICDs/CRTDs, and its correlation with all-cause mortality and cardiac death in old population over 75 years old was investigated.
Methods
Population
Home monitoring (HM) transmission data archived between June 2010 and August 2014 from the SUMMIT registry (Study of Home Monitoring System Safety and Efficacy in Cardiac Implantable Electronic Device-Implantable Patients) in China were retrospectively analysed. For continuous patient monitoring, the HM setting on all devices was turned “on”. The present study complied with the principles of the Declaration of Helsinki and was approved by ethics committee of Fuwai Hospital (the chief institute) and all other participating organizations (Zhongshan Hospital Fudan University et al.). All patients signed informed consent forms before enrollment.
Among those participants, patients with an age older than or equal to 75 years, eligibility for an ICD or a CRTD according to the recommended indications, an implanted ICD/CRTD (Biotronik, Germany) device with HM and surviving more than 3 months after the device implantation were selected. While patients who could not be followed up or had missing HM data, patients with neoplastic diseases or a life expectancy of less than 1 year or patients with disabilities and other diseases that restricted their daily activities were excluded.
Baseline characteristics
Baseline characteristics for all admitted patients in this study were obtained from the patients’ medical records before implantation, including demographic characteristics (age, sex, body mass index (BMI), the New York Heart Association (NYHA) class, comorbidities (ischemic cardiomyopathy, hypertension, diabetes (DM), atrial fibrillation (AF), stroke, syncope), echocardiographic indices (left ventricular ejection fraction (LVEF), left ventricular end diastolic diameter (LVEDD), and medication (renin angiotensin system blocker, beta blocker, diuretic, and amiodarone). All data were obtained and assesed by two independent physicians who were blinded to the other results.
PA definition and measurement
PA in ICD/CRT-D was measured through an integrated circuit accelerometer embedded in the pulse generator. PA was measured as the time during which the Biotronik devices’ motion sensors delivered rates higher than the devices’ basic rates. The threshold value judged as “active” was corresponding to 2 metabolic equivalents (METs) [11, 12]. The percentage of active time per day was recorded as the daily PA; for example, 10% PA indicated 2.4 h of daily PA. The Biotronik remote monitoring system automatically transmitted the data stored in the implantable devices to the Biotronik service centre every day.
As the level of PA after implantation was considered to be less than usual, the data were collected during the first 30–60 days after ICD/CRTD implantation, in accordance with the previous study, and the mean value of the 30-day PA data was used as the baseline PA for each patient.
Follow-up and endpoints
Follow-up was conducted regularly for all patients enrolled. If the patient’s daily transmission was interrupted, the clinical research coordinator immediately contacted his or her family to confirm the patient’s condition. The primary endpoint of this study was all-cause mortality, and the secondary endpoint was cardiac death. The cause of death was obtained by contact with the family and was determined by the death certificate.
Statistical methods
Software SPSS Statistics version 23.0 (IBM Corp., Armonk, New York) and GraphPad Prism software version 7.0 (GraphPad Software, La Jolla, California) were used to perform all statistical analysis. Continuous variables were presented as the mean ± SD and compared using Student’s t-test of variance. Categorical data were presented as numbers (percentages) and compared with Chi-square tests. Dot plot was conducted to compare PA levels between patients who died and survived. Receiver operating characteristic curves were plotted to obtain a cut-off value for quantitative variables. The categories of PA ≤ 6.47% and PA > 6.47% were used for the calculations performed. We performed Kaplan-Meier curves to compare the difference between groups regarding the endpoints and the Log-rank test was used. Then Cox proportional hazard models were used and all variables that had a statistically significant effect were introduced into a multivariate Cox proportional hazards model. Hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated to determine the impact. Two-sided P value < 0.05 was considered significant in all tests.
Results
Baseline characteristics
In total, 133 patients over 75 years old with ICDs/CRTDs were included in the present study. The average age was 79.52 ± 3.68 years old. The study cohort predominantly comprised males (74.4%). The mean baseline PA was 7.85% ± 4.64%. Receiver operating characteristic curve analysis determined that a PA cut-off value of 6.47% (93 min/day) can predict all-cause mortality. The area under the curve was 0.670 (95% CI: 0.573–0.767, P = 0.001), with a sensitivity of 67.4% and a specificity of 66.7% (Fig. 1).
All eligible patients were grouped by the PA cut-off value. Comparisons of patients’ baseline characteristics are presented in Table 1. Patients whose PA ≤ 6.47% had poorer NYHA classifications, had more cases of DM, and had taken more diuretics, although the difference did not reach statistical significance. There were no differences between the two groups in the other baseline characteristics.
Clinical outcomes
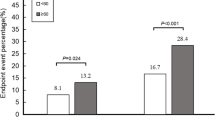
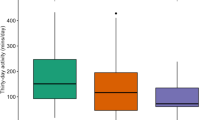
The average follow-up period was 57.1 ± 24.2 months (range: from 4 to 96 months). In total, 46 all-cause deaths (34.6%) and 23 cardiac deaths (17.3%) occurred. Dot plot was conducted and the result showed that PA levels were significantly higher in patients who survived [8.51 (5.7–10.9)%] than in those died [5.48(3.82–7.68)%], P = 0.0029 (Fig. 2). Patients with baseline PA ≤ 6.47% had higher rates of all-cause mortality (51.7% vs 20.5%, P < 0.001) and cardiac death (25.0% vs 11.0%, P = 0.040) (Table 2). Moreover, baseline PA ≤ 6.47% was also significantly associated with non-cardiac death (26.7% vs 9.6%, P = 0.01).
Kaplan-Meier survival curves
The estimated Kaplan-Meier survival curves showed that patients with PA ≤ 6.47% had an increased cumulative incidence of all-cause mortality (Log-rank P < 0.0001) (Fig. 3) and cardiac death (Log-rank P = 0.0067) (Fig. 4).
PA is a predictor of cardiac death and all-cause mortality
In the univariate Cox regression models, PA ≤ 6.47% was significantly related to all-cause mortality (HR 3.297, 95% CI: 1.777–6.118, P < 0.001). After the model was adjusted for confounders, including age, sex, LVEF, the presence of ischaemic cardiomyopathy, the presence of diabetes, the presence of AF and β-blocker consumption, the multivariate Cox regression modelling results showed that PA ≤ 6.47% was an independent risk factor for all-cause mortality (HR 3.137, 95% CI: 1.667–5.904, P < 0.001) (Table 3).
Additionally, PA ≤ 6.47% was found to be positively associated with cardiac death (HR 3.095, 95% CI: 1.310–7.310, P = 0.010). After the model was adjusted for confounders, including age, sex, LVEF, the presence of ischaemic cardiomyopathy, the presence of AF and β-blocker consumption, the multivariate Cox regression modelling results showed that PA ≤ 6.47% was an independent risk factor for cardiac death (HR value 3.345, 95% CI: 1.394–8.028, P = 0.007) (Table 3).
Discussion
The results of the present study indicated that objective daily PA measured by the continuous remote monitoring of ICDs/CRTDs is related to the prognosis of cardiovascular diseases in patients with age > 75 years. PA ≤ 6.47% (93 min/day) was significantly associated with the risk of all-cause mortality and cardiac death. Even in patients with age > 75 years and structural heart diseases and a high risk of sudden cardiac death, daily physical activities should be performed regularly. PA monitoring may aid in long-term management of older patients at high risk of SCD.
The present study confirmed there is a correlation between PA and cardiovascular prognoses, which is consistent with the results presented in previous studies. Lear conducted a prospective cohort study in 130,000 participants from 17 countries and demonstrated that higher levels of recreational and non-recreational PA were associated with a lower risk of mortality and cardiovascular disease events in individuals from low-income, middle-income, and high-income countries [13]. Jeong compared the impact of leisure-time PA on mortality in primary versus secondary cardiovascular prevention and found that individuals with cardiovascular disease may benefit from PA to a greater extent than healthy volunteers without cardiovascular disease [14]. However, these studies mentioned above did not use continuous monitoring with CIEDs, and the results that were based on questionnaires may have been biased. Recently, Toshihiko found a correlation between PA and all-cause mortality in patients with age > 75 years and pacemakers [12]. However, this population did not have structural heart diseases and were not at risk of sudden cardiac death. And the study did not further clarify the cause of death of the patients. Conraads found that a lower baseline PA was associated with readmission and cardiac death in patients with heart failure [15]. However, the population included in that study was relatively young, and no cut-off value was provided to predict the clinical outcomes. In the present study, the average value of the baseline PA was determined through continuous remote monitoring of ICDs/ CRTDs, the follow-up time was long-term, and the endpoint events were more reliable. The population studied was old people with an average age of nearly 80 years. The results confirmed there was a relationship between PA and cardiac death in patients with age > 75 years and provided a baseline PA cut-off value for clinical use.
According to the current guidelines, the recommendations for older adults are the same as those for middle-aged adults [16]. Due to the effects of diseases and ageing, patients with age > 75 years are limited in their ability to perform PA. Compared with younger adults, old people have a lower level of PA, and it is more difficult for them to reach the recommended level of PA. Picel etal found that frailty was common in old patients with ICDs and associated with mortality [17]. Research results on the benefits of PA in older people has been inconsistent. Cheng concluded that the benefit of leisure-time PA is lower for those aged over 65 years than for those aged younger than 65 years [18]. Another study showed that older people need to perform more moderate and high intensity exercise to gain benefits [19]. Hupin claimed that the recommended level of PA for adults may exceed the standard for older people, and 15 minutes of daily PA may be the best target for older adults [20]. A previous study by Zhao found that the cut-off value of PA in predicting cardiac death in the general population was 7.84% (113 min/day) [21]. Compared with the cut-off value in the general population, that in patients with age > 75 years was lower. However, compared with pacemaker patients without structural heart disease (50 min/day), the cut-off value in patients with age > 75 years implanted with ICDs/CRTDs was higher, which indicates that this cohort of patients with more severe conditions may need more PA to exhibit survival benefits [12]. Therefore, the best recommended value for patients with age > 75 years deserves further discussion. Recently, there have been studies on dose correlation [6, 22,23,24], and there are still no related studies on the dose relationship in older people with CIED, which can serve as a direction for future research.
Study limitations
First, our study only included the baseline PA from 30 to 60 days after implantation, longitudinal PA and its changes on the prognosis were not investigated. Moreover, despite of adjustment for multiple covariates, we did not take some important factors such as socioeconomic status and echo indexes besides LVEF and LVEDD. In addition, the use of medical therapy was relatively low though this situation was common in China. Last but not least, this study was limited by a relatively small sample size and retrospective design.
Conclusions
Daily PA measured by continuous remote monitoring was related to the prognoses of patients with age > 75 years and structural heart diseases and a high risk of sudden death. Daily PA of about 1.5 h was associated with lower all-cause mortality and cardiac death risk in patients with age > 75 years and high risk of SCD with ICDs/CRTDs. PA monitoring may aid in long-term management of those patients.
Availability of data and materials
The datasets generated and analysed during the current study can be found at Fuwai Hospital but not publicly available due to related regulations. Data and materials are however available from the authors upon reasonable request and with permission of SUMMIT study.
Abbreviations
- PA:
-
Physical activity
- SCD:
-
Sudden cardiac death
- CIED:
-
Cardiovascular implantable electronic devices
- HM:
-
Home monitoring
- ICD:
-
Implantable cardioverter-defibrillator
- CRTD:
-
Cardiac resynchronization therapy defibrillator
- HR:
-
Hazard ratio
- CI:
-
Confidence interval
- ACEIs:
-
Angiotensin-converting enzyme inhibitors
- ARBs:
-
Angiotensin receptor blockers
- BMI:
-
Body Mass Index
- LVEDD:
-
Left ventricular enddiastolic dimension
- LVEF:
-
Left ventricular ejection fraction
- NYHA:
-
New York Heart Association
References
Beard JR, Officer A, de Carvalho IA, Sadana R, Pot AM, Michel J-P, et al. The world report on ageing and health: a policy framework for healthy ageing. Lancet. 2016;387(10033):2145–54.
Prince MJ, Wu F, Guo Y, Gutierrez Robledo LM, O'Donnell M, Sullivan R, et al. The burden of disease in older people and implications for health policy and practice. Lancet. 2015;385(9967):549–62.
Al-Khatib SM, Stevenson WG, Ackerman MJ, Bryant WJ, Callans DJ, Curtis AB, et al. 2017 AHA/ACC/HRS guideline for Management of Patients with Ventricular Arrhythmias and the prevention of sudden cardiac death: executive summary. J Am Coll Cardiol. 2018;72(14):1677–749.
Kolodziejczak M, Andreotti F, Kowalewski M, Buffon A, Ciccone MM, Parati G, et al. Implantable cardioverter-defibrillators for primary prevention in patients with ischemic or nonischemic cardiomyopathy. Ann Intern Med. 2017;167(2):103.
Lee IM, Shiroma EJ, Lobelo F, Puska P, Blair SN, Katzmarzyk PT. Effect of physical inactivity on major non-communicable diseases worldwide: an analysis of burden of disease and life expectancy. Lancet. 2012;380(9838):219–29.
Ekelund U, Tarp J, Steene-Johannessen J, Hansen BH, Jefferis B, Fagerland MW, et al. Dose-response associations between accelerometry measured physical activity and sedentary time and all cause mortality: systematic review and harmonised meta-analysis. BMJ. 2019;366:l4570.
Harris TJ, Owen CG, Victor CR, Adams R, Cook DG. What factors are associated with physical activity in older people, assessed objectively by accelerometry? Br J Sports Med. 2008;43(6):442–50.
Orkaby AR, Forman DE. Physical activity and CVD in older adults: an expert’s perspective. Expert Rev Cardiovasc Ther. 2017;16(1):1–10.
Warren JM, Ekelund U, Besson H, Mezzani A, Geladas N, Vanhees L. Assessment of physical activity – a review of methodologies with reference to epidemiological research: a report of the exercise physiology section of the European Association of Cardiovascular Prevention and Rehabilitation. Eur J Cardiovasc Prev Rehabil. 2010;17(2):127–39.
Cleland C, Ferguson S, Ellis G, Hunter RF. Validity of the international physical activity questionnaire (IPAQ) for assessing moderate-to-vigorous physical activity and sedentary behaviour of older adults in the United Kingdom. BMC Med Res Methodol. 2018;18(1):176.
Rosman L, Lampert R, Sears SF, Burg MM. Measuring physical activity with implanted cardiac devices: a systematic review. J Am Heart Assoc. 2018;7(11):e008663.
Goto T, Mori K, Nakasuka K, Kato M, Nakayama T, Banno T, et al. Physical activity and mortality in older patients with a pacemaker. Geriatr Gerontol Int. 2019;20(2):106–11.
Lear SA, Hu W, Rangarajan S, Gasevic D, Leong D, Iqbal R, et al. The effect of physical activity on mortality and cardiovascular disease in 130 000 people from 17 high-income, middle-income, and low-income countries: the PURE study. Lancet. 2017;390(10113):2643–54.
Jeong S-W, Kim S-H, Kang S-H, Kim H-J, Yoon C-H, Youn T-J, et al. Mortality reduction with physical activity in patients with and without cardiovascular disease. Eur Heart J. 2019;40(43):3547–55.
Conraads VM, Spruit MA, Braunschweig F, Cowie MR, Tavazzi L, Borggrefe M, et al. Physical activity measured with implanted devices predicts patient outcome in chronic heart failure. Circ Heart Fail. 2014;7(2):279–87.
Piercy KL, Troiano RP, Ballard RM, Carlson SA, Fulton JE, Galuska DA, et al. The physical activity guidelines for Americans. JAMA. 2018;320(19):2020.
Picel K, Vo TN, Kealhofer J, Anand V, Ensrud KE, Adabag S. Implications of frailty among men with implantable cardioverter defibrillators. South Med J. 2020;113(9):427–31.
Cheng W, Zhang Z, Cheng W, Yang C, Diao L, Liu W. Associations of leisure-time physical activity with cardiovascular mortality: a systematic review and meta-analysis of 44 prospective cohort studies. Eur J Prev Cardiol. 2018;25(17):1864–72.
Emerson KG, Gay J. Physical activity and cardiovascular disease among older adults: the case of race and ethnicity. J Aging Phys Act. 2017;25(4):505–9.
Hupin D, Edouard P, Gremeaux V, Garet M, Celle S, Pichot V, et al. Physical activity to reduce mortality risk. Eur Heart J. 2017;38(20):1534–7.
Zhao S, Chen K, Su Y, Hua W, Chen S, Liang Z, et al. Association between patient activity and long-term cardiac death in patients with implantable cardioverter-defibrillators and cardiac resynchronization therapy defibrillators. Eur J Prev Cardiol. 2017;24(7):760–7.
Joseph G, Marott JL, Torp-Pedersen C, Biering-Sørensen T, Nielsen G, Christensen A-E, et al. Dose-response association between level of physical activity and mortality in Normal, elevated, and high blood pressure. Hypertension. 2019;74(6):1307–15.
Pandey A, Garg S, Khunger M, Darden D, Ayers C, Kumbhani DJ, et al. Dose–response relationship between physical activity and risk of heart failure. Circulation. 2015;132(19):1786–94.
Mañas A, del Pozo-Cruz B, Rodríguez-Gómez I, Leal-Martín J, Losa-Reyna J, Rodríguez-Mañas L, et al. Dose-response association between physical activity and sedentary time categories on ageing biomarkers. BMC Geriatr. 2019;19(1):270.
Acknowledgements
None.
Funding
This study was supported by Beijing Municipal Science and Technology Commission (Z191100006619120). There was no role of the funding body in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Author information
Authors and Affiliations
Contributions
XYL, SZ1 and SZ2 contributed to the conception or design of the work. XYL and ZYL contributed to the acquisition, analysis, and interpretation of data for the work. XYL drafted the manuscript. KPC, WH, YGS, JFY, ZGL, WX, SZ2 critically revised the manuscript. All gave final approval and agree to be accountable for all aspects of work ensuring integrity and accuracy.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The present study was a secondary data analysis of the SUMMIT study which was approved by SUMMIT study investigators and the ethics committee of Fuwai Hospital (the chief institute). All methods were carried out in accordance with relevant guidelines and regulations.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Li, X., Chen, K., Hua, W. et al. Implantable device measured objective daily physical activity as a predictor of long-term all-cause mortality and cardiac death in patients with age > 75 years and high risk of sudden cardiac death: a cohort study. BMC Geriatr 22, 130 (2022). https://doi.org/10.1186/s12877-022-02813-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12877-022-02813-1