Abstract
Introduction
The diagnosis of intestinal tuberculosis is challenging even nowadays. This study aims to report the positivity rates of new diagnostic methods such as immunohistochemistry and Real-Time Polymerase Chain Reaction in patients with intestinal tuberculosis, as well as describe the pathological and endoscopic features of intestinal tuberculosis in our population.
Methods
This was a retrospective observational study conducted in patients diagnosed with intestinal tuberculosis, between 2010 to 2023 from the Hospital Nacional Daniel Alcides Carrion and a Private Pathology Center, both located in Peru. Clinical data was obtained, histologic features were independently re-evaluated by three pathologists; and immunohistochemistry and real-time Polymerase Chain Reaction evaluation were performed. The 33 patients with intestinal tuberculosis who fulfilled the inclusion criteria were recruited.
Results
Immunohistochemistry was positive in 90.9% of cases, while real-time Polymerase Chain Reaction was positive in 38.7%. The ileocecal region was the most affected area (33.3%), and the most frequent endoscopic appearance was an ulcer (63.6%). Most of the granulomas were composed solely of epithelioid histiocytes (75.8%). Crypt architectural disarray was the second most frequent histologic finding (78.8%) after granulomas, but most of them were mild.
Conclusion
Since immunohistochemistry does not require an intact cell wall, it demonstrates higher sensitivity compared to Ziehl–Neelsen staining. Therefore, it could be helpful for the diagnosis of paucibacillary tuberculosis.
Similar content being viewed by others
Introduction
Tuberculosis is a common disease with a rising incidence in recent years, particularly in developing countries such as Perú [1]. It can affect the gastrointestinal system eventually. In fact, intestinal tuberculosis (intestinal TB) ranks as the sixth most common site of extra-pulmonary involvement and it is the prevailing form of abdominal tuberculosis reported in tertiary care centers [2, 3]. Unfortunately, diagnosing intestinal tuberculosis is quite difficult due to its similarity to other diseases like colon carcinoma or Crohn’s disease. The diagnosis can be delayed because the microbiologic culture results often take 4 to 6 weeks to obtain [4]. Nonetheless, despite that, currently culture remains as the gold standard [5, 6]. The paucibacillary nature of Mycobacterium tuberculosis (MT) hinders its detection, which increases the risk of false negatives.
These diagnostics difficulties could lead to empiric treatments [7], patient hospitalization, prolonged illness, and finally death [8]. Consequently, novel diagnostic methods have come out that may help in the diagnosis of intestinal TB like immunohistochemistry or real-time Polymerase Chain Reaction (real-time PCR).
The histological diagnosis of intestinal TB is based on the identification of granulomas and acid-fast bacilli stain positive in the biopsy (Ziehl–Neelsen positive). However, the sensitivity of this test is low and operator-dependent. Furthermore, the granulomas can also be found in other pathologies like Crohn's disease or systemic mycosis [9].
Real-time PCR could be used in tuberculosis diagnosis, and has a higher sensitivity and specificity than culture or stains [10]. IS6110 is a DNA target sequence present only in mycobacteria, that is commonly used for Mycobacterium tuberculosiscomplex detection by real-time PCR [11]. Real-time PCR amplifies and detects specific DNA sequences via fluorescent dyes, which are linked to oligonucleotide probes that bind specifically to the amplified product. The identification and quantification of the accumulating product is achieved by rating the fluorescence intensities during the real-time PCR procedure [12]. However, real-time PCR is still expensive for low-income populations who are precisely the most affected by tuberculosis.
Immunohistochemical study for MT in biopsies is an alternative diagnostic tool that can be considered. This technique is based on the detection of MT antigens in paraffin blocks through polyclonal or monoclonal antibodies [13]. The sensitivity of this test can be extremely high, but the specificity is quite variable according to different authors. On the other hand, compared to real-time PCR, immunohistochemistry is lower cost and easier to perform [14].
The aim of this study is to report the positivity rates of immunohistochemistry and real-time PCR for MT in patients with intestinal TB and describe the pathological features of this disease in our population.
Methods
This was a retrospective observational study which was conducted at the Hospital Nacional Daniel Alcides Carrion (HNDAC) and Histodiagnóstico Gastrointestinal Private Pathology Center, both located in Lima-Peru; from 2010 to 2023. From which medical records from patients with a diagnosis of intestinal TB (small bowel, colon or rectum) were selected.
We included patients with the diagnosis of intestinal tuberculosis who underwent upper endoscopy or colonoscopy with biopsy, whose diagnosis of intestinal tuberculosis met at least one of the following criteria: (1) evidence of the acid-fast bacilli (AFB) in histologic sections; (2) positive culture in the endoscopy/colonoscopy sample; and (3) prompt response to antituberculosis treatment. This antituberculosis treatment response was defined as the absence of symptomatology (pain and/or diarrhea) after 6 months of antituberculosis treatment and was assessed by 2 gastroenterologists and one tuberculosis expert from the Peruvian National Program of Tuberculosis (these 3 specialists agreed on the clinical response of the patients). Neither upper endoscopy nor colonoscopy improvement was evaluated. We excluded cases with scarce tissue or insufficient clinical data.
We found a total of 43 patients with the diagnosis of intestinal tuberculosis, but only 33 patients fulfilled the inclusion criteria and thus were recruited for our study.
We collected clinical and endoscopic data of all the patients recruited. The demographic data included: age, sex, diagnosis of lung or multisystemic disseminated tuberculosis, and HIV status. The endoscopic data collected included: the site involved (duodenum, ileum, ileocecal region, right colon, left colon, rectum, or multiple locations), and the type of lesion (erosion, ulcers, polyps, nodular “cobblestone” appearance, or tumor).
The tissue from these patients was subjected to histological examination and ancillary studies (histochemical, immunohistochemical and real-time PCR).
The histologic features were assessed in hematoxylin and eosin (H&E) slides. Each case was re-evaluated by three pathologists separately. The microscopic study evaluated the following histologic features: 1) Granuloma defined as a round or oval collection of epithelioid immune cells in response to a chronic inflammatory stimulus [15]. 2) Confluent granuloma defined as the structures formed by the merge of adjacent granulomas [16]. 3) Microgranuloma: Small nodules composed of 5 to 15 clustered epithelioid histiocytes [17]. 4) Caseation necrosis defined as encapsulated or oval necrotic debris [15]. 5) Giant cells operationalized as present or absent [18]. 6) Crypt architectural disarray defined as elongated, dilated, or branched crypts [19]. 7) Cryptitis is defined as the presence of neutrophils into the surface epithelium and the colonic crypts [20]. 8) Crypt abscess defined as clusters of neutrophils in the crypt lumen [20]. 9) Eosinophils operationalized as the number of eosinophils per high power field in the intestinal lamina propria [21]. 10) Lymphoid follicles or lymphoid aggregated, the first defined as aggregates of lymphocytes with germinal center, and the lymphoid aggregated defined as a collection of lymphocytes and plasma cells without a germinal center [22]. 11) Villus atrophy (evaluated only in the small bowel): It is defined as the decrease in villous height, loss of the normal crypt/villous ratio (3:1), until the complete flattening of the villi [23, 24]. 12) Pyloric metaplasia: Defined as glands in the intestinal mucosa with the characteristics of mucin-secreting distal stomach glands [25].
Paraffin-embedded blocks were stained using the standard protocol of Ziehl–Neelsen to identify an acid-fast bacillus. With this stain, the bacilli stain red, and the background tissue light blue under the effect of methylene blue. A lung tuberculosis sample was used as control tissue.
The immunohistochemistry (IHC) was carried out using the IgG1 type rabbit polyclonal antibody against the BCG antigen of the MT complex (BIO SB inc. Lab, CA, United States). Sections of 3–4 micron were cut from the tissue block and incubated overnight at room temperature. After that, the tissue was deparaffinized and rehydrated. Antigen retrieval solution (DAKO lab) was applied to the tissue, in water bath (98°C) for 30 min, and then cooled for 20 min at room temperature. To remove endogenous peroxidase, methanol and 3% hydrogen peroxide solution were used for 5 min. Primary antibody (anti-Mycobacterium tuberculosis rabbit polyclonal antibody in 1:80 dilutions) reacted in a wet environment at room temperature for 60 min and after rinsing the specimens with phosphate buffer saline. A secondary antibody was applied and was left for 30 min to react. Then, the sections were subjected to a polymer-detection complex (DAKO envision) for 30 min. Finally, the Chromogen was applied for 5 min. Positive staining was defined as coarse granular cytoplasmic strong staining.
Real-time PCR is based on the amplification of a fragment of IS6110, which is specific for the Mycobacterium tuberculosis complex and found in almost all members of the MT complex. Most MT strains harbor 10–15 copies, located at different chromosomal sites. For real-time PCR sample preparation, the formalin-fixed paraffin-embedded (FFPE) specimens were sliced with disposable sterile blades in each paraffin block and deparaffinized. DNA was extracted from 25–50 mg. tissues using a High Pure PCR Template Preparation kit (Roche Diagnostics, Dresden Germany). The extracted DNA was then used as DNA template for the DiaPlexQ™ MTC/NTM Detection assay (Seegene, Seoul, South Korea). This assay relies on real-time multiplex PCR and distinguishes between MT and non-tuberculosis mycobacteria. Primers for insertion sequence IS6110 for MT detection and the Pan-Mycobacterium 16S rRNA gene for NTM detection were amplified with Bio-Rad CFX96 Touch™ real-time PCR detection system. The real-time PCR mixture was prepared in a total volume of 25 µL, which contained 1–5 µL DNA sample. Real-time PCR was performed under the following conditions: UDG reaction at 50˚C for 3 min, initial PCR activation at 95˚C for 15 min followed by 40 cycles of denaturation at 95˚C for 10 s and annealing at 58˚C for 40 s. Amplifications were performed and results analyzed according to the manufacturer’s instructions.
Descriptive statistics were used to report our findings.
Results
A retrospective study was carried out at the Hospital Nacional Daniel Alcides Carrion (HNDAC) and Histodiagnóstico Gastrointestinal Private Pathology Center. The 33 patients who fulfilled the inclusion criteria were recruited in the study. 25 patients (75.7%) met the inclusion criteria by the evidence of the AFB in histologic sections, and 8 (24.24%) by the response to antituberculosis treatment, according to the inclusion criteria described in methods. The clinical parameters assessed are shown in Table 1.
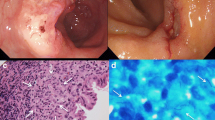
Ziehl–Neelsen staining was positive for acid-fast bacilli in most of the patients; nevertheless, most of them showed very few mycobacteria, making the bacilli detection difficult (Fig. 1).
In IHC, the staining was mainly observed in the inflammatory cells, while the necrotic centers were negative for the IHC study (Fig. 2). Occasionally, the surrounding tissue showed weak staining considered as negative background.
IHC was positive in more than 90% of the 33 cases. IHC was also positive in 24 of the 25 (96%) of the Ziehl–Neelsen positive cases, and in 11 of the 12 (91,6%) real-time PCR positive cases. Only 3 IHC negative cases were found, out of which 2 were also real-time PCR negative, and only 1 IHC negative case was real-time PCR and Ziehl–Neelsen positive. Real-time PCR was positive in 38.7% of the cases. All real-time PCR positive cases except one were also positive for acid-fast bacteria Ziehl–Neelsen staining.
Colonoscopy was performed in 28 patients; upper endoscopy, in 4 patients; and both (upper endoscopy and colonoscopy), in 2 patients. The endoscopic findings assessed are shown in Table 2 and the histologic findings in Table 3.
The ileocecal region was the most affected area (33.3%) (Table 4); and the cases with multiple involvement of the intestine comprised the ileocecal region as well. The rectal compromise is rare, but it was identified in 3 patients (9%), one of whom was presented with fistulae.
The most frequent endoscopic appearance (in all the intestine segments evaluated) was an ulcer (Fig. 3); and we found two cases with a superficially depressed presentation (Type O-IIc).
Histological examination revealed the presence of granulomas in almost all the cases studied. The only case without granulomas was initially misdiagnosed as Crohn's disease. Most of the granulomas were composed solely of epithelioid histiocytes (75.8%). Most granulomas were confluent and found in the submucosa or granulation tissue (Fig. 4). While the glandular architecture of the intestinal mucosa was preserved in 7 patients and most of the cases had mild disarray, a severe crypt disarray with branched crypts was noted in 10 patients (31%) (Fig. 5).
The ancillary tests for the detection of Mycobacterium tuberculosis performed on the tissue sample are shown in Table 3.
Discussion
Our study was focused on determining the positivity rates of auxiliar tissue tests (IHC and real-time PCR) performed for the diagnosis of intestinal tuberculosis, and to describe the endoscopic and histologic findings of these patients. We found a high IHC positivity rate (90.9%); however, the real-time PCR positivity rate was surprisingly low (38.7%). The most affected area was the ileocecal region with the ulcer being the most predominant endoscopic appearance (63.6%). The most frequent histologic finding was the presence of granulomas, which were mostly confluent without giant cells or caseous necrosis, followed by alterations in the architecture of the crypts, which were mild in most cases.
IHC has a better sensitivity than AFB Ziehl–Neelsen staining because it does not require an intact wall cell to be positive, which is very helpful for the diagnosis of paucibacillary tuberculosis [26]; this can explain the high positivity of IHC in our findings (90.9%). However, the specificity of this test is lower, which can result in false-positive reactions due to false-positive background staining. To improve the specificity of IHC some authors recommend proper elimination of background staining, because fine granular staining in the background can cause false-positive reaction [7]. Also, it has been reported that the sensitivity and specificity of IHC could be improved using antibodies against MPT64 antigens rather than antibodies against BCG antigens [27]. MPT64 is a secretory protein of MT, and a specific antigen that differentiates the MT complex from the mycobacteria other than tuberculosis [28]. Currently, the detection of MPT64 protein is performed mainly by immunochromatography. Nevertheless, in immunohistochemistry, the application is limited [29], for this reason, we did not have access to this antibody in our study. To improve the specificity of IHC, the use of monoclonal antibodies instead of polyclonal antibodies has also been recommended [14, 27]. Although, the high price of monoclonal antibodies in Peru made it difficult to access to it. Even so, the IHC employed in our study was the ancillary tissue test with a greater positive percentage in relation to real-time PCR or Ziehl–Neelsen (90.9% versus 38.7% and 75.8%, respectively). A greater percentage of positive cases from the anti-BCG IHC versus the Ziehl–Neelsen stain was previously reported [30].
The immunohistochemistry manifests as a coarse granular staining in the cytoplasm of mainly macrophages and other cells such as neutrophils, lymphocytes, endothelial cells, and dendritic cells [7, 31]. In this study, our immunohistochemistry staining was limited only to macrophages, highlighting the importance of these cells in tuberculosis pathogenesis.
In our study, the real-time PCR positivity rate (38.7%) was lower than that reported in previous studies, which indicated a positivity rate that varies from 60 to 70%, but only in acid-fast bacilli (AFB) positive samples [10, 12]. The low PCR-positivity of our results could be attributed to the small size of the sample [14]; absence or fewer copies of sequence IS6110 in some strains of Mycobacterium tuberculosis [10]; or DNA structural changes of paraffin-embedded tissues due to prolonged formalin fixation [12]. These findings raise the possibility that some local mycobacteria might not harbor the sequence IS6110. Better designes studies are required to evaluate this idea.
Ziehl–Neelsen staining for the identification of acid-fast bacilli is the most trustworthy histologic finding in tuberculosis. It should be noted that most of our cases (75%) were positive, which is higher than the findings of previous reports, but this is related to the fact that Ziehl–Neelsen positivity was one of the inclusion criteria of our study. The sensitivity of Ziehl–Neelsen staining for the detection of MT in histological specimens is variable, ranging from 0 to 60% [2, 7, 32, 33]. The difficulty to detect mycobacterium can be explained by its paucibacillary nature [5], the CDC points out that “there must be between 5000–10000 bacilli per milliliter of specimen to allow the detection of bacteria in stained smears” [34], and for each slide at least 10 [4] bacilli [7, 30]. For that reason, it is recommended that at least 8 biopsies should be taken to ensure an optimal microscopic evaluation [6].
In our study, the ileocecal region was the most frequent site affected by tuberculosis which is consistent with previous studies. In fact, TB can compromise any level of the gastrointestinal tract, from the esophagus to the anus [35]; however, the ileocecal region is the most affected area, accounting for 44%-84% of all gastrointestinal tuberculosis cases. [35,36,37]. Most authors believe that in these areas, the presence of lymphoid tissue and the physiologic stasis of the cecum promotes mycobacterial growth. [38] This predilection for ileocecal region complicates the differentiation of tuberculosis from Crohn’s disease, where ileocecal involvement is similar [39].
While upper gastrointestinal tract involvement by tuberculosis is rare [35], we found a greater rate (12.1%) of duodenum involvement than previously reported (1%-6%) [35, 40,41,42]. Multisegmented involvement of the colon has been rarely reported [26, 35]. Only one report indicates the colonic involvement distribution in single and multiple site lesions (31% and 36%, respectively) [36]. In our study, multisegmented colonic involvement was found in 21.2% of cases.
In our study, ulcer was the most frequent endoscopic finding, mostly reported by the gastroenterologists as multiple, consistent with previous reports [14, 36, 43]. However, the endoscopic presentation of intestinal tuberculosis is quite variable and also includes tumors, strictures, erosions, and nodularity of the mucosa [36, 43, 44]. These endoscopic findings, however, were present only in a small group of patients in our study. The distinction between intestinal TB from Crohn´s disease ulcer can be a true challenge, especially when nodularity of the mucosa layer is described as “cobblestone” [36, 45,46,47,48]. This endoscopic finding has been regularly related to Crohn´s disease, which makes the differential diagnosis even more difficult; however, four parameters have been proposed. These parameters would be more common in Crohn's disease than in intestinal TB, and they are: aphthous ulcers, longitudinal ulcers, anorectal lesions, and cobblestone appearance [49]. Additionally, the mucosa surrounding intestinal TB ulcers tends to be abnormal (edematous, erythematous, irregular, or nodular), whereas the mucosa surrounding Crohn´s disease ulcers tends to be normal [36].
Stricture and tumor appearance are the least frequent endoscopic findings [2], but they are the most concerning due to their similarity with colon neoplasia. We found only 4 cases described as a “tumor” in the colonoscopy report. In this scenario, a meticulous histological study of the sample will be essential for diagnosis as reported in previous papers [50,51,52,53].
Histological evaluation of intestinal tuberculosis poses a diagnostic challenge for pathologists, since it must be differentiated from Crohn´s disease. Both diseases can exhibit granulomatous reaction. Crohn's disease granulomas are composed mainly of clusters of epithelioid cells without giant cells or necrosis [54, 55]. Conversely, tuberculosis granulomas are usually reported as large (> 400 µm), confluents, and contain giant cells called “Langhans giant cells” [56], along with caseous necrosis [48, 57, 58]. Interestingly, a study reported that only between 13 to 33% of the patients with intestinal tuberculosis showed those findings [5]. Our group of patients with intestinal tuberculosis presented mostly incomplete granulomas; only 7 (21.2%) cases had complete granulomas with caseum necrosis. This finding differs from other publications that found caseating granulomas in 70.6% and 40% respectively [2, 48]. We suggest considering non-caseating granulomas as a common histologic finding in intestinal tuberculosis in our population.
Other histologic features such as ulcers lined by bands of epithelioid histiocytes, disproportionate submucosal inflammation, and submucosal granulomas are more frequent in intestinal tuberculosis than in Crohn’s disease [45]. Moreover, in intestinal tuberculosis, ulcers usually do not penetrate beyond the muscularis, and granulomas are commonly located under the ulcer bed [9]. Most of our cases presented granulomas located in the submucosa rather than in the mucosa, thus it is recommendable that deeper biopsy should be taken from the margins of the ulcers to reach the submucosa [4].
Crypts disarray, presence of lymph follicles, and eosinophils counts were other histologic features assessed in our study. We found a predominantly mild crypt disarray and a very few crypts branching. Intestinal tuberculosis has a chronic course, which explains the alteration of the normal architecture; however, these changes are not as prominent as in inflammatory bowel disease [35]. Lymph follicles could play a role in tuberculosis pathogenesis since they can harbor granulomas. Nearly all patients in our study had lymph follicles in the mucosa, and rarely in the submucosal layer.
Although tuberculosis cases at the national and global level continue to increase [59, 60],
its diagnosis remains a challenge, particularly in cases with intestinal involvement, where histologic findings could be similar to other diseases like colon carcinoma or Crohn’s disease [36, 39, 45]. Additionally, microbiologic culture, the gold standard in the diagnosis of Mycobacterium tuberculosis [5, 6]; takes several weeks to be obtained, and the paucibacillary nature of these microorganism could lead to report a false negative [61]. This highlights the importance of using other ancillary tests for the diagnosis on intestinal tuberculosis. In that regard, our study is the first retrospective study conducted in Latin America to evaluate the positivity rates of IHC and real-time PCR in tissue sample obtained from endoscopy in the diagnosis of intestinal TB. In Peru, these tests are infrequently performed due to their high cost, which can be up to three times higher for IHC or thirteen times higher for real-time PCR compared to a conventional study (histologic study + Ziehl Neelsen).
It should be noted that there are some limitations in the present study: the small sample size, and the retrospective nature (which lacks a systematic prospective follow-up) therefore definitive conclusions from the study cannot be made. Besides, including “prompt response to antituberculosis treatment” as an inclusion criterion could have increased the false positivity rate, although it must be highlighted that most of our cases were diagnosed with Ziehl–Neelsen positive in histology.
In conclusion, despite these limitations, our data shows a high positivity rate of anti-BCG IHC in the diagnosis of intestinal tuberculosis, even in Ziehl–Neelsen negative cases. Thus, it should be considered especially in difficult cases with a Ziehl–Neelsen negative staining to avoid misleading the diagnosis; unlike real-time PCR, which had a low positive rate in our study. Finally, we found that a multicentric analytic study should be performed to establish the sensitivity and specificity of the histologic tests used in our study (anti-BCG IHC, real-time PCR and Ziehl–Neelsen stain), and other ancillary tests not evaluated in this study such as anti-MPT64 IHC.
Availability of data and materials
The data can be provided by the corresponding author on reasonable request.
References
Gutierrez Y, Dorantes R, Medina H, Téllez I. Diagnostic approach of intestinal tuberculosis: Case report and literature review. Endoscopia. 2014;26(4):132–5. https://doi.org/10.1016/j.endomx.2014.11.002.
Cheng W, Zhang S, Li Y, Wang J, Li J. Intestinal tuberculosis: clinico-pathological profile and the importance of a high degree of suspicion. Trop Med Int Health. 2019;24(1):81–90. https://doi.org/10.1111/tmi.13169.
Jha DK, Pathiyil MM, Sharma V. Evidence-based approach to diagnosis and management of abdominal tuberculosis. Indian J Gastroenterol. 2023;42(1):17–31. https://doi.org/10.1007/s12664-023-01343-x. Epub 2023 Mar 11. PMID: 36899289; PMCID: PMC10005918.
Arnold C, Moradpour D, Blum HE. Tuberculous colitis mimicking Crohn’s disease. Am J Gastroenterol. 1998;93(11):2294–6. https://doi.org/10.1111/j.1572-0241.1998.00644.x.
Maulahela H, Simadibrata M, Nelwan EJ, et al. Recent advances in the diagnosis of intestinal tuberculosis. BMC Gastroenterol. 2022;22(1):89. https://doi.org/10.1186/s12876-022-02171-7. Published 2022 Mar 1.
Mehta V, Desai D, Abraham P, Rodrigues C. Making a positive diagnosis of intestinal Tuberculosis with the aid of new biologic and histologic features: How far have we reached? Inflamm Intest Dis. 2019;3(4):155–60. https://doi.org/10.1159/000496482.
Karimi S, Shamaei M, Pourabdollah M, et al. Histopathological findings in immunohistological staining of the granulomatous tissue reaction associated with tuberculosis. Tuberc Res Treat. 2014;2014:858396. https://doi.org/10.1155/2014/858396.
Tedla K, Medhin G, Berhe G, et al. Delay in treatment initiation and its association with clinical severity and infectiousness among new adult pulmonary tuberculosis patients in Tigray, northern Ethiopia. BMC Infect Dis. 2020;20:456. https://doi.org/10.1186/s12879-020-05191-4.
Tandon HD, Prakash A. Pathology of intestinal tuberculosis and its distinction from Crohn´s disease. Gut. 1972;13:260–9. https://doi.org/10.1136/gut.13.4.260.
Sekar B, Selvaraj L, Alexis A, Ravi S, Arunagiri K, Rathinavel L. The utility of IS6110 sequence based polymerase chain reaction in comparison to conventional methods in the diagnosis of extra-pulmonary tuberculosis. Indian J Med Microbiol. 2008;26(4):352–5. https://doi.org/10.4103/0255-0857.43575.
Sánchez-Carvajal JM, Galán-Relaño Á, Ruedas-Torres I, et al. Real-Time PCR Validation for Mycobacterium tuberculosis Complex Detection Targeting IS6110 Directly From Bovine Lymph Nodes. Front Vet Sci. 2021;8:643111. https://doi.org/10.3389/fvets.2021.643111.
Mishra PK, Gorantla VR, Bhargava A, Varshney S, Vashistha P, Maudar KK. Molecular detection of Mycobacterium tuberculosis in formalin-fixed, paraffin-embedded tissues and biopsies of gastrointestinal specimens using real-time polymerase chain reaction system. Turk J Gastroenterol. 2010;21(2):129–34. https://doi.org/10.4318/tjg.2010.0070.
Goel M, Budhwar P. Immunohistochemical localization of mycobacterium tuberculosis complex antigen with antibody to 38 kDa antigen versus Ziehl Neelsen staining in tissue granulomas of extrapulmonary tuberculosis. Indian J Tuberc. 2007;54(1):24–9 PMID: 17455420.
Ihama Y, Hokama A, Hibiya K, Kishimoto K, Nakamoto M, Hirata T, Kinjo N, Cash HL, Higa F, Tateyama M, Kinjo F, Fujita J. Diagnosis of intestinal tuberculosis using a monoclonal antibody to Mycobacterium tuberculosis. World J Gastroenterol. 2012;18(47):6974–80. https://doi.org/10.3748/wjg.v18.i47.6974.
Williams O, Fatima S. Granuloma. 2022 Sep 19. In: StatPearls. Treasure Island: StatPearls Publishing; 2024.
Du J, Ma YY, Xiang H, Li YM. Confluent granulomas and ulcers lined by epithelioid histiocytes: new ideal method for differentiation of ITB and CD? A meta analysis. PLoS ONE. 2014;9(10):e103303. https://doi.org/10.1371/journal.pone.0103303. PMID:25299041;PMCID:PMC4191941.
Alimchandani M, Lai JP, Aung PP, Khangura S, Kamal N, Gallin JI, Holland SM, Malech HL, Heller T, Miettinen M, Quezado MM. Gastrointestinal histopathology in chronic granulomatous disease: a study of 87 patients. Am J Surg Pathol. 2013;37(9):1365–72. https://doi.org/10.1097/PAS.0b013e318297427d. PMID:23887163;PMCID:PMC3787986.
Pagán AJ, Ramakrishnan L. The Formation and Function of Granulomas. Annu Rev Immunol. 2018;26(36):639–65. https://doi.org/10.1146/annurev-immunol-032712-100022. Epub 2018 Feb 5 PMID: 29400999.
Rubio CA, Schmidt PT. Morphological Classification of Corrupted Colonic Crypts in Ulcerative Colitis. Anticancer Res. 2018;38(4):2253–9. https://doi.org/10.21873/anticanres.12469. PMID: 29599347.
Fabian O, Bajer L. Histopathological assessment of the microscopic activity in inflammatory bowel diseases: What are we looking for? World J Gastroenterol. 2022;28(36):5300–12. https://doi.org/10.3748/wjg.v28.i36.5300. PMID:36185628;PMCID:PMC9521520.
Yantiss R. Eosinophils in the GI tract: How many is too many and what do they mean? Mod Pathol. 2015;28(Suppl 1):S7–21. https://doi.org/10.1038/modpathol.2014.132.
Shah N, Thakkar B, Shen E, Loh M, Chong PY, Gan WH, Tu TM, Shen L, Soong R, Salto-Tellez M. Lymphocytic follicles and aggregates are a determinant of mucosal damage and duration of diarrhea. Arch Pathol Lab Med. 2013;137(1):83–9. https://doi.org/10.5858/arpa.2011-0430-OA. PMID: 23276179.
Rossi C, Simoncelli G, Arpa G, Stracuzzi A, Parente P, Fassan M, Vanoli A, Villanacci V. Histopathology of intestinal villi in neonatal and paediatric age main features with clinical correlation Part II. Pathologica. 2022;114(1):22–31. https://doi.org/10.32074/1591-951X-338. Epub 2021 Dec 2. PMID: 34856605; PMCID: PMC9040546.
Villanacci V, Vanoli A, Leoncini G, Arpa G, Salviato T, Bonetti LR, Baronchelli C, Saragoni L, Parente P. Celiac disease: histology-differential diagnosis-complications. A practical approach. Pathologica. 2020;112(3):186–96. https://doi.org/10.32074/1591-951X-157. PMID: 33179621; PMCID: PMC7931573.
Goldenring JR. Pyloric metaplasia, pseudopyloric metaplasia, ulcer-associated cell lineage and spasmolytic polypeptide-expressing metaplasia: reparative lineages in the gastrointestinal mucosa. J Pathol. 2018;245(2):132–7. https://doi.org/10.1002/path.5066. PMID: 29508389; PMCID: PMC6026529.
Ramesh J, Banait G, Ormerod L. Abdominal tuberculosis in a district general hospital: a retrospective review of 86 cases. QJM. 2008;101(3):189–95. https://doi.org/10.1093/qjmed/hcm125.
Purohit MR, Mustafa T, Wiker HG, Mørkve O, Sviland L. Immunohistochemical diagnosis of abdominal and lymph node tuberculosis by detecting Mycobacterium tuberculosis complex specific antigen MPT64. Diagn Pathol. 2007;25(2):36. https://doi.org/10.1186/1746-1596-2-36.
Cao XJ, Li YP, Wang JY, et al. MPT64 assays for the rapid detection of Mycobacterium tuberculosis. BMC Infect Dis. 2021;21:336. https://doi.org/10.1186/s12879-021-06022-w.
Hoel IM, Sviland L, Syre H, Dyrhol-Riise AM, Skarstein I, Jebsen P, Jørstad MD, Wiker H, Mustafa T. Diagnosis of extrapulmonary tuberculosis using the MPT64 antigen detection test in a high-income low tuberculosis prevalence setting. BMC Infect Dis. 2020;20(1):130. https://doi.org/10.1186/s12879-020-4852-z. PMID:32050915;PMCID:PMC7014701.
Ulrichs T, Lefmann M, Reich M, Morawietz L, Roth A, Brinkmann V, Kosmiadi GA, Seiler P, Aichele P, Hahn H, Krenn V, Göbel UB, Kaufmann SH. Modified immunohistological staining allows detection of Ziehl-Neelsen-negative Mycobacterium tuberculosis organisms and their precise localization in human tissue. J Pathol. 2005;205(5):633–40. https://doi.org/10.1002/path.1728. PMID: 15776475.
Brees DJ, Reimer SB, Cheville NF, Florance A, Thoen CO. Immunohistochemical detection of Mycobacterium paratuberculosis in formalin-fixed, paraffin-embedded bovine tissue sections. J Vet Diagn Invest. 2000;12(1):60–3. https://doi.org/10.1177/104063870001200111.
Sua LF, Bolaños JE, Maya J, Sánchez A, Medina G, Zúñiga-Restrepo V, Fernández-Trujillo L. Detection of mycobacteria in paraffin-embedded Ziehl-Neelsen-Stained tissues using digital pathology. Tuberculosis (Edinb). 2021;126:102025. https://doi.org/10.1016/j.tube.2020.102025. Epub 2020 Nov 25.
Fukunaga H, Murakami T, Gondo T, Sugi K, Ishihara T. Sensitivity of acid-fast staining for Mycobacterium tuberculosis in formalin-fixed tissue. Am J Respir Crit Care Med. 2002;166(7):994–7. https://doi.org/10.1164/rccm.2111028.
Centers for Disease Control and Prevention. Laboratory Examination: Acid-Fast Bacilli (AFB) Smears. The Division of Tuberculosis Elimitation. Available in: https://www.cdc.gov/tb/education/corecurr/pdf/chapter4.pdf. Cited: 05/19/2023.
Al-Zanbagi AB, Shariff MK. Gastrointestinal tuberculosis A systematic review of epidemiology, presentation, diagnosis and treatment. Saudi J Gastroenterol. 2021;27(5):261–74. https://doi.org/10.4103/sjg.sjg_148_21.
Mukewar S, Mukewar S, Ravi R, Prasad A, Dua KS. Colon tuberculosis: endoscopic features and prospective endoscopic follow-up after anti-tuberculosis treatment. Clin Transl Gastroenterol. 2012;3(10):e24. https://doi.org/10.1038/ctg.2012.19.
Moka P, Ahuja V, Makharia G. Endoscopic features of gastrointestinal tuberculosis and Crohn’s disease. Journal of Digestive Endoscopy. 2017;8:1. https://doi.org/10.4103/jde.JDE_48_16.
Chatzicostas C, Koutroubakis IE, Tzardi M, Roussomoustakaki M, Prassopoulos P, Kouroumalis EA. Colonic tuberculosis mimicking Crohn's disease: case report. BMC Gastroenterol. 2002; 2:10. Published 2002 May 13. https://doi.org/10.1186/1471-230x-2-10
Abadir AP, Han JY, Youssef FA. Intestinal Tuberculosis Masquerading as Crohn’s disease? A case of disseminated tuberculosis after Anti-TNF Therapy for Suspected Crohn’s Disease. Case Rep Gastrointest Med. 2019;14(2019):6053503. https://doi.org/10.1155/2019/6053503.
Chalya PL, Mchembe MD, Mshana SE, Rambau PF, Jaka H, Mabula JB. Clinicopathological profile and surgical treatment of abdominal tuberculosis: a single centre experience in northwestern Tanzania. BMC Infect Dis. 2013;8(13):270. https://doi.org/10.1186/1471-2334-13-270.
Chien K, Seemangal J, Batt J, Vozoris NT. Abdominal tuberculosis: A descriptive case series of the experience in a Canadian tuberculosis clinic. Int J Tuberc Lung Dis. 2018;22:681–5. https://doi.org/10.5588/ijtld.17.0685.
Singh A, Sahu MK, Panigrahi M, Behera MK, UthanSingh K, Kar C, et al. Abdominal tuberculosis in Indians Still very pertinent. J Clin Tuberc Other Mycobact Dis. 2019;15:100097.
Naga MI, Okasha HH, Ismail Z, El-Fatatry M, Hassan S, Monir BE. Endoscopic diagnosis of colonic tuberculosis. Gastrointest Endosc. 2001;53(7):789–93. https://doi.org/10.1067/mge.2001.114965.
Sato S, Yao K, Yao T, et al. Colonoscopy in the diagnosis of intestinal tuberculosis in asymptomatic patients. Gastrointest Endosc. 2004;59(3):362–8. https://doi.org/10.1016/s0016-5107(03)02716-0.
Kirsch R, Pentecost M, Hall Pde M, Epstein DP, Watermeyer G, Friederich PW. Role of colonoscopic biopsy in distinguishing between Crohn’s disease and intestinal tuberculosis. J Clin Pathol. 2006;59(8):840–4. https://doi.org/10.1136/jcp.2005.032383.
Pulimood AB, Amarapurkar DN, Ghoshal U, et al. Differentiation of Crohn’s disease from intestinal tuberculosis in India in 2010. World J Gastroenterol. 2011;17:433–43. https://doi.org/10.3748/wjg.v17.i4.433.
Pulimood AB, Peter S, Ramakrishna B, et al. Segmental colonoscopic biopsies in the differentiation of ileocolic tuberculosis from Crohn’s disease. J Gastroenterol Hepatol. 2005;20:688–96. https://doi.org/10.1111/j.1440-1746.2005.03814.X.
Pulimood AB, Ramakrishna BS, Kurian G, Peter S, Patra S, Mathan VI, Mathan MM. Endoscopic mucosal biopsies are useful in distinguishing granulomatous colitis due to Crohn’s disease from tuberculosis. Gut. 1999;45(4):537–41. https://doi.org/10.1136/gut.45.4.537.
Lee YJ, Yang SK, Byeon JS, Myung SJ, Chang HS, Hong SS, Kim KJ, Lee GH, Jung HY, Hong WS, Kim JH, Min YI, Chang SJ, Yu CS. Analysis of colonoscopic findings in the differential diagnosis between intestinal tuberculosis and Crohn’s disease. Endoscopy. 2006;38(6):592–7. https://doi.org/10.1055/s-2006-924996.
Yu SM, Park JH, Kim MD, Lee HR, Jung P, Ryu TH, Choi SH, Lee IS. A case of sigmoid colon tuberculosis mimicking colon cancer. J Korean Soc Coloproctol. 2012;28(5):275–7. https://doi.org/10.3393/jksc.2012.28.5.275.
Suárez-Noya A, González-Bernardo O, Riera-Velasco JR, Suárez A. Tuberculosis intestinal como simuladora de una neoplasia de colon. Rev Gastroenterol Mex. 2022. https://doi.org/10.1016/J.RGMX.2022.06.005.
MosquedaCala I, Dallas Veranes IB, DíazNordet JC. Tuberculosis intestinal simulando tumor de colon Informe de un caso. Rev Inf Cient. 2018;97(3):652–9.
Garcia JM, Javier R, López M, Hidalgo C, Lopez MA, Jimenez J. Tuberculosis intestinal que simula carcinoma colorrectal diseminado. Gastroenterología y Hepatología VL. 2013;36(7):461–3. https://doi.org/10.1016/j.gastrohep.2013.01.013.
Feakins RM. Ulcerative colitis or Crohn’s disease? Pitfalls and problems. Histopathology. 2014;64(3):317–35. https://doi.org/10.1111/his.12263.
Yantiss RK, Odze RD. Diagnostic difficulties in inflammatory bowel disease pathology. Histopathology. 2006;48(2):116–32. https://doi.org/10.1111/j.1365-2559.2005.02248.x.
Damjanov I. Chapter 2 - Inflammation and Repair. In: Pathology Secrets. Third Edition. Mosby. 2009. P. 19–37. https://doi.org/10.1016/B978-0-323-05594-9.00002-7.
Khor TS, Fujita H, Nagata K, Shimizu M, Lauwers GY. Biopsy interpretation of colonic biopsies when inflammatory bowel disease is excluded. J Gastroenterol. 2012;47:226–48. https://doi.org/10.1007/s00535-012-0539-6.
Lamps LW. Infective disorders of the gastrointestinal tract. Histopathology. 2007;50(1):55–63. https://doi.org/10.1111/j.1365-2559.2006.02544.x.
Global tuberculosis report 2023. Geneva: World Health Organization; 2023. Accessed from https://www.who.int/teams/global-tuberculosis-programme/tb-reports/global-tuberculosis-report-2023. 1st March 2024.
Vigilancia de tuberculosis. Peru. Centro Nacional de Epidemiología, Prevención y Control de Enfermedades. Accessed from https://www.dge.gob.pe/portalnuevo/vigilancia-epidemiologica/vigilancia-de-tuberculosis/#:~:text=En%20el%20Per%C3%BA%20anualmente%20se,de%20tuberculosis%20en%20las%20Am%C3%A9ricas. 1st March 2024.
Liu C, Lyon CJ, Bu Y, Deng Z, Walters E, Li Y, Zhang L, Hesseling AC, Graviss EA, Hu Y. Clinical Evaluation of a Blood Assay to Diagnose Paucibacillary Tuberculosis via Bacterial Antigens. Clin Chem. 2018;64(5):791–800. https://doi.org/10.1373/clinchem.2017.273698. Epub 2018 Jan 18. PMID: 29348166; PMCID: PMC5996152.
Funding
The funding was provided by Histodiagnostico Gastrointestinal.
Private Pathology Center.
Author information
Authors and Affiliations
Contributions
Fernando Arévalo: Study design, Interpretation of histology samples, discussion, conclusion and approval of the final draft, Soledad Rayme: Interpretation of histology samples, discussion, conclusion and approval of the final draft. Rocío Ramírez: Interpretation of histology samples, discussion, conclusion and approval of the final draft. Romy Rolando: Interpretation of histology samples, discussion, conclusion and approval of the final draft. Jaime Fustamante: Collecting the cases and performing endoscopies, discussion, conclusion and approval of the final draft. Mario Monteghirfo: study and interpretation of PCR, discussion, conclusion and approval of the final draft. Rocío Chavez: Collecting the cases and performing endoscopies, discussion, conclusion and approval of the final draft. Eduardo Monge: Collecting the cases and performing endoscopies, discussion, conclusion and approval of the final draft.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The present study was approved by the Ethics Committee of Hospital Nacional Daniel A. Carrión and Histodiagnostico Gastrointestinal.
Private Pathology Center.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Arevalo, F., Rayme, S., Ramírez, R. et al. Immunohistochemistry and real-time Polymerase Chain Reaction: importance in the diagnosis of intestinal tuberculosis in a Peruvian population. BMC Gastroenterol 24, 166 (2024). https://doi.org/10.1186/s12876-024-03235-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12876-024-03235-6