Abstract
Background
The incidence of gastric cancer ranks the first among digestive tract tumors in China. However, there are no specific symptoms in the early stage of the tumor and the diagnosis process is complex, so more effective detection methods are very needed. In this study, a novel long noncoding RNA (lncRNA) was introduced as a diagnostic biomarker for gastric cancer, which brought new thinking to the exploration of its pathological mechanism and clinical prediction.
Methods
The level of lncRNA EPB41L4A-AS1 (EPB41L4A-AS1) in gastric cancer serum and cells was verified via real-time quantitative polymerase chain reaction (RT-qPCR). Receiver operating characteristic (ROC) curve was performed based on the EPB41L4A-AS1 level, and the diagnostic possibility of EPB41L4A-AS was analyzed. The chi-square test evaluated the correlation between EPB41L4A-AS expression and clinical information. The cells were cultured and transfected in vitro, and the mediations of abnormal EPB41L4A-AS level on the viability and motility of gastric cancer cells were verified through cell counting kit-8 (CCK-8) and Transwell assay. Furthermore, luciferase activity assay was performed to confirm the sponge molecule microRNA-17-5p (miR-17-5p) of EPB41L4A-AS1.
Results
EPB41L4A-AS1 was decreased in gastric cancer, and low EPB41L4A-AS1 level indicated resultful diagnostic value. Overexpression of EPB41L4A-AS1 inhibited the activity of gastric cancer cells, while knockdown of EPB41L4A-AS1 promoted tumor deterioration. EPB41L4A-AS1 directly targeted and regulated the expression ofmiR-17-5p.
Conclusion
This study elaborated that EPB41L4A-AS1 is lowly expressed in gastric cancer. Silencing EPB41L4A-AS1 was beneficial to cell proliferation, migration, and invasion. EPB41L4A-AS1 provides a new possibility for the diagnosis of gastric cancer patients by targeting miR-17-5p.
Similar content being viewed by others
Background
Gastric cancer starts from the gastric mucosa, mostly occurs in a single center, and may occur in any part of the stomach. More than 70% of gastric cancer cases worldwide occur in developing countries, especially in East Asia [1]. In China, an annual number of new gastric cancer patients reached 400,000, and the vast majority are diagnosed in advanced stages [2]. The etiology of gastric cancer has not been fully elucidated at present, but the investigation through domestic and foreign epidemiological has found some risk factors that may be highly correlated with the occurrence of gastric cancer, such as dietary patterns, underlying gastric diseases, and helicobacter pylori infection [3, 4]. However, the treatment of gastric cancer varies from person to person, and no systematic standard has been formed, and the prognostic effect is very general. Therefore, how to prevent the occurrence and development of gastric cancer is the focus of our research and attention.
Although long noncoding RNAs (lncRNAs) have not capable of translating proteins, they have shown functions in molecular principles and regulatory mechanisms in diseases [5]. In the discussion of gastric cancer, a large amount of evidence has revealed that lncRNAs may affect tumor progression by binding to microRNAs (miRNAs). For example, Huang et al. claimed that lncRNA TPTEP1 targeting miR-548d-3p suppressed the development of gastric cancer and may provide a new opportunity for subsequent therapy [6]. Yang et al. found that lncRNA CCAT1/miR-140-3p axis drives gastric cancer progression by increasing cell autophagy [7]. In addition, many lncRNAs have been elaborated to be associated with the diagnosis and prediction of gastric cancer [8].
EPB41L4A-AS1 is located in the chromosome 5q22.2 region, which is more than 3.8 kb in length and contains three exons [9]. In the previous literature, EPB41L4A-AS1 was described as a promising biomarker in the exploration of non-small cell lung cancer (NSCLC), chronic obstructive pulmonary disease, and type 2 diabetes [10,11,12]. Additionally, EPB41L4A-AS1 has regulatory capabilities in tumor metabolic reprogramming (glycolysis and glutaminolysis), which may serve as an effective target for cancer therapy [13]. However, the regulation of EPB41L4A-AS1 on gastric cancer has not been reported. Here, we examined the EPB41L4A-AS1 level in sample serum and cells, while evaluated the association of abnormal expression with clinical characteristics of patients and its diagnostic value. Furthermore, the regulation of EPB41L4A-AS1 sponge downstream miRNA on gastric cancer progression was revealed according to the cell viability and motility level. It further provides a new reference and theoretical basis for the development of diagnostic methods for gastric cancer.
Methods
Patient recruitment and sample acquisition
During the period from January to December 2022, blood samples were obtained from 110 gastric cancer patients recruited from The Second Affiliated Hospital of Xi’an Jiaotong University, while healthy blood samples were acquired from matched volunteers. The collected blood samples were centrifuged at 2000 r/pm for 15 min, and the extracted serum was stored in an ultra-low temperature refrigerator. The experiments mentioned were all conducted with the approval and supervision of the hospital Ethics Committee. Informed consent has been obtained from the participants involved. Under the premise of voluntary participation of all gastric cancer patients and volunteers, patients with multiple diseases, patients with adjuvant chemotherapy, and patients with cardiovascular and cerebrovascular diseases were excluded, and the clinicopathological data were recorded.
Cell culture
Two gastric cancer cell lines (AGS and MGC-803) and gastric epithelial cell lines (GES-1) were derived from ATCC. RPMI 1640 medium was used to provide nutrients for the growth of the cells, and 10% fetal bovine serum (FBS; GE Healthcare Life Sciences, USA) was transferred at the time of configuration. The incubation environment for the cells was a humidified 37°C chamber containing 5% CO2.
Transfection of cells
AGS and MGC-803 cells were transferred to culture plates and continued to incubate for about 12 h. Then, in the presence of Lipofectamine 3000 reagent (Invitrogen, USA), the overexpression plasmid (pcDNA3.1-EPB41L4A-AS1), the silencing plasmid (si-EPB41L4A-AS1) and the matched control group were transferred into AGS and MGC-803 cells, and the reconstructed cells were obtained after 48 h.
The growth and motility properties of cells
The proliferation level of the cells was determined by CCK-8 kit (Beyotime, China). Specifically, 100 µL of the cell suspensions absorbed by a pipettes gun (Eppendorf, Germany) were inoculated into 96-well plates, followed by the addition of CCK-8 solution every 24 h, and the optical density (OD)value (450 nm) was measured after 2 h of incubation in the culture chamber. The motility ability of the cells was identified by Transwell assay. For migration assays, RPMI 1640 medium (200 µL) and cells with completed transfection were distributed above, and RPMI 1640 medium (800 µL) containing FBS was placed below. The cells that had moved below were counted after 48 h. The assessment of the cell invasion level requires the Matrigel-coated (BD Biosciences, USA) operation on the top of the system.
Detection of PCR
RNA in sample serum and cells was extracted after the addition of TRIzol reagent (Thermo Fisher Scientific, USA), and its concentration was determined as soon as possible. Then cDNA was obtained by adding reagents on TaqMan Reverse Transcription kit (Applied Biosystems, USA) with RNA as template. SYBR Green Master Mix kit (Thermo Fisher, USA) was configured with RT-qPCR system for detection, and GAPDH and U6 were used for normalization of expression.
Molecular mechanism study and luciferase activity assay
Bioinformatics analysis of EPB41L4A-AS1 was performed on the meridian database, and the prediction was further verified with the help of luciferase activity assays. wild type (WT)-EPB41L4A-AS1 was generated with the involvement of pmirGLO vector, and mutant type (MUT)-EPB41L4A-AS1 was constructed after mutating the binding site of EPB41L4A-AS1 and miR-17-5p. Based on the presence of Lipofectamine 3000 reagent, WT/MUT-EPB41L4A-AS1 was co-transfected into AGS cells with miR-17-5p mimic, miR-17-5p inhibitor or negative control. Finally, the luciferase activity of AGS cells was evaluated by the assay system.
Statistical analysis
The data were processed by GraphPad Prism software 7.0 and IBM SPSS software 20.0 and displayed as mean ± standard deviation (SD). Comparison between different groups was performed by t-test or one-way analysis of variance. The chi-square test was used to evaluate the relationship between EPB41L4A-AS1 expression and clinicopathological features. In addition, the ROC curve demonstrated the sensitivity and specificity of EPB41L4A-AS1 in the diagnosis of gastric cancer. More than three biological replicates were performed for each set of experiments. P < 0.05 was statistically significant.
Results
EPB41L4A-AS1 is down-regulated in gastric cancer
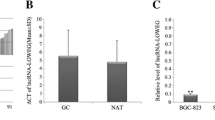
The decreased EPB41L4A-AS1 level in gastric cancer serum was determined by RT-qPCR in Fig. 1A. Similarly, EPB41L4A-AS1 was downregulated in gastric cancer AGS and MGC-803 cells compared with GES-1 cells (Fig. 1B). It is concluded that EPB41L4A-AS1 is negatively expressed in gastric cancer.
Diagnostic potential and clinical relevance of EPB41L4A-AS1
The ROC curve was plotted based on EPB41L4A-AS1 expression in normal and tumor serum. As shown in Fig. 1C, the AUC was 0.949, and the high sensitivity and specificity (94.5% and 83.6%) suggested the diagnostic ability of EPB41L4A-AS1 in gastric cancer. Next, we explored the clinical relevance of EPB41L4A-AS1. In Table 1, the included gastric cancer patients were categorized into low-group (n = 66) and high-group (n = 44) by the mean value of EPB41L4A-AS1 expression. Abnormal EPB41L4A-AS1 expression was prominently associated with TNM stage (P = 0.024) and lymph node metastasis (P = 0.035) in gastric cancer patients.
Effect of aberrant EPB41L4A-AS1 expression on the viability of gastric cancer cells
To clarify the influence of aberrant EPB41L4A-AS1 expression on gastric cancer progression, we designed and obtained overexpression and silencing EPB41L4A-AS1 (pcDNA3.1-EPB41L4A-AS1 and si-EPB41L4A-AS1) fragments and transfected them into AGS and MGC-803 cells, and the transfection results were shown in Fig. 2A and B. In Fig. 2C, pcDNA3.1-EPB41L4A-AS1 reduced the proliferation rate of AGS cells, while si-EPB41L4A-AS1 promoted the cell proliferation, as did MGC-803 cells (Fig. 2D). In addition, pcDNA3.1-EPB41L4A-AS1 also suppressed the number of migration (Fig. 2E and F) and invasion (Fig. 2G and H), whereas si-EPB41L4A-AS1 had the opposite effect in AGS and MGC-803 cells. These results strongly confirmed that knockdown EPB41L4A-AS1 enhanced the cell viability and motility capacity.
Mediation of aberrant EPB41L4A-AS1 expression to gastric cancer cells. Transfection results in AGS and MGC-803 cells (A and B). pcDNA3.1-EPB41L4A-AS1 reduced the proliferation rate (C and D), migration (E and F) and invasion (G and H) number of gastric cancer cells, while si-EPB41L4A-AS1 promoted the cell activity. (*P < 0.05, **P < 0.01, ***P < 0.001)
EPB41L4A-AS1 targets and regulates the mir-17-5p expression
The binding sites between EPB41L4A-AS1 and miR-17-5p were predicted by Starbase website in Fig. 3A. Therefore, we further verified the fact that EPB41L4A-AS1 sponge miR-17-5p via luciferase activity assay. Figure 3B illustrates that luciferase activity was downregulated when co-transfected with WT-EPB41L4A-AS1 and miR-17-5p mimic and upregulated when transfected with miR-17-5p inhibitor. miR-17-5p was upregulated in serum and cell samples as shown in Fig. 3C and D. Moreover, elevated EPB41L4A-AS1 inhibited the miR-17-5p expression (Fig. 3E), suggesting thatEPB41L4A-AS1 had a negative regulatory effect on miR-17-5p.
EPB41L4A-AS1 targets and regulates the miR-17-5p level. (A) EPB41L4A-AS1 binds to miR-17-5p and has binding targets. (B) EPB41L4A-AS1 directly targeted miR-17-5p. (C-D) miR-17-5p was actively expressed in gastric cancer serum and cells. (E) After overexpression of EPB41L4A-AS1, the miR-17-5p level in cells decreased. (*P < 0.05, ***P < 0.001)
Overexpression of EPB41L4A-AS1 inhibited the miR-17-5p expression, while transfection with miR-17-5p mimic restored EPB41L4A-AS1 levels (Fig. 4A). Similarly, co-transfection of pcDNA3.1-EPB41L4A-AS1 and miR-17-5p mimic reversed the repressive effect of EPB41L4A-AS1 upregulation on the biological functions of gastric cancer cells (Fig. 4B and D).
miR-17-5p mimic restored the inhibition of pcDNA3.1-EPB41L4A-AS1 on gastric cancer cell activity. (A) miR-17-5p content in cells after co-transfection with pcDNA3.1-EPB41L4A-AS1 and miR-17-5p mimic. (B-D) Overexpression of miR-17-5p improved the suppression of pcDNA3.1-EPB41L4A-AS1 on the biological function of gastric cancer cells. (*P < 0.05, **P < 0.01, ***P < 0.001, vs. control; #P < 0.05, ##P < 0.01, vs. pcDNA3.1-EPB41L4A-AS1)
Discussion
Gastric cancer is a fatal tumor with high incidence, and the age of onset tends to be younger with the change of daily dietary structure [14]. In clinical application at the present stage, ultrasound gastroscopy and other related imaging examinations are generally used for early screening of patients [15], and surgical treatment, targeted therapy and chemotherapy are used for ameliorative treatment. As the early symptoms of patients are not obvious and they mainly show mild discomfort symptoms such as abdominal distension and dyspepsia, so they are often ignored as ordinary gastritis. As a result, a considerable number of patients with gastric cancer enter the middle and late stage when they are diagnosed, and their postoperative survival conditions are not ideal [16]. Therefore, it is still a serious challenge to develop a convenient and efficient prediction method for gastric cancer.
LncRNAs mediating the occurrence of various diseases have been accepted by most researchers. Through our investigation, multiple lncRNAs have been confirmed to play an effective role in the diagnosis, prognosis, and cure of gastric cancer. For example, lncRNA CRART16 promoted the formation of blood vessels in patients through the miR-122-5p/FOS axis, which can be regarded as a prognostic factor for gastric cancer [17]. LncRNA TP53TG1 was low expressed in gastric cancer, and TP53TG1 obstructed the cell cycle and process, which broaden the field of treatment for gastric cancer [18]. In addition, downregulation of lncRNA APTR has been confirmed to be closely related to the diagnosis and prognosis of gastric cancer, and knockdown of APTR was conducive to the proliferation and migration of gastric cancer cells [5]. Consistent with the previous study, EPB41L4A-AS1 was found to be a tumor suppressor and its expression was reduced both in serum samples, AGS and MGC-803 cells, which was associated with lymph node metastasis and TNM stage. In addition, EPB41L4A-AS1 was also downregulated in type 2 diabetes mellitus, Alzheimer’s disease and several cancers [19,20,21]. In the subsequent cell assays, overexpression of EPB41L4A-AS1 markedly inhibited the viability of AGS and MGC-803 cells, while silencing EPB41L4A-AS1 facilitated the growth and movement level of cells. This is similar to the effect of EPB41L4A-AS1 in NSCLC, and increased EPB41L4A-AS1 reduced the proliferation level of NSCLC cells [10].
Importantly, miR-17-5p was a target molecule of EPB41L4A-AS1 and was enriched in gastric cancer. miR-17-5p has been proposed as a key miRNA involved in tumor growth. It has been reported that outstanding expression of hsa_circ_0017252 may suppress the deterioration of gastric cancer through directly targets miR-17-5p [22]. Yang et al. previously indicated that miR-17-5p was elevated in colon cancer, and DNMBP-AS1 targeted miR-17-5p to inhibit tumor metastasis [23]. Positive expression of miR-17-5p that binds to lncRNAs has also been reported in rheumatoid arthritis, myocardial injury, and hepatocellular carcinoma [24,25,26]. In the latest literature, LINC01798/miR-17-5p axis improved the prognosis of patients with lung adenocarcinoma, and miR-17-5p level decreased after transfection of pcDNA3.1-LINC01798 [27]. This study also demonstrated that miR-17-5p expression was downregulated when EPB41L4A-AS1 was highly expressed. More deeply, silencing miR-17-5p controlled the carcinogenesis rate of gastric cancer by targeting the downstream protein PTEN, which brought new hope for patients, as confirmed by Sun et al [28]. Meanwhile, the recovery assay clarified that transfection with miR-17-5p mimic counteracted the inhibitory effect of pcDNA3.1-EPB41L4A-AS1 on the biological function of gastric cancer cells.
Inevitably, our study still has some shortcomings and regrets. First, the included sample size was limited and there were regional constraints. Secondly, there is a lack of in vivo experimental evidence, and the target gene was single. In future studies, we will increase the diversity and generalization of the sample population when possible, and further systematically explore the pathological mechanism of gastric cancer to better confirm our findings.
Conclusions
In summary, the present study illustrated that EPB41L4A-AS1 was remarkably downregulated in gastric cancer, and knockdown EPB41L4A-AS1 accelerated the growth and motility of the cells through sponging miR-17-5p, leading to the deterioration of the tumor. EPB41L4A-AS1 has high diagnostic ability and may become a biological factor for the prediction and treatment of gastric cancer.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- EPB41L4A:
-
AS1 lncRNA EPB41L4A-AS1
- lncRNAs:
-
long noncoding RNAs
- miRNAs:
-
microRNAs
- NSCLC:
-
non-small cell lung cancer
References
Marimuthu SP, Vijayaragavan P, Moysich KB, Jayaprakash V. Diabetes mellitus and gastric carcinoma: is there an association? J Carcinog. 2011;10:30.
Lim C, Xu JC, Chen TY, Xu JX, Chen WF, Hu JW, et al. Ubiquitin-specific peptide 22 acts as an oncogene in gastric cancer in a son of sevenless 1-dependent manner. Cancer Cell Int. 2020;20:45.
Zheng GL, Zhang GJ, Zhao Y, Zheng ZC. Screening protein prognostic biomarkers for stomach adenocarcinoma based on the Cancer Proteome Atlas. Front Oncol. 2022;12:901182.
Martínez-Barriocanal Á, Arango D, Dopeso H. PVT1 long non-coding RNA in gastrointestinal Cancer. Front Oncol. 2020;10:38.
Ren A, Li Q, Guo Y, Cui X, Wang L, Huo Y, et al. Low expression of lncRNA APTR promotes gastric cancer progression. Transl Oncol. 2022;25:101506.
Huang Y, Wang J, Zhang H, Xiang Y, Dai Z, Zhang H, et al. LncRNA TPTEP1 inhibits the migration and invasion of gastric cancer cells through miR-548d-3p/KLF9/PER1 axis. Pathol Res Pract. 2022;237:154054.
Yang F, Peng ZX, Ji WD, Yu JD, Qian C, Liu JD, et al. LncRNA CCAT1 Upregulates ATG5 to Enhance Autophagy and promote gastric Cancer Development by absorbing miR-140-3p. Dig Dis Sci. 2022;67(8):3725–41.
Ghanei M, Poursheikhani A, Aarabi A, Taghechian N, Abbaszadegan MR. Inconsistency in the expression pattern of a five-lncRNA signature as a potential diagnostic biomarker for gastric cancer patients in bioinformatics and in vitro. Iran J Basic Med Sci. 2022;25(6):704–14.
Zhu Y, Liu Q, Liao M, Diao L, Wu T, Liao W, et al. Overexpression of lncRNA EPB41L4A-AS1 induces metabolic reprogramming in trophoblast cells and placenta tissue of Miscarriage. Mol Ther Nucleic Acids. 2019;18:518–32.
Dong B, Zhang F, Zhang W, Gao Y. IncRNA EPB41L4A-AS1 mitigates the proliferation of Non-small-cell Lung Cancer cells through the miR-105-5p/GIMAP6 Axis. Crit Rev Eukaryot Gene Expr. 2023;33(2):27–40.
Liu S, Fu Y, Ziebolz D, Li S, Schmalz G, Li F. Transcriptomic analysis reveals pathophysiological relationship between chronic obstructive pulmonary disease (COPD) and periodontitis. BMC Med Genomics. 2022;15(1):130.
Yu T, Xu B, Bao M, Gao Y, Zhang Q, Zhang X, et al. Identification of potential biomarkers and pathways associated with carotid atherosclerotic plaques in type 2 diabetes mellitus: a transcriptomics study. Front Endocrinol (Lausanne). 2022;13:981100.
Liao M, Liao W, Xu N, Li B, Liu F, Zhang S, et al. LncRNA EPB41L4A-AS1 regulates glycolysis and glutaminolysis by mediating nucleolar translocation of HDAC2. EBioMedicine. 2019;41:200–13.
Yu H, Dong A, Zhao L, Li P, Zhang Q, Lu J, et al. Intervention effect of Probiotics in Gastric Cancer patients with complications of Coronary Heart Disease and Heart failure. J Oncol. 2021;2021:1620891.
Liu Q, Zeng X, Wang W, Huang RL, Huang YJ, Liu S, et al. Awareness of risk factors and warning symptoms and attitude towards gastric cancer screening among the general public in China: a cross-sectional study. BMJ Open. 2019;9(7):e029638.
Zeng C, He R, Dai Y, Lu X, Deng L, Zhu Q, et al. Identification of TGF-β signaling-related molecular patterns, construction of a prognostic model, and prediction of immunotherapy response in gastric cancer. Front Pharmacol. 2022;13:1069204.
Zhang J, Pang X, Lei L, Zhang J, Zhang X, Chen Z, et al. LncRNA CRART16/miR-122-5p/FOS axis promotes angiogenesis of gastric cancer by upregulating VEGFD expression. Aging. 2022;14(9):4137–57.
Fang D, Ou X, Sun K, Zhou X, Li Y, Shi P, et al. m6A modification-mediated lncRNA TP53TG1 inhibits gastric cancer progression by regulating CIP2A stability. Cancer Sci. 2022;113(12):4135–50.
Wang Z, Liao W, Liu F, Yang T, Xie W, Liao M, et al. Downregulation of lncRNA EPB41L4A-AS1 mediates activation of MYD88-Dependent NF-κB pathway in diabetes-related inflammation. Diabetes Metab Syndr Obes. 2021;14:265–77.
Yang T, Wang Y, Liao W, Zhang S, Wang S, Xu N, et al. Down-regulation of EPB41L4A-AS1 mediated the brain aging and neurodegenerative diseases via damaging synthesis of NAD(+) and ATP. Cell Biosci. 2021;11(1):192.
Krstic J, Deutsch A, Fuchs J, Gauster M, Gorsek Sparovec T, Hiden U et al. (Dis)similarities between the decidual and Tumor Microenvironment. Biomedicines. 2022;10(5).
Song J, Xu X, He S, Wang N, Bai Y, Li B, et al. Exosomal hsa_circ_0017252 attenuates the development of gastric cancer via inhibiting macrophage M2 polarization. Hum Cell. 2022;35(5):1499–511.
Yang L, Yang T, Wang H, Dou T, Fang X, Shi L, et al. DNMBP-AS1 regulates NHLRC3 expression by sponging miR-93-5p/17-5p to inhibit Colon cancer progression. Front Oncol. 2022;12:765163.
Duan B, Yu Z, Liu R, Li J, Song Z, Zhou Q, et al. Tetrandrine-induced downregulation of lncRNA NEAT1 inhibits rheumatoid arthritis progression through the STAT3/miR-17-5p pathway. Immunopharmacol Immunotoxicol. 2022;44(6):886–93.
Li Q, Bu Y, Shao H, Li W, Zhao D, Wang J. Protective effect of bone marrow mesenchymal stem cell-derived exosomes on cardiomyoblast hypoxia-reperfusion injury through the HAND2-AS1/miR-17-5p/Mfn2 axis. BMC Cardiovasc Disord. 2023;23(1):114.
Mao Z, Nie Y, Jia W, Wang Y, Li J, Zhang T et al. Revealing Prognostic and Immunotherapy-sensitive characteristics of a Novel cuproptosis-related LncRNA model in Hepatocellular Carcinoma patients by Genomic Analysis. Cancers (Basel). 2023;15(2).
Li X, Zhu G, Li Y, Huang H, Chen C, Wu D, et al. LINC01798/miR-17-5p axis regulates ITGA8 and causes changes in tumor microenvironment and stemness in lung adenocarcinoma. Front Immunol. 2023;14:1096818.
Yifei S, Chunxiao H, Dinuo L. MiR-17-5p inhibits the proliferation and metastasis of gastric Cancer cells by targeting PTEN protein. Altern Ther Health Med. 2022;28(8):23–9.
Acknowledgements
Not Applicable.
Funding
This study was funded by Shaanxi Natural Science Basic Research Program (No. 2020JQ-555).
Author information
Authors and Affiliations
Contributions
J.C. M, Y.Y. F and H. G designed the research study. J.K. X, Z.Y. L and J.Y. L performed the research. J.C. M, Y.Y. F analyzed the data. H. G wrote the manuscript. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The experiments mentioned were all conducted with the approval and supervision of the hospital Ethics Committee. Informed consent has been obtained from the participants involved.
Consent for publication
Not Applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Ma, J., Feng, Y., Xu, J. et al. Downregulation of lncRNA EPB41L4A-AS1 promotes gastric cancer cell proliferation, migration and invasion. BMC Gastroenterol 24, 136 (2024). https://doi.org/10.1186/s12876-024-03216-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12876-024-03216-9