Abstract
Objective
The aim of this study was to determine whether serum vitamin D levels are associated with H. pylori infection and whether low serum vitamin D levels are an independent risk factor for H. pylori infection.
Methods
We conducted a retrospective analysis of a multicenter cohort study from 2017 to 2019. A total of 415 H. pylori+ patients and 257 H. pylori− patients aged between 18 and 75 years with both 13 C-urea breath test and serum vitamin D level results were included from four hospitals. A questionnaire was used to collect information on potential factors influencing H. pylori infection.
Results
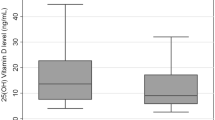
Serum vitamin D levels were significantly lower in the H. pylori+ group than in the H. pylori− group (16.7 ± 6.6 ng/ml vs. 19.2 ± 8.0 ng/ml, p < 0.05). Using a cutoff value of 20 ng/ml, the H. pylori infection rate was significantly higher in the vitamin D-deficient group (< 20 ng/ml) than in the vitamin D-nondeficiency group (≥ 20 ng/ml) (66.5% vs. 51.0%, p < 0.001). Ordered logistic regression analysis showed that serum vitamin D levels < 20 ng/ml (OR: 1.652, 95% CI: 1.160–2.351, p = 0.005), higher education levels (OR: 1.774, 95% CI: 1.483–2.119, p < 0.001), family size ≥ 4 (OR: 1.516, 95% CI: 1.081–2.123, p = 0.016), and lower annual income (OR: 1.508, 95% CI: 1.289–1.766, p < 0.001) were independent risk factors for H. pylori infection.
Conclusion
Lower serum vitamin D levels may be associated with an increased risk of H. pylori infection, and lower serum vitamin D levels are an independent risk factor for increasing H. pylori infection rates. Randomized controlled trials are needed to determine whether supplementation with vitamin D can reduce H. pylori infection rates.
Similar content being viewed by others
Introduction
Helicobacter pylori (H. pylori) infection is currently the most important controllable risk factor for gastric cancer, with 90% of noncardia gastric cancers being associated with H. pylori infection [1,2,3,4,5]. China is one of the countries with a high prevalence of H. pylori infection, with an overall infection rate of 56.22%. The infection rate of H. pylori in Tibet is as high as 84.62%, ranking first in the world [6]. There are variations and trends in H. pylori infection rates among different regions in China. Using the carbon-urea breath test as the diagnostic method for current infection, the lowest current infection rate was found in Guangdong Province at 42%, while the highest was in Shaanxi Province at 64%. Therefore, preventing H. pylori infection is crucial for reducing the incidence of gastric cancer in China.
Vitamin D is a micronutrient that regulates bone metabolism. However, several studies have found that vitamin D3 decomposition product 1 (VDP1) can induce cell membrane collapse, leading to the lysis of H. pylori cells [7, 8]. Additionally, vitamin D3 can reactivate the acidification and degradation function of autolysosomes through the activation of the PDIA3-STAT3-MCOLN3-Ca2+ axis, thereby eliminating the survival of H. pylori hidden in the autophagy of cells [9]. Vitamin D3 can also induce the VDR-CAMP signaling pathway to eradicate H. pylori in the stomach [10]. Furthermore, it can protect gastric mucosal epithelial cells against H. pylori infection-induced apoptosis through the VDR-dependent c-Raf/MEK/ERK pathway [11].
However, there is currently controversy regarding the relationship between serum vitamin D levels and H. pylori infection in clinical studies. In a cross-sectional study conducted in 2007 on end-stage renal disease patients undergoing maintenance dialysis, a significant positive correlation was found between serum vitamin D levels and serum H. pylori− specific IgG antibody titers (r = 0.36, P = 0.043) [12]. This suggests that vitamin D analogs may have antibacterial effects against H. pylori. Another study conducted in Japan on healthy elderly women aged 70–99 years in nursing homes found that the prevalence of H. pylori infection was significantly lower in subjects receiving vitamin D3 supplementation than in unheated individuals (p < 0.05), indicating a suppressive effect of long-term vitamin D3 intake on H. pylori infection [13]. Antonio Antico et al. also found that serum vitamin D levels were significantly lower in H. pylori− related gastric inflammation patients than in healthy individuals, suggesting that individuals with lower serum vitamin D levels may be more susceptible to H. pylori infection [14]. A clinical study conducted in Italy found that the proportion of vitamin D deficiency in the H. pylori+ group was significantly higher than that in the H. pylori− group (86% vs. 67.3%, P = 0.014) [15]. A cross-sectional study of 294 patients who visited a hospital in Lebanon in 2016 for dietary habits and H. pylori infection found that participants with a university degree or higher education (OR = 2.74; CI = 1.17–6.44), patients with a history of peptic ulcer disease (OR = 3.80; CI = 1.80–8.01), stomach adenocarcinoma (OR = 3.99; CI = 1.35–11.83), and those with vitamin D levels below normal (OR = 29.14; CI = 11.77–72.13) had a higher risk of H. pylori infection [16]. A cross-sectional study of individuals aged 65 and older found that the proportion of patients with H. pylori+ and vitamin D deficiency (< 20 ng/mL) was higher than that of the H. pylori− group (86% vs. 67.3%, p = 0.014). The proportion of H. pylori+ patients decreased with increasing serum vitamin D levels (p = 0.010) [17]. A large cross-sectional study conducted in infants and young children found that the prevalence of vitamin D deficiency in the H. pylori+ and H. pylori− groups was 20.7% and 12.1%, (P < 0.001) [18]. A study of a large electronic database of medical records from the Israeli population’s health maintenance organization found a negative correlation between serum vitamin D levels and H. pylori infection (p < 0.001). The odds of H. pylori detection being positive were 31% higher in patients with serum vitamin D levels < 20 ng/mL than in those with levels ≥ 20 ng/mL (OR 1.31, 99% CI 1.22–1.4, p < 0.001) [19]. However, a community-based study from Taiwan, which included 1126 H. pylori+ and 987 H. pylori− patients, found no significant difference in the average serum vitamin D levels between the two groups. Further stratification by age also did not reveal any differences [20].
Due to the limited and controversial clinical studies on the relationship between serum vitamin D levels and H. pylori infection, we conducted a retrospective analysis of a cohort study to determine the impact of serum vitamin D levels on H. pylori infection and whether low serum vitamin D levels independently increase the risk of H. pylori infection.
Data and methods
Study subjects
We conducted a retrospective analysis of case data from a multicenter cohort study conducted between 2017 and 2019. The study included a total of 496 H. pylori − positive (H. pylori+) and 257 H. pylori − negative (H. pylori−) patients between the ages of 18 and 75 who had 13 C-urea breath test results and serum levels of vitamin D measured using chemiluminescence assay. The study was conducted at Xijing Hospital of Air Force Medical University, Xianyang Central Hospital, The First Affiliated Hospital of Zhengzhou University, and West Zone General Hospital. Information on H. pylori+ and H. pylori− patients was collected using a questionnaire on the factors affecting H. pylori infection. Exclusion criteria included the following: (a) age less than 18 years old or over 75 years old; (b) previous history of H. pylori eradication therapy; (c) previous history of gastric surgery; (d) pregnancy or lactation; (e) major systemic diseases (e.g., hypertension, diabetes, hypothyroidism, metabolic syndrome, adrenal insufficiency, inflammatory bowel disease, systemic lupus erythematosus, rheumatoid arthritis, systemic vasculitis, HIV/AIDS, etc.); (f) diseases affecting serum vitamin D levels (e.g., hyperthyroidism, malabsorption, rickets, bone tumors, Cushing’s syndrome, severe liver disease with Child‒Pugh class B or C, renal failure with serum creatinine > 177 µmol/L, alcoholism); (g) use of antibiotics, acid-suppressing drugs or bismuth preparations within the past 8 weeks; (h) daily supplementation of vitamin D; and (i) incomplete case data. Finally, a total of 415 H. pylori+ and 257 H. pylori− patients were included.
Statistical analysis
Statistical analysis was conducted using SPSS version 22.0 software (IBM, Armonk, NY, USA). Double-entry and validation were used to ensure the accuracy of the data collected from the cohort study. The normality of continuous variables was tested using the Kolmogorov‒Smirnov test, and nonnormally distributed variables are presented as medians (range), while normally distributed variables are presented as the means ± SDs. Group comparisons were performed using t tests, Wilcoxon rank-sum tests, or chi-square tests as appropriate. Two-tailed P < 0.05 was considered statistically significant. Univariate analysis was used with H. pylori infection as the dependent variable and various factors as independent variables, and ordered logistic regression was used to determine if lower levels of serum vitamin D were independent risk factors for increased H. pylori infection risk. The strength of association was measured using odds ratios (ORs) and 95% confidence intervals (95% CIs).
Results
Patient characteristics
As shown in Table 1, information on H. pylori+ and H. pylori− patients was collected using a questionnaire on the factors affecting H. pylori infection, including sex, age, occupation, residential area, body type, marital status, education level, family size, annual income, cigarette smoking, alcohol consumption, history of periodontal disease, hygiene of dining place, main source of drinking water, drinking unheated water, and vitamin D serum level. The hygiene of dining place was self-assessed by the patients using the questionnaire as either clean and hygienic or relatively poor. The main source of drinking water for patients was assessed using the questionnaire. If it came from purified water or tap water, it was defined as clean. If it came from well water, river water, or spring water, it was defined as probably polluted.
As shown in Fig. 1, there were a total of 496 H. pylori+ and 257 H. pylori− patients in the cohort. After excluding 81 H. pylori+ patients with incomplete data, a total of 415 H. pylori+ and 257 H. pylori− patients were included in the analysis. Comparing the serum vitamin D levels between the two groups, the H. pylori+ group had significantly lower levels than the H. pylori− group (16.7 ± 6.6 ng/ml vs. 19.2 ± 8.0 ng/ml, p < 0.001), as shown in Table 2.
Difference in H. pylori infection rate between patients with serum vitamin D levels < 20 ng/ml and ≥ 20 ng/ml
The patients were divided into a vitamin D deficiency group (< 20 ng/ml) and a nondeficiency group (≥ 20 ng/ml) according to a cutoff value of 20 ng/ml for serum vitamin D levels. The difference in the H. pylori infection rate between the two groups was compared, as shown in Table 3. The vitamin D deficiency group had a significantly higher H. pylori infection rate than the nondeficiency group (66.5% vs. 51.0%, p < 0.001), suggesting a possible association between vitamin D deficiency and a higher H. pylori infection rate.
Univariate analysis of factors influencing the H. pylori Infection rate
Univariate analysis was conducted on all factors collected using a questionnaire on the factors affecting H. pylori infection in both the H. pylori+ and H. pylori− groups. As shown in Table 1, there were no significant differences in sex, age, occupation, residential area, body type, cigarette smoking, alcohol consumption, history of periodontal disease, hygiene of dining place, main source of drinking water and drinking unheated water between the H. pylori+ and H. pylori− groups. However, there were significant differences in marital status (married vs. single, 60.1% vs. 84.8%, p = 0.001), education level (elementary school and below vs. middle school vs. university and above, 42.9% vs. 60.1% vs. 72.5%, p < 0.001), family size (< 4 vs. ≥ 4, 57.2% vs. 65.3%, p = 0.033), annual income (low vs. medium low vs. medium vs. medium high vs. high, 93.3% vs. 69.4% vs. 62.2% vs. 55.3% vs. 55.7%, p < 0.001), and serum vitamin D levels (< 20 ng/ml vs. ≥ 20 ng/ml, 66.5% vs. 51.0%, p < 0.001) between the two groups.
Multivariate analysis of factors influencing H. pylori Infection
As shown in Table 4, ordered logistic regression analysis was conducted on the factors with significant differences in the univariate analysis. The results revealed that serum vitamin D level < 20 ng/ml (OR: 1.652, 95% CI: 1.160–2.351, p = 0.005), higher education level (OR: 1.774, 95% CI: 1.483–2.119, p < 0.001), family size ≥ 4 (OR: 1.516, 95% CI: 1.081–2.123, p = 0.016), and lower annual income (OR: 1.508, 95% CI: 1.289–1.766, p < 0.001) were independent risk factors influencing H. pylori infection. Additionally, married was not found to be an independent protective factor for H. pylori infection (OR: 0.430, 95% CI: 0.182–1.012, p = 0.053).
Discussion
H. pylori infection is a significant risk factor for the development of gastric cancer in the majority of patients [1]. H. pylori infection accounts for 90% of noncardia gastric cancer cases [2]. Therefore, active prevention and treatment of H. pylori infection are essential for reducing the incidence of gastric cancer. Vitamin D is an important micronutrient that affects bone metabolism. However, basic research has demonstrated that vitamin D3 metabolites can induce the collapse of H. pylori cell membranes and eliminate H. pylori by activating multiple signaling pathways [7,8,9,10,11]. Serval clinical research has also shown that Vitamin D influences H. pylori infection and eradication [12,13,14,15,16,17,18,19,20,21,22,23,24,25].
Due to conflicting results on the association between serum vitamin D levels and H. pylori infection and the lack of large sample, multicenter, and multivariable case‒control studies, we designed this study to further explore whether serum vitamin D levels are an independent risk factor for H. pylori infection. Our study found that the H. pylori+ group had significantly lower serum vitamin D levels than the H. pylori− group (16.7 ± 6.6 ng/ml vs. 19.2 ± 8.0 ng/ml, p < 0.001). Defining vitamin D deficiency as serum vitamin D levels < 20 ng/ml, the prevalence of H. pylori infection was significantly higher in the vitamin D-deficient group than in the nondeficient group (66.5% vs. 51.0%, p < 0.001). Furthermore, ordered logistic regression analysis showed that serum vitamin D levels < 20 ng/ml (OR: 1.652, 95% CI: 1.160–2.351, p = 0.005), higher education level (OR: 1.774, 95% CI: 1.483–2.119, p < 0.001), larger family size (≥ 4 members) (OR: 1.516, 95% CI: 1.081–2.123, p = 0.016), and lower annual income (OR: 1.508, 95% CI: 1.289–1.766, p < 0.001) were independent risk factors for H. pylori infection. This suggests that lower serum vitamin D levels may be associated with an increased risk of H. pylori infection, and lower levels of serum vitamin D are independent risk factors for increased H. pylori infection rates. Interestingly, our study, along with another study, indicated that higher education levels were associated with higher rates of H. pylori infection. This could be due to higher education levels leading to a greater likelihood of undergoing H. pylori testing, resulting in relatively higher rates of H. pylori infection being detected. Although our sample size was relatively large, the lack of specific data on the impact of vitamin D on H. pylori infection rates hindered our ability to calculate the exact sample size. Additionally, our study did not include vitamin D supplementation; therefore, further evaluation in larger randomized controlled trials is required to assess whether vitamin D supplementation can reduce the rate of H. pylori infection.
Data Availability
The datasets used and analyzed during the current study are available from the corresponding author upon reasonable request.
References
Loor A, Dumitraşcu DL. Helicobacter pylori Infection, gastric cancer and gastropanel. Rom J Intern Med. 2016;54(3):151–6.
Herrero R, Park JY, Forman D. The fight against gastric cancer—the IARC Working Group report. Best Pract Res Clin Gastroenterol. 2014;28(6):1107–14.
Malfertheiner P, Megraud F, O’morain CA, et al. Management of Helicobacter pylori Infection—the Maastricht V/Florence consensus report. Gut. 2017;66(1):6–30.
Sugano K, Tack J, Kuipers EJ, et al. Kyoto global consensus report on Helicobacter pylori gastritis. Gut. 2015;64(9):1353–67.
Shiotani A, Cen P, Graham DY. Eradication of gastric cancer is now both possible and practical. Semin Cancer Biol. 2013;23(6 Pt B):492–501.
Zhang WD, Hu FL, Xiao SD, et al. Prevalence of Helicobacter pylori Infection in China [in Chinese]. Mod Dig Intervent. 2010;15(5):265–70.
Hosoda K, Shimomura H, Wanibuchi K, et al. Identification and characterization of a vitamin D3 decomposition product bactericidal against Helicobacter pylori. Sci Rep. 2015;5:8860. https://doi.org/10.1038/srep08860.
Wanibuchi K, Hosoda K, Ihara M, et al. Indene compounds synthetically derived from vitamin D have selective antibacterial action on Helicobacter pylori. Lipids. 2018;53(4):393–401.
Hu W, Zhang L, Li MX, Shen J, Liu XD, Xiao ZG, Wu DL, Ho IHT, Wu JCY, Cheung CKY, Zhang YC, Lau AHY, Ashktorab H, Smoot DT, Fang EF, Chan MTV, Gin T, Gong W, Wu WKK, Cho CH. Vitamin D3 activates the autolysosomal degradation function against Helicobacter pylori through the PDIA3 receptor in gastric epithelial cells. Autophagy. 2019;15(4):707–25.
Zhang Y, Wang C, Zhang L, Yu J, Yuan W, Li L. Vitamin D3 eradicates Helicobacter pylori by inducing VDR-CAMP signaling. Front Microbiol. 2022;13:1033201.
Zhao S, Wan D, Zhong Y, Xu X. 1α, 25-Dihydroxyvitamin D3 protects gastric mucosa epithelial cells against Helicobacter pylori-infected apoptosis through a vitamin D receptor-dependent c-Raf/MEK/ERK pathway. Pharm Biol. 2022;60(1):801–9.
Nasri H, Baradaran A. The influence of serum 25-hydroxy vitamin D levels on Helicobacter pylori Infections in patients with end‐stage Renal Failure on regular hemodialysis. Saudi J Kidney Dis Transpl. 2007;18(2):215–9.
Kawaura A, Takeda E, Tanida N, et al. Inhibitory effect of long term 1α-hydroxyvitamin D3 administration on Helicobacter pylori infection[J]. J Clin Biochem Nutr. 2006;38(2):103–6.
Antico A, Tozzoli R, Giavarina D, et al. Hypovitaminosis D as predisposing factor for atrophic type a gastritis: a case–control study and review of the literature on the interaction of vitamin D with the immune system[J]. Clin Rev Allergy Immunol. 2012;42(3):355–64.
Mut DS, Surmeli ZG, Bahsi R, et al. Vitamin D deficiency and risk of Helicobacter pylori Infection in older adults: a cross-sectional study[J]. Aging clinical and experimental research; 2018.
Assaad S, Chaaban R, Tannous F, Costanian C. Dietary habits and Helicobacter pylori Infection: a cross sectional study at a Lebanese hospital. BMC Gastroenterol. 2018;18(1):48.
Mut Surmeli D, Surmeli ZG, Bahsi R, Turgut T, Selvi Oztorun H, Atmis V, Varli M, Aras S. Vitamin D deficiency and risk of Helicobacter pylori Infection in older adults: a cross-sectional study. Aging Clin Exp Res. 2019;31(7):985–91.
Gao T, Zhao M, Zhang C, Wang P, Zhou W, Tan S, Zhao L. Association of Helicobacter pylori Infection with vitamin D Deficiency in infants and toddlers. Am J Trop Med Hyg. 2020;102(3):541–6.
Shafrir A, Shauly Aharonov M, Katz LH, Paltiel O, Pickman Y, Ackerman Z. The Association between Serum Vitamin D Levels and Helicobacter pylori Presence and Eradication. Nutrients. 2021;13(1):278.
Chen LW, Chien CY, Hsieh CW et al. The associations between Helicobacter pylori Infection, serum vitamin D, and metabolic syndrome: a community-based study[J]. Medicine, 2016, 95(18).
Yildirim O, Yildirim T, Seckin Y, Osanmaz P, Bilgic Y, Mete R. The influence of vitamin D deficiency on eradication rates of Helicobacter pylori. Adv Clin Exp Med. 2017;26(9):1377–81.
Huang B, Yan S, Chen C, Ye S. Effect of 25-hydroxyvitamin D on Helicobacter pylori eradication in patients with type 2 Diabetes. Wien Klin Wochenschr. 2019;131(3–4):75–80.
El Shahawy MS, Hemida MH, El Metwaly I, Shady ZM. The effect of vitamin D deficiency on eradication rates of Helicobacter pylori Infection. JGH Open. 2018;2(6):270–5.
Surmeli DM, Surmeli ZG, Bahsi R et al. Vitamin D deficiency and risk of Helicobacter pylori Infection in older adults: a cross-sectional study [Published online ahead of print Septebmer 28, 2018]. Aging Clin Exp Res. https://doi.org/10.1007/s40520-018-1039-1.
Han C, Ni Z, Yuan T, Zhang J, Wang C, Wang X, Ning HB, Liu J, Sun N, Liu CF, Shi M, Lu WQ, Shi YQ. Influence of serum vitamin D level on Helicobacter pylori eradication: a multi-center, observational, prospective and cohort study. J Dig Dis. 2019;20(8):421–6.
Acknowledgements
We thank Dr Yong Quan Shi from the Xijing Hospital of Digestive Diseases and Dr Yong Xi Wang from Xianyang Central Hospital for their contributions to the study. This work was supported by the grants from National Natural Science Foundation of China (No. 81873554) and Hospital management of The General Hospital Of Western Theater Command (No. 41C416AT).
Funding
This work was supported by the grants from National Natural Science Foundation of China (No. 81873554) and Hospital management of The General Hospital Of Western Theater Command (No. 41C416AT). The funders had no role in the study design, data collection, and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
Chuan Han, Dan Liu, Li Ren, Da Peng Zhong and Wei Zhang wrote the main manuscript text. Wen Wen Li and Jie Liu prepared Tables 1, 2, 3 and 4. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Ethical approval and consent to participate
This study was approved by the Ethics Committee of Xijing Hospital of Air Force Medical University (Approval No. KY20173035-1), Xianyang Central Hospital (Approval No. KY20171011-1), The First Affiliated Hospital of Zhengzhou University (Approval No. KY2017-33), and West Zone General Hospital (Approval No. KY20171023-1). The study was conducted in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration (revised in 2013 in Brazil). Written informed consent was obtained from all participants in the study.
Consent for publication
Not applicable.
Conflict of interest
There are no conflicts of interest.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Liu, D., Ren, L., Zhong, D. et al. Association of serum vitamin D levels on Helicobacter pylori infection: a retrospective study with real-world data. BMC Gastroenterol 23, 391 (2023). https://doi.org/10.1186/s12876-023-03037-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12876-023-03037-2