Abstract
Background
Adenosquamous carcinoma is a rare sub-type of colorectal cancer with a poor prognosis. Little is known about its clinicopathological and molecular characteristics in Asian populations. This study aimed to investigate these features in a cohort of patients with adenosquamous carcinoma in the colorectum.
Methods
Tumor cases pathologically diagnosed with colorectal adenosquamous carcinoma were retrieved from the Sixth Affiliated Hospital, Sun Yat-sen University tissue archive between December 2012 and June 2020. Clinicopathological features, molecular characteristics, and oncology outcomes were analyzed.
Results
Among 18,139 cases of colorectal cancer, 11 were diagnosed with adenosquamous carcinoma, providing an incidence rate of 0.061%. The median overall survival (OS) was 14 months, and the expected 3-year OS rate was 29.6%. As of October 14, 2022, four cases had local recurrence and five had distant metastasis. KRAS gene mutations were found in four of seven patients (57.1%), and three out of eleven (27.3%) patients had mismatch repair-deficient (dMMR) tumors.
Conclusions
Adenosquamous carcinoma is associated with a poor prognosis. Compared to other sub-types of colorectal cancer, a higher proportion of patients with dMMR and KRAS mutations were observed. These findings suggested that more patients with adenosquamous carcinoma could benefit from targeted therapies, such as immunotherapy.
Similar content being viewed by others
Background
Colorectal cancer is a significant medical and economic burden worldwide, particularly in developed countries [1]. Adenosquamous carcinoma is a rare subtype of colorectal cancer that accounts for only 0.06-0.18% of tumor incidence and has a poor prognosis [2]. This subtype contains both adenocarcinoma and squamous carcinoma components. Compared to adenocarcinoma, colorectal adenosquamous carcinoma (CASC) has a worse prognosis and is primarily treated with surgery [3].
Since CASC tumors are rare, most studies have been retrospective analyses. The most established retrospective studies have been database-based studies, such as the first NCI SEER database study, which found that patients with adenosquamous carcinoma had poor survival [4]. Another study by Masoomi found higher overall and colorectal-specific mortality of CASC compared with adenocarcinoma [3]. The latest database study based on the SEER cohort concluded that adenosquamous carcinoma tends to present with an advanced stage, poor differentiation, and poor overall survival (OS) [5]. However, studies based on Asian populations have been limited, and the molecular characteristics of CASC have seldom been mentioned in previous literature [6].
In the present study, we analyzed the molecular characteristics (KRAS, BRAF, and PIK3CA mutations, and the status of MMR) of CASC in Asian patients. Additionally, we investigated the clinicopathological characteristics of CASC patients by conducting a single-center cohort study with detailed follow-up information.
Materials and methods
Patients and clinical information
This retrospective, single-center study analyzed 11 patients with adenosquamous carcinoma from The Sixth Affiliated Hospital, Sun Yat-sen University, Guangzhou, China, identified between 14 and 2011 and 14 October 2020. Demographic characteristics, preoperative imaging, surgical procedures, preoperative and postoperative treatments, and other related information were collected.
Follow-up information
Follow-up started on the date of surgery and continued until October 14, 2022, with visits scheduled every 3 months during the first year, biannually from years 1 to 5, and annually after 5 years. Follow-up methods included a re-examination of outpatients and inpatients with medical records and telephone follow-ups. Imagine detection including CT (computed tomography), MRI (magnetic resonance imaging), pathological biopsy findings, and other collected data were used to define recurrence and metastasis. During the telephone follow-up, the patient’s family informed death, and the date of death was recorded according to the information provided by the receipt of a death certificate from the hospital. All the work was assisted by the Oncology Follow-up Center of The Sixth Affiliated Hospital, Sun Yat-sen University.
Pathology assessment
Individual cases were identified via surgical indices subjected to conventional processing. Two experienced pathologists individually reviewed specimens from CASC patients. Microscopically, the cancerous tissue contained both squamous cells and gland-like cells, which exhibited an irregular glandular duct-like and pore-like arrangement (Fig. 1). Either squamous carcinoma or adenocarcinoma accounts for at least 10% of the total tumor. predominantly squamous type of CASC refers to the squamous component accounting for 60% or more of the total tumor tissues. The patients were staged according to the American Joint Committee on Cancer (AJCC) staging (eighth edition)/International Union Against Cancer (UICC) TNM staging.
Pathology assessment (H&E staining) Top: post-surgery H&E stain of the sigmoid colon tumor resection specimen from a 59-year-old man with a stage IVc / T4bN2bM1c. The tumor tissue (arrowheads) consisted of both an adenocarcinoma component (10%) and a squamous cell carcinoma component (90%) Bottom: pathological response after neoadjuvant chemotherapy in a 59-year-old woman with stage IIB / T4bN0M0 - residual tissue contained mid-differentiated adenocarcinoma and mid-differentiated squamous cell carcinoma
Immunohistochemistry (IHC) for MMR status
Immunohistochemistry (IHC) was mainly used to make a definitive diagnosis and analyze MMR status. Antigens retrieved from silanized glass slides consist of tumor tissues. The sections were treated with the primary antibodies diluted in the background-reducing solution for CK5/6, p40, CK20, CDX2, and Ki-67. All negative control reactions were phosphate-buffered saline (PBS). Tumor makers for both components (Fig. 2): squamous cell carcinoma markers (CK5/6, p40) and adenocarcinoma markers (CK20, CDX2); MMR status: when there was a complete absence of nuclear staining in tumor cells, tumors were considered negative for MMR proteins (MLH1, MSH2, PMS2, or MSH6). Tumors that maintained expression of all MMR proteins were considered pMMR, otherwise, tumors were considered dMMR (Fig. 3). MMR status would be further confirmed by PCR-based MSI testing if the IHC result was uncertain,
KRAS, NRAS, BRAF, and PIK3CA mutation analysis
The assessments of KRAS, BRAF, and PIK3CA mutations were conducted by an adequate quality-control procedure in the Molecular Diagnostic Laboratory of the Sixth Affiliated Hospital of Sun Yat-sen University. Exon 2 (codon 12 and 13), exon 3 and exon 4 of KRAS, exon 2 (G12D) and exon 3 (Q61R/K) of NRAS, exon 9 (codon 542 and 545) and exon 20 (codon 1047) of PIK3CA, and exon 15 (codon 600) of BRAF were assessed. An ABI 9700 PCR system was used to conduct PCR amplification. Amplification was done in a 20µL reaction containing 50-100ng of DNA template and 500nM primers, with the following program: 5 min at 98 °C for initial denaturation followed by 45 cycles of 25 s at 95 °C, 25 s at 58 °C, and 25 s at 72 °C, and a final extension at 72 °C for 10 min. PCR products were purified and sequenced by using BigDye Terminator v3.1 Sequencing Standard Kit (Thermo Fisher Scientific, USA) with an ABI Prism 3500Dx Genetic Analyzer (Applied Biosystems, Foster City, CA).
Statistical analysis
The primary outcomes of this study were overall survival (OS) and disease-free survival (DFS). DFS was defined as the time from surgery to local or systemic recurrence or death, while OS was defined as the time from surgery to death from any cause. The survival curves for OS and DFS were estimated by the Kaplan-Meier method.
The reporting of this study conforms to the STROBE statement [7].
Results
Clinicopathological features
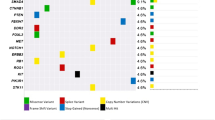
Out of the 18,139 patients diagnosed with colorectal cancer during the 10 years from 2011 to 2020, 11 patients were identified with colorectal adenosquamous carcinoma, a prevalence of 0.061% (Table 1). The tumors were predominately located on the left side (63.6%), with 8 cases in the colon and three cases in the rectum. The majority of patients were in an advanced clinical stage, with four cases (36.4%) classified as stage III and five cases (45.5%) as stage IV. The median tumor diameter was 6 cm, and only one case of adenosquamous carcinoma was detected through preoperative colonoscopy (Fig. 4). Microscopic examination revealed a predominantly squamous type (squamous cell carcinoma was the main tumor component) in six patients. Poorly differentiated components were seen in only two patients and the rest presented well or moderately differentiated regions of both components. Lymphovascular invasion was exhibited in two patients and Perineural invasion was in three, other histopathological characteristics: microabscess formation [8] was observed in four patients, tumor necrosis [9] was found in two cases and acellular mucin pools [10] were detected in one patient. Lymph node metastasis was noted in seven cases (63.64%). One patient (male, 22y, T1N0M0) had a family history of polyposis, and another patient (female, 64y, T4aN0M0) had a history of teratoma.
Molecular characteristics
Out of the 11 patients, eight were found to have pMMR (72.7%) and three had dMMR (27.3%). Only one patient was detected as Her-2 positive. The tumor cell nuclear proliferation index Ki-67 ranged from 25 to 70%, with a median of 40%. Among the seven patients who were tested for gene mutations: KRAS gene mutations were detected in four patients (57.1%). No mutations were detected in the NRAS gene, BRAF gene, and PIK3CA gene (Table 2).
Oncological outcomes and prognostic analysis
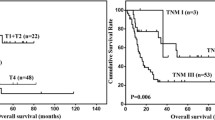
As of 14 October 2022, one case was lost to follow-up, three were alive, and seven had died. The median overall survival (OS) period was 14 months (Fig. 5), and the expected 3-year overall survival rate was 29.6%. Local recurrence and distant metastasis were observed in four (Fig. 6) and five cases (one developed distant metastasis preoperatively and two developed distant metastases after local recurrence), respectively, and three cases achieved disease-free survival. The median disease-free survival (DFS) period was 6 months. Among the 11 cases, six were treated with surgery alone, three underwent a second surgery, five received adjuvant chemotherapy (2–8 cycles), one case received adjuvant radiotherapy, and one had neoadjuvant chemotherapy plus immunotherapy (Fig. 6).
Discussion
The incidence rate of colorectal adenosquamous carcinoma (CASC) in this study was 0.061%, which is consistent with previous reports [2,3,4]. Compared to adenocarcinoma, CASC patients demonstrated a poorer prognosis. This study is the first to report on the molecular characteristics of CASC patients, including a higher proportion of patients with dMMR (27.3%) and the discovery of KRAS-gene mutations in four patients.
The incidence data of CASC has been derived from population-based research, including Cagir’s study [4], the earliest database study that determined an adenosquamous carcinoma incidence rate of 0.06%. Moreover, Masoomi et al. showed an adenosquamous cell carcinoma incidence of 0.09% in 111,263 patients with colorectal adenocarcinoma or adenosquamous carcinoma [3]. Further, the Roswell Park Cancer Institute showed that adenosquamous cell carcinoma accounted for 0.18% of colorectal tumors [11]. According to the latest database study, adenosquamous cell carcinoma accounts for 0.06% of colorectal tumors [2], and an article by Nasseri cites the incidence of adenosquamous carcinoma of the colon as 0.025–0.1% [5]. The CASC incidence derived from our study was consistent with these studies. However, our study is the first to research the incidence of CASC in an Asian population.
The clinicopathological features of patients with adenosquamous carcinoma of the colorectum include a higher incidence in the elderly population [2]. Clinical presentations are dominated by non-specific symptoms such as abdominal pain, diarrhea, and weight loss. Hypercalcemia has been observed in several case reports [12,13,14,15,16], but it is rarely observed in clinical practice. Radiographic examinations helped assess outcomes [17], with recurrence detected in four of our patients by CT. Most patients were diagnosed by pathology after surgical resection. There was a single report of adenosquamous carcinoma diagnosis by colonoscopy with endoscopic resection pathology [18], and one of seven patients reported adenosquamous carcinoma by colonoscopy preoperatively in our study. However, CT and colonoscopy are of limited value in clarifying the preoperative diagnosis of adenosquamous carcinoma, which may be due to the difficulty of detecting the components of squamous and adenocarcinoma simultaneously.
Our research found a higher proportion of patients (27.3%) with dMMR status than previous data (10–20%) showed for colorectal cancer. Akahoshi reported a patient with PD-L1 overexpression in adenosquamous tissue and systematically higher expression of dMMR in adenosquamous carcinoma of other cancer types [19], which indicated that adenosquamous cancer has a different pathogenesis and tumor environment compared with adenocarcinoma. A study by Hirsch showed that colorectal cancer patients with dMMR responded to immunotherapy [20]. In addition, several articles have also shown that colorectal cancer with dMMR status responded to immunotherapy and Pembrolizumab [21,22,23]. The aforementioned immunotherapy research targets are basically adenocarcinoma, while few reports have been on CASC because it is an uncommon type of cancer. A report from Evert [24] of successful treatment with pembrolizumab in metastatic CASC provided an excellent therapeutic direction. Deficient MMR protein expression is currently not a routine examination for colorectal patients in many countries. Investigating the proportion of dMMR status might provide variable treatment and management for CASC patients.
We also found that a high proportion of patients exhibited mutations in KRAS and that missense mutations occurred at a different codon than in colorectal adenocarcinoma. KRAS mutations account for 40% of all genetic mutations in colorectal tumors [25], however, due to the small number of patients, there were no previous reports of mutant KRAS gene in CASC [26]. Our finding supplements the involvement of KRAS in this aggressive histological sub-type of colon cancer. Similar involvement was mentioned in lung cancer in the literature [27]. Furthermore, one study concluded that a KRAS mutation is associated with suppressed Th1/cytotoxic immunity in colorectal cancer [28], which provided a basis for interpreting the association between KRAS mutations and colorectal adenosquamous carcinoma. KRASSG12Callele-specific inhibitors have been developed due to the need for more precise therapies [29]. Lenkiewicz’s study of adenosquamous carcinoma of the pancreas suggested that mutations in the KRAS gene provide a therapeutic target for adenosquamous carcinoma [30], which may provide new directions for the treatment of colorectal adenosquamous carcinoma.
The present study has some limitations. First, this was a single-center study, and although the hospital is one of the largest gastrointestinal specialist hospitals in Asia, the patients are likely not representative of the entire Asian population. Second, the population size was relatively small, which may limit the generalizability of the findings. Lastly, the follow-up period was limited, which may underestimate the long-term oncological behavior and prognosis of adenosquamous carcinoma. Therefore, future multi-center studies with larger sample sizes and longer follow-up periods are needed to validate our findings and provide more comprehensive insights into adenosquamous carcinoma’s clinical characteristics, molecular features, and therapeutic options.
Conclusions
In conclusion, CASC is a rare cancer with a poor prognosis and nonspecific clinical presentations that is often diagnosed at an advanced stage. Moreover, we identified a high proportion of dMMR status and KRAS mutations in our CASC patients, which informs the development of targeted therapies like immunotherapy.
Data Availability
Data and materials were available by contacting corresponding authors upon reasonable requests.
Abbreviations
- dMMR:
-
mismatch repair-deficient
- CASC:
-
colorectal adenosquamous carcinoma
References
Xie Y, Shi L, He X, Luo Y. Gastrointestinal cancers in China, the USA, and Europe. Gastroenterol Rep. 2021;9(2). https://doi.org/10.1093/gastro/goab010.
Khan AH, Gao X, Goffredo P, et al. Presentation, treatment, and prognosis of colorectal adenosquamous carcinoma: a contemporary analysis of the surveillance, epidemiology, and end results database. Am J Surg. 2022;223(5):957–62. https://doi.org/10.1016/j.amjsurg.2021.09.004.
Masoomi H, Ziogas A, Lin BS, et al. Population-based evaluation of adenosquamous carcinoma of the colon and rectum. Dis Colon Rectum. 2012;55(5):509–14. https://doi.org/10.1097/DCR.0b013e3182420953.
Cagir B, Nagy MW, Topham A, Rakinic J, Fry RD. Adenosquamous carcinoma of the colon, rectum, and anus: epidemiology, distribution, and survival characteristics. Dis Colon Rectum. 1999;42(2):258–63. https://doi.org/10.1007/BF02237138.
Nasseri Y. Adenosquamous carcinoma: an aggressive histologic sub-type of colon cancer wth poor prognosis.:5.
Lan YT, Huang KH, Liu CA, et al. A nation-wide Cancer Registry-Based study of Adenosquamous Carcinoma in Taiwan. PLoS ONE. 2015;10(10):e0139748. https://doi.org/10.1371/journal.pone.0139748.
von Elm E, Altman DG, Egger M, et al. Strengthening the reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ. 2007;335(7624):806–8. https://doi.org/10.1136/bmj.39335.541782.AD.
Uehara K, Nakanishi Y, Shimoda T, Taniguchi H, Akasu T, Moriya Y. Clinicopathological significance of microscopic abscess formation at the invasive margin of advanced low rectal cancer. Br J Surg. 2007;94(2):239–43. https://doi.org/10.1002/bjs.5575.
Ling YH, Chen JW, Wen SH, Huang CY, Li P, Lu LH, et al. Tumor necrosis as a poor prognostic predictor on postoperative survival of patients with solitary small hepatocellular carcinoma. BMC Cancer. 2020;20(1):607.
de Campos-Lobato LF, Dietz DW, StocchiL, et al. Clinical implications of acellular mucin pools in resected rectal cancer with pathological complete response to neoadjuvant chemoradiation. Colorectal Dis. 2012;14:62–7.
Comer TP, Beahrs OH, Dockerty MB. Primary squamous cell carcinoma and adenoacanthoma of the colon. Cancer. 1971;28(5):1111–7. https://doi.org/10.1002/1097-0142(1971)28:5%3C1111::AID-CNCR2820280504%3E3.0.CO;2-V.
Links M, Ho H, Clingan P, Diamond T. Hypercalcaemia in a patient with fatal adenosquamous carcinoma of the colon. Med J Aust. 1994;160(5):286–7.
Berkelhammer CH, Baker AL, Block GE, Bostwick DG, Michelassi F. Humoral hypercalcemia complicating adenosquamous carcinoma of the proximal colon. Dig Dis Sci. 1989;34(1):142–7. https://doi.org/10.1007/BF01536171.
Moll UM, Ilardi CF, Zuna R, Phillips ME. A biologically active parathyroid hormone-like substance secreted by an adenosquamous carcinoma of the transverse colon. Hum Pathol. 1987;18(12):1287–90. https://doi.org/10.1016/s0046-8177(87)80415-x.
Thompson JT, Paschold EH, Levine EA. Paraneoplastic hypercalcemia in a patient with adenosquamous cancer of the colon. Am Surg. 2001;67(6):585–8.
Chevinsky AH, Berelowitz M, Hoover HCJ. Adenosquamous carcinoma of the colon presenting with hypercalcemia. Cancer. 1987;60(5):1111–6. https://doi.org/10.1002/1097-0142(19870901)60:5%3C1111::aid-cncr2820600532%3E3.0.co;2-1.
Elmas N, Killi RM, Sever A. Colorectal carcinoma: radiological diagnosis and staging. Eur J Radiol. 2002;42(3):206–23. https://doi.org/10.1016/S0720-048X(02)00036-0.
Okabayashi K, Hasegawa H, Ishii Y, Endo T, Kitagawa Y. Adenosquamous carcinoma of the sigmoid colon treated by the less invasive procedures of endoscopy and laparoscopy: report of a case. Surg Today. 2009;39(11):994–7. https://doi.org/10.1007/s00595-009-3961-5.
Akahoshi S, Yamamura K, Komohara Y, et al. A Case Report of Metachronous multiple adenosquamous carcinoma of the Colon over-expressing PD-L1 and a literature review. Anticancer Res. 2021;41(11):5847–54. https://doi.org/10.21873/anticanres.15404.
Hirsch D. Clinical responses to PD-1 inhibition and their molecular characterization in six patients with mismatch repair-deficient metastatic cancer of the digestive system. J Cancer Res Clin Oncol. 11.
Le DT, Kim TW, Van Cutsem E, et al. Phase II open-label study of Pembrolizumab in Treatment-Refractory, microsatellite Instability–High/Mismatch repair–deficient metastatic colorectal Cancer: KEYNOTE-164. J Clin Oncol. 2020;38(1):11–9. https://doi.org/10.1200/JCO.19.02107.
Overman MJ, Lonardi S, Wong KYM, et al. Durable clinical benefit with Nivolumab Plus Ipilimumab in DNA Mismatch Repair–Deficient/Microsatellite instability–high metastatic colorectal Cancer. J Clin Oncol. 2018;36(8):773–9. https://doi.org/10.1200/JCO.2017.76.9901.
Chalabi M, Fanchi LF, Dijkstra KK, et al. Neoadjuvant immunotherapy leads to pathological responses in MMR-proficient and MMR-deficient early-stage colon cancers. Nat Med. 2020;26(4):566–76. https://doi.org/10.1038/s41591-020-0805-8.
Evert K, Stiegler C, Schäfer C, et al. [Successful pembrolizumab therapy in metastasized adenosquamous carcinoma of the colon]. Pathol. 2019;40(5):540–5. https://doi.org/10.1007/s00292-018-0546-3.
Dienstmann R, Connor K, Byrne AT, et al. Precision Therapy in RAS Mutant Colorectal Cancer. Gastroenterology. 2020;158(4):806–11. https://doi.org/10.1053/j.gastro.2019.12.051.
Lu SB, Ge FS, Liu C, Hua YW. Adenosquamous carcinoma of sigmoid colon in an adolescent: a case report and literature review. Asian J Surg. 2022;45(4):1055–6. https://doi.org/10.1016/j.asjsur.2022.01.055.
Jia XL, Chen G. EGFR and KRAS mutations in chinese patients with adenosquamous carcinoma of the lung. Lung Cancer Amst Neth. 2011;74(3):396–400. https://doi.org/10.1016/j.lungcan.2011.04.005.
Lal N, White BS, Goussous G, et al. KRAS Mutation and Consensus Molecular Subtypes 2 and 3 are independently Associated with reduced Immune Infiltration and Reactivity in Colorectal Cancer. Clin Cancer Res. 2018;24(1):224–33. https://doi.org/10.1158/1078-0432.CCR-17-1090.
Zhu G, Pei L, Xia H, Tang Q, Bi F. Role of oncogenic KRAS in the prognosis, diagnosis and treatment of colorectal cancer. Mol Cancer. 2021;20(1):143. https://doi.org/10.1186/s12943-021-01441-4.
Lenkiewicz E, Malasi S, Hogenson TL, et al. Genomic and epigenomic landscaping defines new therapeutic targets for Adenosquamous Carcinoma of the pancreas. Cancer Res. 2020;80(20):4324–34. https://doi.org/10.1158/0008-5472.CAN-20-0078.
Acknowledgements
Not applicable.
Funding
This study was supported by the National Key Clinical Discipline, Science and technology key research and development plan project of Guangzhou (China) (202103000072), Natural Science Foundation of Guangdong Province (China) (2018A030313621), Science and Technology Projects in Guangzhou (202206010062), Sun Yat-sen University Clinical Research 5010 Program (2016005), Shenzhen “San Ming Projects” Research (Grant No.lc202002).
Author information
Authors and Affiliations
Contributions
Conceptualization: [Liang Huang], [Qin Guo]; Methodology: [Fujin Ye], [Mian Chen]; Analysis and investigation: [Huashan Liu], [Xiaobin Zheng], [Hao Xie]; Writing - original draft preparation: [Fujin Ye]; Writing - review and editing: [Liang Huang], [Xiaobin Zheng]; Funding acquisition: [Liang Huang]; Resources: [Wei Xiao], [Pinzhu Huang]; Supervision: [Liang Huang], [Chao Wang], [Qin Guo]
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Clinical Research Ethics Committee of the Sixth Affiliated Hospital of Sun Yat-sen University, Guangzhou, China (approval number: E2019052). All the methods included in this study are in accordance with the declaration of Helsinki. Written Informed consent for participation was obtained from all subjects and/or their legal guardian(s) in our institutional consent form.
Consent for publication
Informed consent for publication was obtained from all subjects and/or their legal guardian(s) in our institutional consent form. This consent form informed of the clinical information invoked (including the use of tissue samples).
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Ye, F., Chen, M., Zheng, X. et al. Clinicopathological and molecular characteristics of colorectal adenosquamous carcinoma in an Asian population. BMC Gastroenterol 24, 36 (2024). https://doi.org/10.1186/s12876-023-02989-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12876-023-02989-9