Abstract
Background
The purpose of this study was to investigate the differences between the clinical characteristics and the factors influencing liver injury in patients with the Omicron subvariant BA.5.2 (Omicron BA.5.2) and the prototype of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
Methods
Between December 30, 2019 and November 30, 2022, 157 patients infected with the SARS-CoV-2 prototype and 199 patients infected with the Omicron BA.5.2 were included in this case-control, single-center, retrospective study. Differences in clinical characteristics and liver injury between the Omicron BA.5.2 patients and the prototype patients were subsequently analyzed.
Results
None of the Omicron BA.5.2 patients reached the critical state, and showed relatively milder symptoms including fever, cough, headache, muscle soreness, nausea or vomiting, diarrhea, anorexia and hypoxia. The Omicron BA.5.2 had a lower effect on body temperature (T), white blood cell (WBC) count, hematocrit (HCT), C-reactive protein (CRP) level, D-dimer, finger pulse oxygen saturation (SpO2) and lung lesions. The differences in liver injury between the two groups were related to the severity of the disease, T, blood oxygen levels, albumin (ALB), CRP, and medication usage. Gender, body mass index, and CRP levels influenced liver damage in the Omicron BA.5.2 patients. In particular, CRP was an independent risk factor for liver injury. Because the severity of liver function damage was considerably low, only a small number of Omicron BA.5.2 patients required liver-protective treatment.
Conclusion
Liver injury is expected in the COVID-19 patients. The Omicron BA.5.2 patients showed milder symptoms of liver injury than the prototype patients. However, dynamic monitoring of liver function is warranted, especially for individuals presenting with elevated levels of CRP.
Similar content being viewed by others
Introduction
The first case of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection was first reported in Wuhan, China (December 2019), and the resulting condition was officially named coronavirus infectious disease 2019 (COVID-19) by the World Health Organization (WHO) (January 2020). COVID-19 has posed a serious threat to global public health [1–2]. As of November 8, 2022, more than 638 million confirmed cases and 6.8 million COVID-19-related deaths have been reported [1]. The increasing accumulation of mutations in the virus genome has resulted in the formation of new lineages. Five variants of concerns (VOCs) have been reported thus far, including Alpha (B.1.1.7) (prototype), Beta (B.1.351), Gamma (P.1), Delta (B.1.617.2), and Omicron (B.1.1.529) [3,4,5,6].
The SARS-CoV-2 variant named Omicron (B.1.1.529) was first detected in South Africa on November 14, 2021, as the fifth VOC [7]. Numerous Omicron variants have subsequently emerged and spread globally, with these variants being classified into five primary lineages: BA.1, BA.2, BA.3, BA.4, and BA.5. Some of the sublineages include BA.1.1, BA.2.12.1, BA.2.11, BA.2.75, BA.4.6, BA.5.1, and BA.5.2. All Omicron lineages show multiple mutations, of which 31–37 mutations were observed in spike proteins, leading to relatively higher rates of infection and morbidity than previous VOCs [3, 8,9,10]. The first case of Omicron subvariant BA.5.2 (Omicron BA.5.2), which was accidentally imported into Beijing, China, was discovered on July 4, 2022. Since then, this SARS-CoV-2 variant rapidly spread within the country and has reportedly become the dominant strain in some cities [11].
According to existing research, SARS-CoV-2 is the most well-known etiological agent for substantial respiratory pathology; furthermore, it may lead to several extrapulmonary manifestations, including gastrointestinal and liver injury, acute kidney injury, and neurological and psychiatric illnesses. Among these, liver injury is relatively the most common [12,13,14]. COVID-19-associated liver injury occurs as a result of the cumulative effects of multiple factors. SARS-CoV-2 RNA expression has been detected in liver tissue, potentially causing hepatocellular lesions directly. Moreover, liver injury may be associated with drug-induced liver injury, hypoxic reperfusion, immune stress, and inflammatory factor storms [15–16]. The data of 12 studies showed that the pooled prevalence of liver injury, the increased alanine aminotransferase (ALT), increased aspartate aminotransferase (AST), and decreased albumin (ALB) levels were 19%, 18%, 21%, and 6%, respectively [17]. Additionally, liver injury is more prevalent in severe cases than in mild cases of COVID-19 [18]. The Omicron variants reported here may not have been as severe as the previous episodes; however, additional evidence is needed to determine whether the Omicron variants are relatively more benign [10]. The characteristics of the impact of the earliest COVID-19 (prototype) patients on liver function have been analyzed in the past [19]. In this study, we aim to further analyze differences in clinical characteristics and liver injury between patients diagnosed with the Omicron BA.5.2 and the prototype.
Methods
Study design and patients
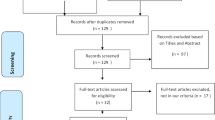
In the single-center retrospective study, patients admitted to the First Hospital of Yangtze University and infected with the prototype and Omicron BA.5.2 were included; thus, patients aged < 18 years, with serious underlying diseases, and pregnant women were excluded. The COVID-19 diagnosis was established based on the New Coronavirus Pneumonia Prevention and Control Program (9th edition) published by the National Health Commission of China and the interim guidance from the WHO [19–20]. A positive COVID-19 PCR test confirms diagnosis, leading to hospitalization and isolation treatment according to local policies. Nasopharyngeal swab samples from 356 patients who were tested at the Jingzhou Centers for Disease Control laboratory genetic sequencing of the virus were Omicron BA.5.2 and prototype from December 30, 2019, to November 30, 2022.
Data collection
The general information and clinical symptoms of all cases were collected. General information included gender, age, body weight, height, history of smoking and vaccination, and comorbidities—e.g., chronic obstructive pulmonary disease, hypertension, coronary heart disease and/or diabetes, viral hepatitis, and fatty liver. Clinical symptoms involved body temperature (T), finger pulse oxygen saturation (SpO2), respiratory symptoms (e.g., fevers, cough, and sore throat, among others), and digestive symptoms (e.g., diarrhea and anorexia, among others). Laboratory tests included routine blood tests (white blood cell [WBC], lymphocyte [LY], and platelet [PLT] count hematocrit [HCT]), coagulation function (international normalized ratio [INR] and plasma prothrombin time[PT]), liver function (alanine transaminase [ALT], aspartate aminotransferase [AST], prealbumin [PA], albumin [ALB], total bilirubin [TB], alkaline phosphatase [ALP], g-glutamyl transpeptidase [GGT], lactate dehydrogenase [LDH], and cholinesterase [CHE]), C-reactive protein (CRP), D-dimer, IL-6, computed tomography (CT) imaging presentations, therapeutic drugs, and disease prognosis. Reverse transcription-quantitative polymerase chain reaction (RT-qPCR) was used to detect SARS-CoV-2 in samples collected via nasopharyngeal swabs. According to the central laboratory report specification prepared by the authors of the present study, the upper limits of normal (ULN) of ALT, AST, ALP, GGT, and LDH were 40, 42, 128, 50, and 240 U/L, respectively. In addition, the ULN of TB was 20.4 µmol/L, the lower limits of normal (LLN) of ALB was 35 g/L. Liver injury is defined as any exceedance of the ULN for liver function parameters including ALT, AST, ALP, GGT, LDH, and TB, or below the LLN for ALB.
Statistical analyses
Statistical analyses for all data were performed using the SPSS software version 22.0 (IBM Inc., Chicago, IL). Categorical variables were presented as numbers (percentages) and were analyzed using the Chi-Squared test or Fisher exact test. Measurement data were presented as mean ± standard deviation and were analyzed using the Student’s t-test for intergroup comparisons. Multiple factors were analyzed using logistic regression. The histograms were drawn by GraphPad Prism 8. Furthermore, a two-sided P < 0.05 indicated statistical significance.
Results
Epidemiological and clinical characteristics of the Omicron BA.5.2 and prototype patients
The Omicron BA.5.2 patients (n = 199) and prototype patients (n = 157) from December 30, 2019, to November 30, 2022, were included. The demographic and clinical characteristics are shown in Table 1. There were no significant differences between the two groups in terms of gender, age, smoking history, and comorbidities (coronary heart disease, hypertension, pulmonary disease, and liver disease) (P > 0.05) .
The proportion of prototype patients showing symptoms of fever (75.2% vs. 39.2%, P < 0.001), cough (59.9% vs. 39.2%, P < 0.001), fatigue (24.8% vs. 4.0%, P < 0.001), chest tightness/dyspnea (17.8% vs. 1.0%, P < 0.001), muscle soreness (14.0% vs. 4.5%, P = 0.002), diarrhea (10.2% vs. 2.0%, P = 0.017), and anorexia (17.8% vs. 2.5%, P = 0.001) was significantly higher than the Omicron BA.5.2 patients. However, a higher proportion of the Omicron BA.5.2 patients were asymptomatic (31.2% vs. 1.0%, P < 0.001) and more frequently presented with symptoms of headache (10.6% vs. 3.8%, P = 0.012) and sore throat (13.1% vs. 8.3%, P = 0.001) than the prototype patients. In total, among the prototype patients, there were 45 severe or critical cases and 11 deaths. However, none of the Omicron BA.5.2 patients were in a critical condition or had died(P < 0.001).
Laboratory and radiological characteristics of the Omicron BA.5.2 and prototype patients
The prototype patients (92.50 ± 15.00) exhibited significantly lower SpO2 levels than the Omicron BA.5.2 patients (97.51 ± 1.48) (X2 = 4.403, P < 0.001). Regarding pulmonary CT examination, higher ratios of ground-glass opacity (19.1% vs. 6.5%), patchy shadowing (68.2% vs. 34.2%), and consolidation (11.5% vs. 0.0%) were observed in the prototype patients compared to those with the Omicron BA.5.2 patients (X2 = 142.600, P < 0.001). However, none of the 118 Omicron BA.5.2 patients (59.3%) exhibited signs of pneumonia. In terms of routine blood tests, abnormalities in WBC counts, PLT, and HCT were less common in Omicron BA.5.2 patients than in the prototype patients (P < 0.001), though the decrease in lymphocyte counts was higher in the Omicron BA.5.2 patients (P < 0.001). 73 Omicron BA.5.2 patients (36.7%) showed increased CRP levels and 8 patients (4.0%) had increased D-dimer, both lower than the prototype patients (P < 0.001). In terms of coagulation function, INR (1.00 ± 0.15 vs. 0.91 ± 0.09) and PT (11.20 ± 1.68 vs. 10.46 ± 0.82) in the prototype patients were higher compared to Omicron BA.5.2 patients (P < 0.001). In terms of liver function, the prototype patients showed significantly higher rates of abnormalities in PA (81.5% vs. 16.6%), ALB (41.4% vs. 0.5%), TB (33.8% vs. 6.5%), ALT (50.3% vs. 11.1%), AST (37.6% vs. 9.5%), GGT (38.9% vs. 15.1%), ALP (8.3% vs. 1.5%), LDH (45.9% vs. 1.5%) and CHE (14.6% vs. 0.0%) than the Omicron BA.5.2 patients (P < 0.05) (Table 2).
Baseline level of liver function in the Omicron BA.5.2 and prototype patients
Among the 157 prototype patients, the baseline levels of ALT, AST, TB, ALB, LDH, GGT, PA, and CHE were 76.14 ± 108.40 U/L, 46.22 ± 35.75 U/L, 19.74 ± 15.35 µmol/L, 36.70 ± 4.64 g/L, 251.10 ± 154.70 U/L, 88.24 ± 150.00 U/L, 137.50 ± 68.45 mg/L, and 5292.00 ± 2083.00 U/L, respectively. Among the 199 Omicron BA.5.2 patients, the baseline levels of ALT, AST, TB, ALB, LDH, GGT, PA, and CHE were 22.27 ± 21.95 U/L, 28.88 ± 21.06 U/L, 11.95 ± 5.07 µmol/L, 42.97 ± 2.24 g/L, 159.20 ± 31.91 U/L, 34.91 ± 38.54 U/L, 237.20 ± 68.45 mg/L, and 8451.00 ± 1793.00 U/L, respectively. The Omicron BA.5.2 patients exhibited significantly lower levels of ALT (t = 6.839, P < 0.001), AST (t = 5.696, P < 0.001), TB (t = 6.707, P < 0.001), GGT (t = 4.809, P < 0.001), and LDH (t = 6.301, P < 0.001). They also had significantly higher levels of ALB (t = 17.730, P < 0.001), PA (t = 12.540, P < 0.001), and CHE (t = 4.809, P < 0.001). All these differences were statistically significant. However, no intergroup differences were noted in terms of the ALP levels (t = 1.436, P = 0.152) (Table 3).
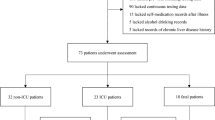
We further analyzed the extent of liver function impairment in both groups. The proportion of ALT ( t = 49.710, P < 0.001), AST (t = 39.770, P < 0.001), TB (t = 10.370, P < 0.001), GGT (t = 25.100, P < 0.001), and LDH (t = 26.860, P < 0.001) levels greater than 2ULN in the prototype patients was significantly higher than in the Omicron BA.5.2 patients. Additionally, the proportion of ALB levels (t = 98.900, P < 0.001) lower than 35 g/L in prototype patients was higher compared to the Omicron BA.5.2 patients (Table 4). A total of 45 the prototype patients (28.7%) and 10 the Omicron BA.5.2 patients (5.0%) were treated with hepatoprotective drugs (t = 31.210, P < 0.001) (Fig. 1).
Influencing factors of abnormal liver function in the Omicron BA.5.2 and prototype patients
We discussed the probable reasons for the differences in liver damage between the Omicron BA.5.2 patients (n = 26) and the prototype patients (n = 77). No significant differences were noted in terms of gender (P = 0.106), age (P = 0.619), and comorbidities (P = 0.069). The Omicron BA.5.2 patients were vaccinated, and none of them were critically ill (P < 0.001). The SpO2 (P = 0.027) and ALB (P < 0.001) levels in the prototype patients were significantly lower than the Omicron BA.5.2 patients, and the temperature (P = 0.041) and CRP levels (P = 0.001) were significantly higher. However, D-dimer levels showed no significant difference (P = 0.161). Besides, the prototype patients received a wider variety of medications. Hormones (P < 0.001), antibiotics (P < 0.001), and chloroquine (P = 0.019) may be associated with liver damage, there was no difference in liver damage among patients using Chinese patent medicine (P = 0.115 ) (Table 5).
Influencing factors of abnormal liver function in the Omicron BA.5.2 patients
We analyzed influencing factors of normal (n = 173) and abnormal (n = 26) liver function in the Omicron BA.5.2 patients. Liver injury in those patients was significantly correlated with gender (P = 0.005), body mass index (BMI) (P = 0.020), and CRP levels (P < 0.001). However, no correlation was found with age, vaccination history, comorbidities, SpO2, T, Alb, WBC count, PT, INR, or therapeutic drugs. CRP was an independent risk factor for liver injury (Waldχ2 = 6.067 P < 0.05) (Table 6).
Discussion
Currently, Omicron has become the dominant global epidemic strain owing to its significant immune escape and higher transmissibility. The Omicron BA.5 variant has become the most prevalent Omicron subvariant worldwide [9, 21−22]; however, the characteristics of liver damage caused by the Omicron BA.5 variant remain unclear. In the study, we aim to examine and compare the clinical features, laboratory test results, and liver injury associated with the Omicron BA.5.2 patients to those of the prototype patients.
Several countries reported mild symptoms related to the Omicron strain with a mortality rate of 0.13–0.5%, which was 83–90% lower than that of the prototype and other VOC [23–24]. The proportion of Omicron–VOC patients with asymptomatic was 16–47.5% [25–26]. Furthermore, similar features were observed in the Omicron BA.5.2. A total of 199 the Omicron BA.5.2 patients and 157 the prototype patients were enrolled in the study. The proportion of asymptomatic Omicron BA.5.2 patients was 31%. Conversely, nearly all the prototype patients presented with various symptoms. Our previous study demonstrated that more serious lung injuries were associated with disease exacerbation, lower blood oxygen, and more serious CT manifestations in prototype patients. The hospitalized patients infected with the Omicron BA.5.2 showed little oxygen depletion, and about two-thirds had no inflammatory response on lung CT, all patients showed mild or common manifestations. The mortality rate of the prototype patients reached 7%, whereas no severe disease or death occurred in the Omicron BA.5.2 patients. This observation aligns with several studies suggesting a milder course for the Omicron. A nationwide data study in South Africa indicated that the risk of severe illness from Omicron infections reduced by 70% compared to earlier Delta infections [27]. Similarly, a retrospective cohort study (Omicron and Delta cohorts) in the United States found that proportions of hospitalization, ICU admission, and mechanical ventilation among Omicron patients were significantly reduced [28]. Interestingly, Kenrie P. Y. Hui et al. discovered that, 24 h after infection, the replication efficiency of the Omicron variant in human bronchi was 70 times higher than the prototype and Delta variant, but was 10 times lower in human lung tissue compared to the prototype [29]. This could also explain an important reason why the majority of the Omicron-infected population experiences relatively mild conditions.
In addition, the data showed that compared with the prototype, the Omicron BA.5.2 had less impact on the following parameters: WBC, PLT, HCT, PT, INR, D-dimer, and CRP. The Omicron BA.5.2 patients predominantly presented with lymphopenia, and a few patients had leukocytosis, thrombocytopenia, and hematocrit reduction. Serum CRP levels are closely related to inflammatory activity [30]. D-dimer may also reflect an inflammatory condition and predict severe and fatal cases of COVID-19 with moderate accuracy [31]. The levels of CRP and D-dimer in the Omicron BA.5.2 patients were significantly lower than in the prototype patients. Therefore, Omicron BA.5.2 patients had a lower inflammatory response than the prototype patients. Omicron BA.5.2 is thought to be less pathogenic than the prototype.
Studies have thoroughly explored the pulmonary lesions of patients with COVID-19; thus, the present study focused on liver injury. Compared with liver function indicators, the baseline levels of ALT, AST, TB, ALB, LDH, GGT, PA, and CHE as well as the proportion of those abnormal indicators were lower in the Omicron BA.5.2 patients than in the prototype patients. Specifically, the proportion of ALT, AST, TB, GGT, and LDH levels exceeding 2ULN was significantly higher in the prototype patients, these findings indicate that the Omicron BA.5.2 was associated with milder liver function impairment and a lower risk of causing liver damage than the prototype. The differences in the severity of liver damage between Omicron BA.5.2 and prototype could be associated with factors such as the severity of the disease, T, blood oxygen levels, ALB and CRP. These factors might involve the different virological characteristics of virus mutant strains, hypoxia reperfusion dysfunction, immune imbalance, and cytokine storms [16]. The foremost direct damage to the liver induced by SARS-CoV-2 is a plausible mechanism. Angiotensin-converting enzyme 2 (ACE2) has been shown to mediate SARS-CoV-2 infection, which is also expressed in cholangiocytes, hepatic sinusoidal endothelial cells (LSECs), and hepatocytes. Direct binding of the virus spike protein to ACE2 of targeted cells may result in hepatocyte and cholangiocyte injury and subsequent bile acid accumulation [16, 32−34]. Omicron variants have been associated with the prototype through multiple mutations. The specific mechanisms underlying the possible effects of different strains require further elucidation.
At present, no specific medicine exists for the treatment of SARS-CoV-2. In the early stages of the outbreak, hormones, antibiotics, arbidol, and Chinese patent medicine, among others were widely used, which may directly or indirectly cause drug-induced liver injury. However, it also has been reported that no liver injury secondary to Favipiravir was detected [35]. In a previous, authors of the current study reported that hormones were associated with liver damage [19]. Moreover, many regression studies have mentioned that the risk and proportion of liver injury were increased in patients with medium-to-large doses of glucocorticoids (≥ 10 mg/d prednisolone or equivalent drugs), antibiotics, and other drugs[36–37]. Only a few of the hospitalized patients infected with the Omicron BA.5.2 were treated with Chinese medicine and antipyretic drugs. Compared with early treatment, the influence of drug therapy on liver function was significantly reduced. However, although Omicron has an immune escape, the vaccine continues to have some protective effects. Even if COVID-19 vaccinations do not provide complete protection against the new variant, they will at least result in less severe infections and lower death rates [37]. Compared with the prototype patients, the Omicron BA.5.2 patients exhibited less severe inflammation and near-normal blood oxygen levels. Consequently, factors such as immune stress, inflammatory factor storms, ischemia, and hypoxia had less effect on liver damage [38]. According to the review, Omicron patients showed milder abnormal liver function.
Furthermore, this article further explored the characteristics of abnormal liver function in Omicron BA.5.2 patients. The main manifestation of liver injury was the mild elevation of AST, ALT, and GGT, which was more likely to occur in male and obese patients. Age, T, ALB, D-dimer, blood oxygen, and therapeutic drugs had little impact on liver damage. Notably, CRP was an independent factor associated with Omicron BA.5.2 patients. Ultimately, a small number of patients required liver-protective treatment. However, patients with elevated CRP still require attention.
This study has some limitations. First, it is a single-center retrospective study with a relatively small sample. Second, the assessment of liver injury is not comprehensive enough, lacking evaluation indicators such as liver biopsy puncture and radiological evaluation. Additionally, as the understanding of the virus evolves, the treatment of patients is continually adjusted. Despite these limitations, our study has successfully revealed the clinical features and liver damage characteristics of the Omicron BA.5.2 patients, and conducted comparative analysis with the prototype patients. Large-scale, multi-center clinical data are still essential to fully comprehend the impact of different SARS-CoV-2 variants on liver function.
Conclusion
In conclusion, our study demonstrates that the Omicron BA.5.2 presents with milder symptoms and lower mortality rates compared to the prototype. The Omicron BA.5.2 patients exhibit less liver damage. Gender, BMI, and CRP may correlate with liver function impairment in the Omicron BA.5.2 patients, with CRP potentially predicting the occurrence of liver function impairment. Yet dynamic monitoring of liver function is necessary, especially among those with elevated CRP during treatment, to ensure proper management and adapt treatment strategies as needed.
Data availability
The datasets used and/or analyzed during the study are available from the corresponding author on reasonable request.
Abbreviations
- ALB:
-
Albumin
- ALT:
-
Alanine transaminase
- AST:
-
Aspartate aminotransferase
- ALP:
-
Alkaline phosphatase
- ACE2:
-
Angiotensin-converting enzyme 2
- BMI:
-
Body Mass Index
- COVID-19:
-
Coronavirus disease 2019
- CRP:
-
C-reactive protein
- CHE:
-
Cholinesterase
- GGT:
-
γ-glutamyl transpeptidase
- HCT:
-
Hematocrit
- INR:
-
International Normalized Ratio
- LY:
-
Lymphocytes
- LSECs:
-
Hepatic sinusoidal endothelial cells
- LDH:
-
Lactate dehydrogenase
- PLT:
-
Platelets
- PA:
-
Prealbumin
- PT:
-
Plasma prothrombin time
- SARS-CoV-2:
-
Severe acute respiratory syndrome coronavirus 2
- SpO2:
-
Finger pulse oxygen saturation
- T:
-
Temperature
- TB:
-
Total bilirubin
- ULN:
-
Upper limit of normal
- WBC:
-
White blood cell
References
WHO. World Health Organization. Coronavirus disease (COVID-19) outbreak. Available at: http://www.who.int.
Chen ATC, Coura-Filho GB, Rehder MHH. Clinical characteristics of Covid-19 in China. N Engl J Med. 2020;382:1859–62.
Tian D, Nie W, Sun Y, Ye Q. The epidemiological features of the SARS-CoV-2 Omicron Subvariant BA.5 and its evasion of the neutralizing activity of vaccination and prior infection. Vaccines (Basel). 2022;10(10):1699.
Rajah M, Hubert M, Bishop E, et al. SARS-CoV-2 alpha, Beta and Delta variants display enhanced spike-mediated syncytia Formation[J]. The EMBO Journal; 2021.
Faria NR, Mellan TA, Whittaker C et al. Genomics and epidemiology of the P.1 SARS-CoV-2 lineage in Manaus, Brazil[J]. Science, 2021:eabh2644.
Tian D, Sun YH, Zhou JM et al. The global epidemic of SARS-CoV-2 variants and their mutational immune escape[J]. Journal of medical virology:https://doi.org/10.1002/jmv.27376.
World Health Organization (WHO). Technical Report. Classification of Omicron (B.1.1.529): SARS-CoV-2 Variant of Concern.2021.
Kandeel M, Mohamed M, Venugopala KN, et al. Omicron variant genome evolution and phylogenetics[J]. J Med Virol. 2022;94(4):1627–32.
Hoffmann M, Krüger N, Schulz S et al. The Omicron variant is highly resistant against antibody-mediated neutralization: implications for control of the COVID-19 pandemic[J]. Cell, 2021(15).
Ren SY, Wang WB, Gao RD et al. Omicron variant (B.1.1.529) of SARS-CoV-2: Mutation, infectivity, transmission, and vaccine resistance[J].
Feng Z, Shen Y, Li S et al. The First Outbreak of Omicron Subvariant BA.5.2—Beijing Municipality,China,July 4,2022[J].
Salome B, Magen A. Dysregulation of lung myeloid cells in COVID-19. Nat Rev Immunol. 2020;20:277.
Gupta A, Madhavan MV, Sehgal K et al. Extrapulmonary manifestations of COVID-19[J]. Nat Med, 2020, 26(7).
Taquet M, Geddes JR, Husain M et al. 6-month neurological and psychiatric outcomes in 236 379 survivors of COVID-19: a retrospective cohort study using electronic health records[J]. The Lancet Psychiatry, 2021, 8(5).
Marjot T, Webb GJ, Barritt AS et al. COVID-19 and liver disease: mechanistic and clinical perspectives[J]. Nature Reviews Gastroenterology & Hepatology, 2021(Suppl. 1).
Nardo AD, Schneeweiss-Gleixner M, Bakail M, et al. Pathophysiological mechanisms of liver injury in COVID-19. Liver Int. 2021;41(1):20–32.
Mao R, Qiu Y, He JS, et al. Manifestations and prognosis of gastrointestinal and liver involvement in patients with COVID-19: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2020;5(7):667–78.
Bangash MN, Patel JM, Parekh D, et al. SARS-CoV-2: is the liver merely a bystander to severe disease? J Hepatol. 2020;73(4):995–6.
Zhang Q, Li J, Zhang Y, et al. Differences in clinical characteristics and liver injury between suspected and confirmed COVID-19 patients in Jingzhou, Hubei Province of China. Med (Baltim). 2021;100(19):e25913.
Committee GOoNH. Notice on the issuance of a program for the diagnosis and treatment of novel coronavirus (2019-nCoV) infected pneumonia (trial ninth edition). Available at: http://www.nhc.gov.cn/yzygj/s7653p/202003/46c9294a7dfe4cef80dc7f5912eb1989.sshtm.
Kannan S, Shaik Syed Ali P, Sheeza A. Omicron (B.1.1.529) - variant of concern - molecular profile and epidemiology: a mini review. Eur Rev Med Pharmacol Sci. 2021;25(24):8019–22.
Meo SA, Meo AS, Al-Jassir FF, et al. Omicron SARS-CoV-2 new variant: global prevalence and biological and clinical characteristics. Eur Rev Med Pharmacol Sci. 2021;25(24):8012–8.
Stlcrantz J, Kristoffersen AB, Bs H et al. Milder disease trajectory among COVID-19 patients hospitalised with the SARS-CoV-2 Omicron variant compared with the Delta variant in Norway[J]. Scand J Public Health, 2022(6):50.
Adjei S, Hong K, Molinari NM, Bull-Otterson L, et al. Mortality risk among patients hospitalized primarily for COVID-19 during the omicron and Delta variant pandemic periods - United States, April 2020-June 2022. MMWR Morb Mortal Wkly Rep. 2022;71(37):1182–9.
Kim MK, Lee B, Choi YY, et al. Clinical characteristics of 40 patients infected with the SARS-CoV-2 Omicron variant in Korea. J Korean Med Sci. 2022;37(3):e31.
Houhamdi L, Gautret P, Hoang VT, et al. Characteristics of the first 1119 SARS-CoV-2 Omicron variant cases, in Marseille, France, November-December 2021. J Med Virol. 2022;94(5):2290–5.
Wolter N, Jassat W, Walaza S, et al. Early assessment of the clinical severity of the SARS-CoV-2 omicron variant in South Africa: a data linkage study. Lancet. 2022 Jan;29(10323):437–46.
Wang L, Berger NA, Kaelber DC et al. Comparison of outcomes from COVID infection in pediatric and adult patients before and after the emergence of Omicron. medRxiv. 2022 Jan 2:2021.12.30.21268495.
Hui KPY, Ho JCW, Cheung MC, et al. SARS-CoV-2 Omicron variant replication in human bronchus and lung ex vivo. Nature. 2022;603(7902):715–20.
Wang L. C-reactive protein levels in the early stage of COVID-19. Med Mal Infect. 2020;50(4):332–4.
Rostami M, Mansouritorghabeh H. D-dimer level in COVID-19 infection: a systematic review. Expert Rev Hematol. 2020;13(11):1265–75.
Hamming I, Timens W, Bulthuis ML, et al. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203(2):631–7.
Pirola CJ, Sookoian S. SARS-CoV-2 virus and liver expression of host receptors: putative mechanisms of liver involvement in COVID-19. Liver Int. 2020;40(8):2038–40.
Zhao B, Ni C, Gao R, et al. Recapitulation of SARS-CoV-2 infection and cholangiocyte damage with human liver ductal organoids. Protein Cell. 2020;11(10):771–5.
Ucan A, Cerci P, Efe S, et al. Benefits of treatment with favipiravir in hospitalized patients for COVID-19: a retrospective observational case-control study. Virol J. 2021;18(1):102.
Guo H, Zhang Z, Zhang Y, et al. Analysis of liver injury factors in 332 patients with COVID-19 in Shanghai, China. Aging. 2020;12(19):18844–52.
Ai J, Zhang H, Zhang Y, et al. Omicron variant showed lower neutralizing sensitivity than other SARS-CoV-2 variants to immune sera elicited by vaccines after boost. Emerg Microbes Infect. 2022;11(1):337–43.
Sonzogni A, Previtali G, Seghezzi M, et al. Liver histopathology in severe COVID 19 respiratory failure is suggestive of vascular alterations. Liver Int. 2020;40(9):2110–6.
Acknowledgements
We sincerely thank the medical staff in Jingzhou City for the treatment of COVID-19 patients.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
Qing Zhang and Xiaoping Tan designed the study. Jie Li, Yan Zhang, Chao Xu, Yan Zhang, Yueyue Lu, and Minghua Ai collected data. Qing Zhang and Jie Li analyzed the data. Qing Zhang and Jie Li wrote the paper. Xiaoping Tan reviewed and edited the manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and Consent to participate
According to the principles expressed in the Declaration of Helsinki, this study was reviewed and approved by the Ethics Committee of Jingzhou Public Health Clinical Center. The need for written informed consent was waived by the Jingzhou Public Health Clinical Center ethics committee due to retrospective nature of the study.
Consent for publication
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Li, J., Zhang, Q., Xu, C. et al. Differences in clinical characteristics and liver injury between patients diagnosed with the Omicron subvariant BA.5.2 and the prototype of SARS-CoV-2: a single center retrospective study. BMC Gastroenterol 23, 271 (2023). https://doi.org/10.1186/s12876-023-02907-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12876-023-02907-z