Abstract
Background
This study was performed to compare a metal stent (MS) and plastic stent (PS) in terms of efficacy and complications during neoadjuvant therapy (NAT) and the perioperative period.
Methods
We performed an electronic search of the following databases until 1 June 2022: PubMed, Embase, Web of Science, Cochrane Central Register of Controlled Trials, and ClinicalTrials.gov. Studies comparing an MS versus PS for PBD in patients with pancreatic cancer undergoing NAT were included.
Results
The meta-analysis showed that use of an MS was associated with lower rates of reintervention (p < 0.00001), delay of NAT (p = 0.007), recurrent biliary obstruction (RBO) (p = 0.003), and cholangitis (p = 0.03). There were no significant differences between the two groups in terms of stent migration (p = 0.31), postoperative complications (p = 0.20), leakage (p = 0.90), and R0 resection (p = 0.50).
Conclusions
Use of an MS for PBD in patients with pancreatic cancer undergoing NAT followed by surgery was associated with lower rates of reintervention, delay of NAT, RBO, and cholangitis compared with use of a PS. However, the postoperative outcomes were comparable between the MS and PS. Further studies on this topic are recommended.
Similar content being viewed by others
Background
Radical surgery is a curative treatment for pancreatic head cancer. However, only about 15–20% of patients are potential candidates for resection at the diagnostic stage [1]. With the development of chemotherapy and radiotherapy, neoadjuvant therapy (NAT) [including neoadjuvant chemotherapy (NAC) or neoadjuvant chemoradiotherapy (NACRT)] has drawn attention regarding its application in resectable and borderline resectable pancreatic cancer because of its ability to reduce the tumor size and increase the R0 resection rate [2,3,4]. However, obstructive jaundice is one of the primary symptoms of pancreatic head cancer, and NAT in such cases would be time-consuming because of the need to maintain biliary drainage. Endoscopic biliary drainage is considered superior to percutaneous biliary drainage in terms of peritoneal dissemination and patient comfort [5]. A metal stent (MS) and plastic stent (PS) are two types of stents commonly used for preoperative biliary drainage (PBD) in patients undergoing endoscopic biliary drainage. An MS appears to be the optimal choice for unresectable pancreatic cancer [6]. Compared with a PS, an MS has the advantages of longer patency and a lower reintervention rate. Nevertheless, although several studies have compared the efficacy and safety of an MS versus PS for NAT in patients with resectable or borderline resectable pancreatic cancer, there is no standard viewpoint on the best type of stent [7, 8]. The preoperative and postoperative outcomes between an MS and PS remain controversial. This study was performed to compare an MS and PS for PBD in patients with pancreatic cancer undergoing NAT.
Materials and methods
This systematic review and meta-analysis were reported in line with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [9].
Search strategy
Two authors independently conducted a thorough electronic search of the PubMed, Embase, Web of Science, Cochrane Central Register of Controlled Trials (CENTRAL), and ClinicalTrials.gov databases until 1 June 2022 to identify studies comparing an MS and PS for PBD in patients with pancreatic cancer undergoing NAT. English-language search terms included but were not limited to the following: “ERCP,” “endoscopic,” “stent,” “metal,” “endoscopic retrograde cholangiopancreatography,” “plastic,” “neoadjuvant,” “chemoradiation,” “neoadjuvant chemoradiation therapy,” and “pancreatic cancer.” The search was restricted to human subjects and English-language articles. The references of the articles identified after the initial search were also manually reviewed. Any discrepancy in article selection was resolved by consensus.
Inclusion and exclusion criteria
Studies that met the following criteria were included in the meta-analysis. (1) The study compared any MS and PS for PBD in patients with pancreatic cancer undergoing NAT. (2) Information was provided on reintervention, delay of NAT, recurrent biliary obstruction (RBO), cholangitis, migration, R0 resection, and postoperative complications. (3) The original article was published in English. Abstracts were included as long as they met the inclusion criteria and provided the data needed for the analysis. We excluded (1) studies that did not provide sufficient data and (2) case series, non-comparison studies, and non-human studies.
Outcome measures and data extraction
The outcome measures were reintervention, delay of NAT, RBO, cholangitis, migration, R0 resection, and postoperative complications. Reintervention was defined as endoscopic biliary drainage necessitated by the appearance of elevated hepatobiliary enzyme and total bilirubin levels and/or concomitant cholangitis. RBO is defined as a re-elevation of total bilirubin. R0 resection was defined as no microscopic or macroscopic tumor. The definition of operative complications adopts the definition of postoperative complications in the original study. We abstracted the data of interest from the included studies onto a standardized form, including the author, year of publication, type of study, country in which the study took place, sample size, patient age, and pancreatic cancer status. Conflicts in data abstraction were resolved by consensus and by referring to the original article. EndNote X8 (Thomson Reuters, Toronto, Ontario, Canada) was used to remove duplicate studies.
Quality assessment
The quality of the included nonrandomized studies was assessed in accordance with the Newcastle–Ottawa scale [10]. The scoring system included the following criteria: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of the results assessment, incomplete results data, selective reporting, and other sources of bias. The Cochrane collaboration tool was used to assess the quality of the randomized controlled trials (RCTs) by evaluating methods of randomization and allocation concealment, performance, and detection of bias [11].
Statistical analysis
All statistical analyses were performed using Review Manager (RevMan) version 5.3 software (The Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen, Denmark). Odds ratios (ORs) with 95% confidence intervals (CIs) were used for dichotomous outcomes. Publication bias was evaluated by the χ2 test and funnel plots. Heterogeneity among studies was evaluated by the χ2 test. A two-tailed p value of < 0.05 was considered statistically significant.
Results
Study selection and trial characteristics
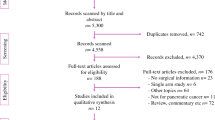
As shown in Fig. 1, we identified 347 articles from the electronic search; of these, 163 articles were excluded because of duplication. After screening the titles and abstracts, an additional 97 articles were excluded for various reasons. Finally, 11 publications that met the inclusion criteria were selected for the current meta-analysis [7, 8, 12,13,14,15,16,17,18,19,20]. A flowchart of the literature search process is shown in Fig. 1.
The characteristics and quality evaluation of the included citations are shown in Table 1. The 11 eligible studied comprised 2 RCTs and 9 retrospective studies. The studies were published from 2012 to 2022 and were performed in Finland, Japan, and the United States. Two studies were published as an abstract and 10 were full-text articles. The participants in the included studies were divided into an MS group (188 participants) and a PS group (308 participants). With respect to NAT, five studies applied NACRT, six studies applied NAC, and one study did not provide the type of NAT. A high risk of bias was not detected in any of the RCTs. According to the Newcastle–Ottawa scale, the study scores ranged from 5 to 7. The characteristics and quality of the included studies are shown in Table 1.
Outcome measures
Reintervention
Four studies involving 333 patients provided data regarding reintervention. The MS group had a lower reintervention rate than the PS group (OR, 0.04; 95% CI, 0.01–0.10; p < 0.00001) (Fig. 2a).
Delay of NAT
Seven studies provided data regarding delay of NAT. Our meta-analysis showed that patients in the MS group had less delay of NAT than those in the PS group (OR, 0.17; 95% CI, 0.05–0.61; p = 0.007) (Fig. 2b).
RBO
Nine studies involving 463 patients provided data regarding RBO. The MS group had a lower rate of RBO than the PS group (OR, 0.09; 95% CI, 0.02–0.40; p = 0.001) (Fig. 2c).
Cholangitis
Four studies involving 218 participants provided data regarding cholangitis after preoperative endoscopic retrograde cholangiopancreatography. Our meta-analysis showed a lower rate of cholangitis in the MS than PS group (OR, 0.16; 95% CI, 0.03–0.79; p = 0.03) (Fig. 2d).
Migration
There was no significant difference in stent migration between the MS group and PS group (OR, 0.52; 95% CI, 0.15–1.81; p = 0.31) (Fig. 2e).
Postoperative complications
Seven studies provided data regarding postoperative complications, and our meta-analysis showed no significant difference between the MS group and PS group (OR, 0.64; 95% CI, 0.36–1.24; p = 0.20) (Fig. 3a). The rate of leakage was also comparable between the two groups (OR, 1.10; 95% CI, 0.25–4.88; p = 0.90) (Fig. 3b).
R0 resection
Five studies involving 180 patients provided data regarding R0 resection. Our analysis showed no significant difference between the two groups (OR, 1.36; 95% CI, 0.56–3.29; p = 0.50) (Fig. 3c).
Sensitivity analysis
The influence of a single study on the overall meta-analysis estimate was investigated by omitting one study at a time. The omission of any study resulted in no significant difference, indicating that our results were statistically reliable.
Publication bias
The graphical funnel plots of most parameters were symmetrical. Egger’s test revealed no significant publication bias.
Discussion
The current meta-analysis compared the use of an MS and PS for PBD in terms of preoperative and postoperative outcomes in patients with pancreatic cancer undergoing NAT. To the best of our knowledge, this is the first meta-analysis to focus on this topic. Our meta-analysis showed that compared with a PS, use of an MS for PBD in patients with pancreatic cancer undergoing NAT followed by surgery was associated with lower rates of reintervention, delay of NAT, RBO, and cholangitis. Additionally, the postoperative outcomes were comparable between an MS and PS. Further studies on this topic are recommended.
Because NAT has only recently gained popularity, there are few reports comparing different types of stents during treatment. In patients with pancreatic cancer undergoing NAT, RBO is often the main reason for delay or interruption of NAT. Our study showed that an MS was associated with a lower rate of RBO than a PS (19.88% vs. 57.53%, respectively). Previous studies have shown that the incidence of RBO with use of an MS ranged from 3 to 35%, whereas that with use of a PS ranged from 20–97% [12, 20,21,22]. The differences in the incidence of RBO among studies may be related to differences in the timing of preoperative NAT. There is no consensus on the optimal time of NAT. The main causes of RBO are stent occlusion and migration. In our study, occlusion occurred less frequently in the MS than PS group. In theory, an MS has a larger diameter than a PS, which can reduce the risk of stent occlusion. Ikezawa et al. [23] demonstrated that a larger-diameter PS may decrease the risk of delayed NAC. Stent migration also plays an important role in occlusion. In the current meta-analysis, the incidence of migration was similar between the MS and PS groups. Consistent with previous studies, our study indicated that reintervention was less frequent with MS than PS placement. This may be related to the fact that MS can reduce the occurrence of RBO. Notably, different MS types may have different impacts on the incidence of stent reintervention. Leone et al. [24] demonstrated that a covered self-expanding MS has longer patency than an uncovered MS. The types of MS varied among the studies included in our meta-analysis, which is one of the shortcomings of our study.
The aim of NAT is to increase the rate of resection and radical surgery. Some studies have shown that MS may be associated with a higher rate of R0 resection because of fewer complications following endoscopic retrograde cholangiopancreatography [16, 25]. Fewer complications may be helpful for enough anticancer agent. However, this conclusion is still debatable. The use of an MS may increase biliary inflammation and fibrosis (adhesion to the bile duct and vessels) [26, 27]. Our study showed no significant difference in R0 resection or postoperative complications between the two groups. Several studies have revealed that an MS was associated with a higher incidence of wound infection and a longer operation time [28, 29]. Tol et al. [30] demonstrated more frequent serious complications with use of a PS than MS. However, the data on the association between an MS and PS on the postoperative complications were limited. Consistent with several previous studies, our meta-analysis showed that an MS did not provide an advantage with respect to postoperative complications. Studies focusing on the effects of an MS and PS on postoperative complications and the R0 resection rate in patients with pancreatic cancer are still limited, and further research on this topic is required.
With respect to cost-effectiveness, an MS appears to have higher cost than a PS; however, this remains controversial. An RCT conducted by Gardner et al. [20] showed that a PS was less expensive than an MS. However, other studies have produced different conclusions. Because costs were calculated differently among previous studies, we were unable to analyze cost-effectiveness in our meta-analysis.
The present study has several limitations. First, most of the included studies were retrospective in nature; only two RCTs were included, which may have led to selection bias. Second, the samples in the included studies were small, and the data provided in the original studies were insufficient. Third, the definitions of reported outcome measures were variable. Finally, the NAT regimens were different, which may have affected the R0 resection rate.
Conclusion
Use of an MS for PBD in patients with pancreatic cancer undergoing NAT followed by surgery was associated with lower rates of reintervention, delay of NAT, RBO, and cholangitis compared with use of a PS. However, the postoperative outcomes were comparable between the two types of stents. Further studies on this topic are recommended.
Data Availability
All the data used in the study can be obtained from the original articles.
Abbreviations
- NAT:
-
Neoadjuvant therapy
- NAC:
-
Neoadjuvant chemotherapy
- NACRT:
-
Neoadjuvant chemoradiotherapy
- MS:
-
Metal stent
- PS:
-
Plastic stent
- PBD:
-
Preoperative biliary drainage
- CI:
-
Confidence interval
- OR:
-
Odds ratios
References
Sternby H, Andersson B. Nationwide trends and outcomes of neoadjuvant chemotherapy in pancreatic cancer - an analysis of the swedish national pancreatic cancer registry. Scand J Gastroenterol 2022:1–6.
Chun YS, Milestone BN, Watson JC, Cohen SJ, Burtness B, Engstrom PF, Haluszka O, Tokar JL, Hall MJ, Denlinger CS, et al. Defining venous involvement in borderline resectable pancreatic cancer. Ann Surg Oncol. 2010;17(11):2832–8.
Andriulli A, Festa V, Botteri E, Valvano MR, Koch M, Bassi C, Maisonneuve P, Sebastiano PD. Neoadjuvant/preoperative gemcitabine for patients with localized pancreatic cancer: a meta-analysis of prospective studies. Ann Surg Oncol. 2012;19(5):1644–62.
Papalezova KT, Tyler DS, Blazer DG 3rd, Clary BM, Czito BG, Hurwitz HI, Uronis HE, Pappas TN, Willett CG, White RR. Does preoperative therapy optimize outcomes in patients with resectable pancreatic cancer? J Surg Oncol. 2012;106(1):111–8.
Wang L, Lin N, Xin F, Ke Q, Zeng Y, Liu J. A systematic review of the comparison of the incidence of seeding metastasis between endoscopic biliary drainage and percutaneous transhepatic biliary drainage for resectable malignant biliary obstruction. World J Surg Oncol. 2019;17(1):116.
Sawas T, Al Halabi S, Parsi MA, Vargo JJ. Self-expandable metal stents versus plastic stents for malignant biliary obstruction: a meta-analysis. Gastrointest Endosc. 2015;82(2):256–267e257.
Tsuboi T, Sasaki T, Serikawa M, Ishii Y, Mouri T, Shimizu A, Kurihara K, Tatsukawa Y, Miyaki E, Kawamura R, et al. Preoperative biliary drainage in cases of Borderline Resectable Pancreatic Cancer treated with neoadjuvant chemotherapy and surgery. Gastroenterol Res Pract. 2016;2016:7968201.
Nakamura K, Sho M, Akahori T, Nagai M, Nishiwada S, Nakagawa K, Tanaka T, Kichikawa K, Tamamoto T, Hasegawa M, et al. A comparison between Plastic and metallic biliary stent Placement in Patients receiving Preoperative Neoadjuvant Chemoradiotherapy for Resectable Pancreatic Cancer. World J Surg. 2019;43(2):642–8.
Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg (London England). 2010;8(5):336–41.
Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–5.
Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA. The Cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ (Clinical research ed). 2011;343:d5928.
Kubota K, Sato T, Watanabe S, Hosono K, Kobayashi N, Mori R, Taniguchi K, Matsuyama R, Endo I, Nakajima A. Covered self-expandable metal stent deployment promises safe neoadjuvant chemoradiation therapy in patients with borderline resectable pancreatic head cancer. Dig Endosc. 2014;26(1):77–86.
Vehviläinen S, Seppänen H, Nurmi A, Haglund C, Mustonen H, Udd M, Kylänpää L. Use of self-expandable metallic stents for endoscopic biliary decompression decreases stent complications in pancreatic cancer patients receiving chemotherapy. Surg Endosc. 2022;36(1):614–20.
Tamura T, Itonaga M, Ashida R, Yamashita Y, Hatamaru K, Kawaji Y, Emori T, Kitahata Y, Miyazawa M, Hirono S, et al. Covered self-expandable metal stents versus plastic stents for preoperative biliary drainage in patient receiving neo-adjuvant chemotherapy for borderline resectable pancreatic cancer: prospective randomized study. Dig Endosc. 2021;33(7):1170–8.
Kobayashi K, Kobara H, Kamada H, Kohno T, Namima D, Fujita N, Yamana H, Fujihara S, Okano K, Masaki T. Comparison of plastic stent versus metal stent in preoperative biliary drainage for pancreatic head cancer with neoadjuvant chemoradiotherapy. J Hepatobiliary Pancreat Sci. 2021;28(10):856–63.
Kuwatani M, Nakamura T, Hayashi T, Kimura Y, Ono M, Motoya M, Imai K, Yamakita K, Goto T, Takahashi K, et al. Clinical outcomes of biliary drainage during a neoadjuvant therapy for pancreatic Cancer: metal versus plastic stents. Gut Liver. 2020;14(2):269–73.
Hasegawa S, Kubota K, Yagi S, Kurita Y, Sato T, Hosono K, Matsuyama R, Endo I, Kobayashi N, Nakajima A. Covered metallic stent placement for biliary drainage could be promising in the coming era of neoadjuvant chemo-radiation therapy for all pancreatic cancer. J Hepatobiliary Pancreat Sci. 2021;28(7):617–24.
Adams MA, Anderson MA, Myles JD, Khalatbari S, Scheiman JM. Self-expanding metal stents (SEMS) provide superior outcomes compared to plastic stents for pancreatic cancer patients undergoing neoadjuvant therapy. J Gastrointest Oncol. 2012;3(4):309–13.
Lewis J, Seminara B. Preoperative biliary drainage with self-expanding metal biliary stents during Neoadjuvant Chemotherapy for the treatment of pancreatic adenocarcinoma reduces the need for repeat endoscopic intervention: 2833. Official J Am Coll Gastroenterol | ACG 2018, 113.
Gardner TB, Spangler CC, Byanova KL, Ripple GH, Rockacy MJ, Levenick JM, Smith KD, Colacchio TA, Barth RJ, Zaki BI, et al. Cost-effectiveness and clinical efficacy of biliary stents in patients undergoing neoadjuvant therapy for pancreatic adenocarcinoma in a randomized controlled trial. Gastrointest Endosc. 2016;84(3):460–6.
Boulay BR, Gardner TB, Gordon SR. Occlusion rate and complications of plastic biliary stent placement in patients undergoing neoadjuvant chemoradiotherapy for pancreatic cancer with malignant biliary obstruction. J Clin Gastroenterol. 2010;44(6):452–5.
Aadam AA, Evans DB, Khan A, Oh Y, Dua K. Efficacy and safety of self-expandable metal stents for biliary decompression in patients receiving neoadjuvant therapy for pancreatic cancer: a prospective study. Gastrointest Endosc. 2012;76(1):67–75.
Ikezawa K, Takada R, Takahashi H, Kiyota R, Imai T, Abe Y, Fukutake N, Nawa T, Ashida R, Katayama K, et al. Efficacy of larger-diameter Plastic Stent Placement for preoperative biliary drainage in patients receiving Neoadjuvant Chemoradiation for Pancreatic Cancer. Pancreas. 2020;49(3):e20–1.
Leone F, Gatti M, Massucco P, Colombi F, Sperti E, Campanella D, Regge D, Gabriele P, Capussotti L, Aglietta M. Induction gemcitabine and oxaliplatin therapy followed by a twice-weekly infusion of gemcitabine and concurrent external-beam radiation for neoadjuvant treatment of locally advanced pancreatic cancer: a single institutional experience. Cancer. 2013;119(2):277–84.
Jang JY, Han Y, Lee H, Kim SW, Kwon W, Lee KH, Oh DY, Chie EK, Lee JM, Heo JS, et al. Oncological benefits of Neoadjuvant Chemoradiation with Gemcitabine Versus Upfront surgery in patients with Borderline Resectable Pancreatic Cancer: a prospective, randomized, Open-label, Multicenter Phase 2/3 Trial. Ann Surg. 2018;268(2):215–22.
Vakil N, Gross U, Bethge N. Human tissue responses to metal stents. Gastrointest Endosc Clin N Am. 1999;9(3):359–65.
Euscher ED, Marsh WL Jr, Lucas JG, Frankel WL. Histologic and immunohistochemical changes in the stented common bile duct. Appl Immunohistochem Mol Morphol. 2007;15(3):299–304.
Cavell LK, Allen PJ, Vinoya C, Eaton AA, Gonen M, Gerdes H, Mendelsohn RB, D’Angelica MI, Kingham TP, Fong Y, et al. Biliary self-expandable metal stents do not adversely affect pancreaticoduodenectomy. Am J Gastroenterol. 2013;108(7):1168–73.
Singal AK, Ross WA, Guturu P, Varadhachary GR, Javle M, Jaganmohan SR, Raju RP, Fleming JB, Raju GS, Kuo YF, et al. Self-expanding metal stents for biliary drainage in patients with resectable pancreatic cancer: single-center experience with 79 cases. Dig Dis Sci. 2011;56(12):3678–84.
Tol JA, van Hooft JE, Timmer R, Kubben FJ, van der Harst E, de Hingh IH, Vleggaar FP, Molenaar IQ, Keulemans YC, Boerma D, et al. Metal or plastic stents for preoperative biliary drainage in resectable pancreatic cancer. Gut. 2016;65(12):1981–7.
Acknowledgements
We thank Angela Morben, DVM, ELS, from Liwen Bianji (Edanz) (www.liwenbianji.cn), for editing the English text of a draft of this manuscript.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Author information
Authors and Affiliations
Contributions
Conception and design: YXL. Data collection: SJY, BW. Quality assessment: YXL, BW. Final approval of studies: YXL. Statistical analysis: YXL. Article writing: YXL, BW. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
Yunxiao Lyu, Bin Wang, and Shenjian Ye have no conflicts of interest or financial ties to disclose.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Lyu, Y., Ye, S. & Wang, B. Comparison of metal versus plastic stent for preoperative biliary drainage in patients with pancreatic cancer undergoing neoadjuvant therapy: a meta-analysis and systematic review. BMC Gastroenterol 23, 235 (2023). https://doi.org/10.1186/s12876-023-02874-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12876-023-02874-5