Abstract
Background
Several pre-clinical studies have reported the usefulness of artificial intelligence (AI) systems in the diagnosis of esophageal squamous cell carcinoma (ESCC). We conducted this study to evaluate the usefulness of an AI system for real-time diagnosis of ESCC in a clinical setting.
Methods
This study followed a single-center prospective single-arm non-inferiority design. Patients at high risk for ESCC were recruited and real-time diagnosis by the AI system was compared with that of endoscopists for lesions suspected to be ESCC. The primary outcomes were the diagnostic accuracy of the AI system and endoscopists. The secondary outcomes were sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and adverse events.
Results
A total of 237 lesions were evaluated. The accuracy, sensitivity, and specificity of the AI system were 80.6%, 68.2%, and 83.4%, respectively. The accuracy, sensitivity, and specificity of endoscopists were 85.7%, 61.4%, and 91.2%, respectively. The difference between the accuracy of the AI system and that of the endoscopists was − 5.1%, and the lower limit of the 90% confidence interval was less than the non-inferiority margin.
Conclusions
The non-inferiority of the AI system in comparison with endoscopists in the real-time diagnosis of ESCC in a clinical setting was not proven.
Trial registration
Japan Registry of Clinical Trials (jRCTs052200015, 18/05/2020).
Similar content being viewed by others
Background
Esophageal cancer is the seventh most common cancer and the sixth most common cause of cancer-related mortality worldwide [1], and squamous cell carcinoma is the predominant type of esophageal cancer in Asia [2]. The prognosis for advanced esophageal squamous cell carcinoma (ESCC) is poor, but a good prognosis can be expected if ESCC is detected at an early stage and treated with endoscopic resection, chemoradiation, and surgical resection [3,4,5,6]. Many studies have reported the usefulness of narrow band imaging (NBI) in the diagnosis of ESCC [7,8,9] and the diagnostic accuracy of magnifying endoscopy (ME) with NBI is comparable to that of diagnosis by biopsy [10]. However, mastering endoscopic diagnosis takes many years of training, and the skills are difficult for general endoscopists to learn because they do not diagnose ESCC frequently. Furthermore, endoscopic diagnosis of ESCC is prone to interobserver differences [11, 12].
Artificial intelligence (AI) systems for medical diagnostic imaging have evolved rapidly in recent years. Many studies report on the usefulness of AI systems for analysis of the gastrointestinal tract, and we have reported several studies demonstrating the usefulness of AI systems in the diagnosis of ESCC [11,12,13,14,15,16]. However, a limitation of these studies is that they were conducted using still images or video images, and not on patients in real-time settings. We therefore conducted this study to evaluate the usefulness of an AI system for real-time diagnosis of ESCC in a real clinical setting.
Methods
Study design and patients
This single-center prospective single-arm non-inferiority study was performed in accordance with the Declaration of Helsinki. Patients at high risk for ESCC were recruited to maximize the number of lesions detected. Because patients with ESCC have been reported to have a high incidence of synchronous or metachronous recurrence [17, 18] and patients with head and neck cancer also often present with synchronous or metachronous ESCC [19], the inclusion criteria were set as: (1) a history of head and neck cancer or ESCC; (2) no prior surgery, chemotherapy, or irradiation for ESCC (including irradiation of cancer in other organs); (3) an age of between 20 and 90 years; and (4) preserved major organ function. The exclusion criteria were as follows: (1) patients with severe esophageal stricture; (2) patients continuing antithrombotic drugs, for whom biopsy should not be carried out according to the Japanese guidelines (e.g., patients on warfarin and with a prothrombin time international normalized ratio not confirmed to be in the therapeutic range) [20] [21]; and (3) pregnancy or suspected pregnancy. Written informed consent was obtained from all participants.
Preparation of the training dataset
This study used a training dataset and an AI system developed in previous studies [12], and the following is a summary of the dataset preparation. A deep learning-based AI system for the diagnosis of superficial ESCCs was developed. Endoscopic still and video images of pathologically proven superficial ESCCs captured at three facilities between December 2005 and June 2019 were collected, as were images of noncancerous lesions and normal esophagi captured at Osaka International Cancer Institute between January 2009 and June 2019. After extracting still images from the video images, all the images were manually marked-up by precisely delineating the boundaries and filling in the areas containing superficial ESCCs or other abnormal lesions. All marked images were checked by a board-certified trainer (R.I.) of the Japan Gastroenterological Endoscopy Society. Finally, the training dataset for the AI system consisted of 25,048 images from 1433 superficial ESCCs and 4746 images from 410 noncancerous lesions and normal esophagi. These images included both non-ME and ME with white-light imaging, and NBI or blue laser imaging.
Construction of the AI system
The AI system was constructed using the same methods as in a previous study [12], and the following provides a summary of this. A BiT-M (ResNet-101 × 1) model pretrained on the ImageNet-21k dataset was used for the AI system. At the transfer learning phase, the model was trained using a BiT-HyperRule to select the most important hyperparameters for tuning. Stochastic gradient descent was used with an initial learning rate of 0.003 and momentum of 0.9. The model was fine-tuned for 3900 steps with a batch size of 32. The learning rate was decayed by a factor of 10 at 30%, 60%, and 90% of the training steps. The model was trained on the dataset and validated using the PyTorch deep learning framework [22]. A difference from the previous study is that to match the quality of the real-time images with that of the training data, a Gaussian filter with a kernel size of 10 was applied and the images were then resized to 512 × 512 for input into the AI system. The trained neural network generated a probability score between 0 and 1 corresponding to the probability of ESCC. A still image was judged as ESCC when the probability score for ESCC surpassed the threshold value in real time.
Examination protocol
The examination protocol consisted of detection by non-ME with NBI, characterization by ME with NBI, and biopsy of detected lesions (Fig. 1). First, the entire esophagus was observed with non-ME with NBI for at least 10 s, followed by non-ME with NBI using the AI system for at least 10 s to detect target lesions. Target lesions were defined as brownish areas detected by endoscopists that required differential diagnosis between ESCC and noncancerous lesions (e.g., abnormal vessels or esophagitis), or that the AI system judged to be positive for ESCC on non-ME with NBI. Then, the endoscopist classified the target lesions as ESCC or noncancerous lesions using ME with NBI, followed by the AI system differentiating the target lesions between ESCC and noncancerous lesions in real time using captured still images of ME with NBI. All target lesions were ultimately biopsied. Lesions diagnosed as ESCC or suspected to be ESCC were treated by endoscopic resection. The reference standards were histological diagnosis of the resected specimen for resected lesions, and histological diagnosis of the biopsy sample for non-resected lesions. Histological assessments were performed according to the Japanese Classification of Esophageal Cancer [23].
The endoscopic procedures were performed using the following equipment: GIF-H290Z, GIF-H260Z, GIF-Q240Z, GIF-XZ1200, or GIF-EZ1500 endoscopes (Olympus, Tokyo, Japan) with an EVIS X1 system (Olympus, Tokyo, Japan). Five endoscopists who had been routinely diagnosing gastrointestinal tumors performed all endoscopic procedures. These five endoscopists were board-certified fellows of the Japan Gastroenterological Endoscopy Society or had equivalent qualifications, and all had diagnosed over 300 cases of ESCCs with endoscopic images. The five endoscopists had a median endoscopy experience of 8 years (range 6–26 years) and the median number of esophagogastroduodenoscopies performed was 4500 (range 3000–25,000).
Sample size
A non-inferiority trial design was chosen to investigate the accuracy of the AI system. Our previous study showed diagnostic accuracy of 83% for the AI system when the diagnostic accuracy of endoscopists was 78% 14. We considered that we could recommend the use of the AI system for general endoscopists if the lower limit of the 90% confidence interval (CI) for the difference between the accuracy of the AI system and that of endoscopists in a cancer hospital was not less than − 10%. Therefore, we calculated the number of biopsies required with a non-inferiority margin of 10%, a power of 90%, and a one-sided significance level of 0.05, which gave the result of 238 biopsies. According to a previous report [24], 160 target lesions could be expected from 350 participants, and the number of required participants was calculated as 520. Considering a decrease in detection power due to unanalyzable lesions, the planned enrollment of participants was 550.
Statistical analysis
The primary outcomes were the diagnostic accuracies of the AI system and endoscopists. The secondary outcomes were sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and adverse events. These parameters were calculated as follows: accuracy = correctly diagnosed lesions/total target lesions; sensitivity = correctly diagnosed ESCCs/total ESCCs; specificity = correctly diagnosed noncancerous lesions/total noncancerous lesions; PPV = correctly diagnosed ESCCs/total lesions diagnosed as ESCC; NPV = correctly diagnosed noncancerous lesions/total lesions diagnosed as noncancerous lesions. The results are shown with 95% CIs. All analyses were performed on a personal computer using the EZR software package, version 1.55 (Saitama Medical Center, Jichi Medical University, Tochigi, Japan) [25].
Results
Patients and lesions
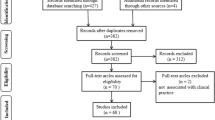
Between May 2021 and July 2022, 437 patients were assessed for study eligibility. Forty-nine patients declined to participate, and the remaining 388 patients were enrolled in this study. Patient recruitment was ended after these 388 patients because we had collected 238 biopsied lesions, the calculated sample size requirement for this study. Eight patients were excluded after enrollment: three because of severe esophageal stricture, two because of a previous history of chemoradiotherapy for ESCC, two because of withdrawn consent, and one with no history of head and neck cancer or ESCC. Of the remaining 380 patients, 370 underwent an endoscopic procedure with a GIF-H290Z endoscope, 4 with a GIF-H260Z, 3 with a GIF-Q240Z, 2 with a GIF-XZ1200, and 1 with a GIF-EZ1500. No serious adverse event was observed, and only one patient received intravenous flumazenil because of prolonged deep sedation after the endoscopic procedure. Finally, a total of 237 detected target lesions were evaluated. A flowchart of patient inclusion is shown in Fig. 2.
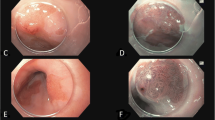
Of the 237 target lesions, 44 were pathologically diagnosed as ESCC. All 44 lesions were superficial. The characteristics of the patients and lesions are shown in Table 1, and a representative case is shown in Fig. 3.
A lesion diagnosed as ESCC by the AI system and an endoscopist
a. The lesion was located on the left wall of the lower thoracic esophagus
b. Magnifying image of the lesion with NBI.
c. Specimen resected by endoscopic submucosal dissection
d. The histopathological diagnosis of the resected specimen was squamous cell carcinoma with invasion into the lamina propria
AI, artificial intelligence; ESCC, esophageal squamous cell carcinoma; NBI, narrow band imaging
Performance of the AI system and endoscopists
The AI system diagnosed 30 of 44 ESCCs (68.2%) as ESCC, and 161 of 193 (83.4%) noncancerous lesions as noncancerous lesion (Table 2). In contrast, the endoscopists diagnosed 27 of 44 ESCCs (61.4%) as ESCC, and 176 of 193 (91.2%) noncancerous lesions as noncancerous lesion (Table 2). The accuracies of the AI system and endoscopists were 80.6% and 85.7%, respectively (Table 2). The difference between the accuracy of the AI system and that of the endoscopists was − 5.1% (90% CI, − 10.7–0.6%). The lower limit of the 90% CI was less than − 10%, and we therefore concluded that the non-inferiority of the AI system to endoscopists was not proven.
Subgroup analyses by lesion size and location
Table 3 lists the accuracy, sensitivity, and specificity of the AI system and the endoscopists with respect to lesion size and location. Except for sensitivity for lesions ≥ 10 mm, the AI system had lower accuracy and specificity but higher sensitivity than the endoscopists.
Discussion
In this study, we evaluated the usefulness of an AI system for real-time diagnosis of ESCC in a clinical setting. However, the non-inferiority of the AI system in comparison with endoscopists in the real-time diagnosis of ESCC was not confirmed.
We attribute the failure to demonstrate the non-inferiority of the AI system to the following reasons. First, the images used for validation of the AI system in the pre-clinical studies [11, 12, 14] were different from those used in the current clinical study. Compressed images were used to train the AI system because the endoscopic images obtained in our routine clinical practice were stored as compressed images. In the validation of the AI system, we used compressed images in the pre-clinical studies, but original uncompressed images in the current clinical study. Although the diagnostic accuracy of the AI system trained on compressed images was high when verified with compressed images in the pre-clinical studies [11, 12, 14], the same high accuracy was not reproduced in the current clinical study using original images. The differences between the original and compressed images are very difficult for humans to discern. However, significant differences do exist between the original and compressed images because we repeatedly confirmed differences in the diagnostic performance of the AI between compressed and uncompressed images from the same dataset. This problem made it difficult to apply our AI system trained on compressed images to original images obtained in the clinical setting. We tried to eliminate this difference between compressed images and original images using various techniques before the start of the study, but were unable to eliminate it completely. We believe that this was the main reason for the negative results of this study. On the basis of the results of this study, we believe that there is a limit to the technical improvements that can be performed to bridge the gap between compressed training images and original images, and that the diagnostic performance of the AI system can be improved in the future by training it with endoscopic images saved in an uncompressed format.
Second, the proportion of ESCC in the target lesions differs from that in previous studies. In previous studies [11, 12, 14], ESCCs accounted for 38.5–56.5% of the validation dataset, whereas in the current study ESCCs accounted for only 18.6% of all target lesions. In the current study, the AI system had high diagnostic accuracy (sensitivity) for ESCC but low diagnostic accuracy (specificity) for noncancerous lesions. The high proportion of noncancerous lesions in this study may have contributed to the lower accuracy of the AI system.
The sensitivity and specificity of the AI system can be altered by adjusting the ESCC threshold, and changing this threshold may improve the overall positive diagnosis rate. However, because sensitivity and specificity have a trade-off relationship, it is not possible to improve both by adjusting this threshold. Because the priority of accuracy, sensitivity, or specificity varies depending on the situation in which an AI system is used in clinical practice, it is necessary to set the optimal threshold based on ROC analysis and the needs of the clinical setting.
In this study, a total of 237 target lesions were detected in 380 participants, which was a higher frequency than expected. We attribute this to the fact that the previous study [24] we used for planning the number of participants was reported more than 10 years ago, and that improvements in endoscopes and endoscopic observation techniques [26] over recent years have increased the number of target lesions detected per participant.
In the subgroup analysis, the sensitivity of the endoscopists was low, especially in lesions < 10 mm. We consider two factors to be the main causes for the low sensitivity of the endoscopists’ diagnoses in our study. One is the prospective study design, which may have resulted in reduced diagnostic performance compared with a retrospective study design [7, 27]. The other reason is the high proportion of cancers that were < 10 mm or that were shallow (cancer invasion depth of epithelium/lamina propria), with the sensitivity for these being generally low [12]. In contrast to the sensitivity, the specificity, which is in a trade-off relationship with sensitivity, was high in this study. Moreover, the diagnostic accuracy for lesions < 10 mm was comparable with that in previous studies [11, 12, 14]. In addition, the difference between the accuracy of the endoscopists and that of the AI system was 3.7% when the lesion size was < 10 mm, but was 11.1% when the lesion size was ≥ 10 mm. In this study, AI diagnosis was made only according to ME findings, while the endoscopists’ diagnoses were also made using non-ME findings as a reference, in addition to ME findings. We speculate that because the significance of non-ME findings is higher in larger lesions, the difference between the endoscopists’ and AI diagnosis was more obvious in lesions ≥ 10 mm than in lesions < 10 mm.
This study is subject to the following limitations. First, this was a single-center study performed at a high-volume center. Second, when the study was planned, we also intended to evaluate the detection and diagnosis of the cancer invasion depth. However, because of the difference between compressed images and original images, estimation of the invasion depth did not improve the accuracy of the AI system, and this feature was not evaluated, thus preventing a comprehensive evaluation of the diagnosis of ESCC. Third, we did not evaluate interobserver agreement or compare diagnostic performance between the endoscopists because the design of this study was not suitable for such assessments. In addition, we have already evaluated these in previous studies [11, 14].
Conclusions
The non-inferiority of the AI system to endoscopists in the real-time diagnosis of ESCC in a clinical setting was not proven.
Data Availability
The datasets generated and analyzed during this study are not publicly available but are available from the corresponding author on reasonable request.
Abbreviations
- AI:
-
artificial intelligence
- CI:
-
confidence interval
- ESCC:
-
esophageal squamous cell carcinoma
- ME:
-
magnifying endoscopy
- NBI:
-
narrow band imaging
- NPV:
-
negative predictive value
- PPV:
-
positive predictive value
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424.
Rustgi AK, El-Serag HB. Esophageal carcinoma. N Engl J Med. 2014;371:2499–509.
Igaki H, Kato H, Tachimori Y, et al. Clinicopathologic characteristics and survival of patients with clinical stage I squamous cell carcinomas of the thoracic esophagus treated with three-field lymph node dissection. Eur J Cardiothorac Surg. 2001;20:1089–94.
Yamashina T, Ishihara R, Nagai K, et al. Long-term outcome and metastatic risk after endoscopic resection of superficial esophageal squamous cell carcinoma. Am J Gastroenterol. 2013;108:544–51.
Minashi K, Nihei K, Mizusawa J et al. Efficacy of endoscopic resection and selective chemoradiotherapy for stage I esophageal squamous cell carcinoma. Gastroenterology. 2019; 157: 382 – 90.e3.
Kato K, Ito Y, Nozaki I, et al. Parallel-group controlled trial of surgery Versus Chemoradiotherapy in patients with stage I esophageal squamous cell carcinoma. Gastroenterology. 2021;161:1878–86. e2.
Ishihara R, Inoue T, Uedo N, et al. Significance of each narrow-band imaging finding in diagnosing squamous mucosal high-grade neoplasia of the esophagus. J Gastroenterol Hepatol. 2010;25:1410–5.
Muto M, Minashi K, Yano T, et al. Early detection of superficial squamous cell carcinoma in the head and neck region and esophagus by narrow band imaging: a multicenter randomized controlled trial. J Clin Oncol. 2010;28:1566–72.
Oyama T, Inoue H, Arima M, et al. Prediction of the invasion depth of superficial squamous cell carcinoma based on microvessel morphology: magnifying endoscopic classification of the Japan Esophageal Society. Esophagus. 2017;14:105–12.
Nagai K, Ishihara R, Ishiguro S, et al. Endoscopic optical diagnosis provides high diagnostic accuracy of esophageal squamous cell carcinoma. BMC Gastroenterol. 2014;14:141.
Fukuda H, Ishihara R, Kato Y, et al. Comparison of performances of artificial intelligence versus expert endoscopists for real-time assisted diagnosis of esophageal squamous cell carcinoma (with video). Gastrointest Endosc. 2020;92:848–55.
Tajiri A, Ishihara R, Kato Y, et al. Utility of an artificial intelligence system for classification of esophageal lesions when simulating its clinical use. Sci Rep. 2022;12:6677.
Nakagawa K, Ishihara R, Aoyama K, et al. Classification for invasion depth of esophageal squamous cell carcinoma using a deep neural network compared with experienced endoscopists. Gastrointest Endosc. 2019;90:407–14.
Ohmori M, Ishihara R, Aoyama K, et al. Endoscopic detection and differentiation of esophageal lesions using a deep neural network. Gastrointest Endosc. 2020;91:301–9e1.
Shimamoto Y, Ishihara R, Kato Y, et al. Real-time assessment of video images for esophageal squamous cell carcinoma invasion depth using artificial intelligence. J Gastroenterol. 2020;55:1037–45.
Waki K, Ishihara R, Kato Y, et al. Usefulness of an artificial intelligence system for the detection of esophageal squamous cell carcinoma evaluated with videos simulating overlooking situation. Dig Endosc. 2021;33:1101–9.
Urabe Y, Hiyama T, Tanaka S, et al. Metachronous multiple esophageal squamous cell carcinomas and lugol-voiding lesions after endoscopic mucosal resection. Endoscopy. 2009;41:304–9.
Yokoyama A, Omori T, Yokoyama T, Sato Y, Kawakubo H, Maruyama K. Risk of metachronous squamous cell carcinoma in the upper aerodigestive tract of japanese alcoholic men with esophageal squamous cell carcinoma: a long-term endoscopic follow-up study. Cancer Sci. 2008;99:1164–71.
Nobre Moura R, Kuboki Y, Baba ER, et al. Long-term results of an endoscopic screening program for superficial esophageal cancer in patients with head and neck squamous cell carcinoma. Endosc Int Open. 2022;10:E200–8.
Fujimoto K, Fujishiro M, Kato M, et al. Guidelines for gastroenterological endoscopy in patients undergoing antithrombotic treatment. Dig Endosc. 2014;26:1–14.
Kato M, Uedo N, Hokimoto S, et al. Guidelines for gastroenterological endoscopy in patients undergoing antithrombotic treatment: 2017 appendix on anticoagulants including direct oral anticoagulants. Dig Endosc. 2018;30:433–40.
Ketkar N. Introduction to PyTorch. In: Ketkar N, editor. Deep learning with Python: a Hands-on introduction. Berkeley, CA: Apress; 2017. pp. 195–208.
Japan Esophageal Socety. Japanese classification of esophageal cancer, 11th Edition: part I. Esophagus. 2017;14:1–36.
Ishihara R, Takeuchi Y, Chatani R, et al. Prospective evaluation of narrow-band imaging endoscopy for screening of esophageal squamous mucosal high-grade neoplasia in experienced and less experienced endoscopists. Dis Esophagus. 2010;23:480–6.
Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48:452–8.
Iwatsubo T, Ishihara R, Yamasaki Y, et al. Narrow band imaging under less-air condition improves the visibility of superficial esophageal squamous cell carcinoma. BMC Gastroenterol. 2020;20:389.
Minami H, Inoue H, Ikeda H, et al. Usefulness of background coloration in detection of Esophago-Pharyngeal Lesions using NBI magnification. Gastroenterol Res Pract. 2012;2012:529782.
Acknowledgements
We thank Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript.
Funding
None.
Author information
Authors and Affiliations
Contributions
Yasuhiro Tani: Drafting article, endoscopic procedure, analysis of data, and final approval of the article. Ryu Ishihara: Conception and design, endoscopic procedure, analysis of data, critical revision of the article for important intellectual content, and final approval of the article. Takahiro Inoue: Conception and design, data collection, and final approval of the article. Yushi Kawakami, Katsunori Matsueda and Muneaki Miyake: Endoscopic procedure, data collection, and final approval of the article. Yuki Okubo, Shunsuke Yoshii, Satoki Shichijo, Takashi Kanesaka, Sachiko Yamamoto, Yoji Takeuchi, Koji Higashino, Noriya Uedo and Tomoki Michida: Data collection and final approval of the article. Yusuke Kato and Tomohiro Tada: Construction and mechanical management of the AI system and final approval of the article.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Certified Review Board of Osaka International Cancer Institute (no. 19221-3) and registered in the Japan Registry of Clinical Trials (jRCT) as jRCTs052200015 on 18/05/2020. This study was performed in accordance with the Declaration of Helsinki and written informed consent was obtained from all participants.
Consent for publication
Not applicable.
Competing interests
Satoki Shichijo has received personal fees from Olympus Corporation, Daiichi-Sankyo, EA Pharma, AstraZeneca, AI Medical Service Inc., and Jannsen Pharmaceutical. Takashi Kanesaka has received personal fees from Olympus Corporation. Yoji Takeuchi has received personal fees from Olympus Corporation, Boston Scientific Japan, FUJIFILM Medical Co., Ltd, Daiichi-Sankyo, Miyarisan Pharmaceutical, Asuka Pharmaceutical, AstraZeneca, EA Pharma, Zeria Pharmaceutical, Fujifilm, Kaneka Medix, and Kyorin Pharmaceutical. Noriya Uedo has received personal fees from Olympus Corporation, Boston Scientific Japan, FUJIFILM Medical Co., Ltd, Daiichi-Sankyo, Takeda Pharmaceutical, EA Pharma, Otsuka Pharmaceutical, AstraZeneca, AI Medical Service Inc., and Miyarisan Pharmaceutical. Ryu Ishihara has received personal fees from Olympus Corporation and FUJIFILM Medical Co., Ltd, Daiichi-Sankyo, Ono Pharmaceutical, EA Pharma, and AstraZeneca. Tomohiro Tada is a shareholder in AI Medical Service Inc. and holds the following patent PCT/JPT2018/040381. The other authors have no financial relationships to disclose.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Tani, Y., Ishihara, R., Inoue, T. et al. A single-center prospective study evaluating the usefulness of artificial intelligence for the diagnosis of esophageal squamous cell carcinoma in a real-time setting. BMC Gastroenterol 23, 184 (2023). https://doi.org/10.1186/s12876-023-02788-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12876-023-02788-2