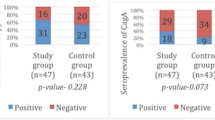
Abstract
Background
Colonoscopy is considered the most effective screening method for colorectal polyps. However, the longevity and complexity of the procedure makes it less desirable to screen for colorectal polyps in the general population. Therefore, it is essential to identify other independent risk factors. In this study, we explored the link between Hp infection, atrophic gastritis, and colorectal polyps to identify a new potential risk factors of colorectal polyps.
Methods
In this study, atrophic gastritis and intestinal polyps were diagnosed by endoscopy and pathology. All the 792 patients in this retrospective study were divided into sub-groups based on the presence of colorectal polyps. The correlation between polyps and atrophic gastritis was analyzed using the chi-square test and Kruskal-Wallis test. The receiver operating characteristic (ROC) curve was used to compare the predictive value for colorectal polyps between Hp infection and atrophic gastritis. Binary logistic regression was utilized to identify independent risk factors for colorectal polyps.
Results
Patients with colorectal polyps were primarily male with advanced age, and the number of patients with colorectal polyps had a higher association with smoking, alcohol drinking, and Hp infection than the control group. A positive correlation between the number of colorectal polyps and the severity of atrophic gastritis was observed. ROC analysis showed that atrophic gastritis was a better risk factors for colorectal polyps. Multivariate analysis identified atrophic gastritis as an independent risk factor for colorectal polyps (OR 2.294; 95% CI 1.597–3.296).
Conclusions
Atrophic gastritis confirmed could be an independent risk factors for colorectal polyps.
Similar content being viewed by others
Introduction
Colorectal cancer is the third most common cancer and the fifth leading cause of cancer-related deaths worldwide [1]. Colorectal polyps, especially adenomatous polyps, are considered pre-cancerous lesions caused by numerous factors such as smoking, age, and excessive red meat intake [2]. Colonoscopy is the gold standard for detecting pre-cancerous lesions such as colorectal polyps. Due to operational and preparation complexity, and the inherent risks involved with the procedure, especially in regions with insufficient medical resources, it is challenging to use colonoscopy for mass screening [3]. Hence a more convenient, efficient way to identify high-risk patients is necessary, which can help physicians to decide whether a patient should be subjected to an invasive procedure like a colonoscopy.
Helicobacter pylori (Hp) is recognized as a type I carcinogen by World Health Organization [4, 5] and plays an important role in the development of gastric cancer [6,7,8]. Long-term Hp infection leads to atrophic gastritis and intestinal epithelial metaplasia, which eventually may progress into gastric cancer [9]. In addition to gastric cancer, Hp infection is associated with various digestive diseases, such as colorectal cancer. Interestingly a report suggests an association between Hp infection and colorectal polyps [10]. Hp infection promotes the secretion of gastric acid and causes colorectal polyps [11, 12]. Whether the correlation between Hp infection and colorectal polyps is mediated by Hp-associated atrophic gastritis is yet to be established. Therefore, this retrospective study was conducted to elucidate the relationship between atrophic gastritis, Hp infection, and colorectal polyps to provide new insight into screening strategies for colorectal polyps.
Methods
Patients
Patients who underwent their first gastroscopy combined with colonoscopy at the Affiliated Hospital of Qingdao University from January 2021 to March 2022 were enrolled for the study. Patients diagnosed with gastric cancer, colon cancer, ulcerative colitis, Crohn’s disease, MALT lymphoma, gastric submucosal masses, and familial adenomatous polyposis were excluded from the study. Further patients who underwent previous gastrointestinal resection were also excluded from the study.
Patients with colorectal polyps were categorized as the polyp group, while patients with no detectable colorectal polyps were classified as the control group.
Data collection
Gastroscopy biopsy specimens and carbon 13 urea breath test was used to detect Hp infection in the patients. Patients confirmed positive for either of these tests were considered positive for Hp infection. Information on previous Hp infection and treatment plans was obtained from all the patients through inquiry and medical records. Two experienced physicians graded the degree of gastric atrophy in patients using the Kimura-Takemoto classification [13] and gastroscopy, and the atrophic gastritis was classified into open and closed types. If the atrophic region was just limited to the lesser curvature of the stomach was regarded as a closed type (C type) and was divided into three grades (C-1, C-2, C-3). If the atrophic region extended to the greater curvature side was regarded as an open type and was classified into three grades (O-1, O-2, O-3). All polyps found were excised carefully and sectioned for pathological evaluation. Hematoxylin and eosin staining was performed on the sections for diagnosis. Polyps were classified into neoplastic polyps, which included tubular adenomas, tubular villous adenomas, choroidal and serrated adenomas. The second type was non-neoplastic polyps, which included hyperplastic polyps and inflammatory polyps. Polyp size was assessed per the Yamada criteria, and the number of polyps was classified as single or multiple.
Statistical analysis
Quantitative data were expressed as mean value ± standard deviation (X ± S), while qualitative data was represented as n (%). Differences between the two sets of qualitative data were compared using the Chi-square test or Fisher’s exact test. The quantitative data were calculated using the parametric test like student’s t-test, and non-parametric tests like Kruskal-Wallis test. The univariate analysis compared the clinicopathological features between the two groups and was calculated using the Chi-square test, Fisher’s exact test, student’s t-test, and Kruskal-Wallis test. Multivariate regression analysis was performed on variables with P < 0.05 and was calculated using the odds ratio (OR) and a 95% confidence interval (95% CI). A binary logistic regression analysis was used to identify independent risk factors for colorectal polyps. Receiver operating characteristic (ROC) curve was used to compare the predictability of colorectal polyps by Hp infection and atrophic gastritis. P < 0 0.05 was considered as statistically significant. The data analysis was performed using SPSS version 26 (SPSS Inc., Chicago, IL, USA).
Results
Demographics of study patients
A total of 792 patients were included in the study. The mean age of patients was 54.34 ± 10.92 years, of whom 381 (48.1%) were males, and 411 (51.9%) were females. Colorectal polyps were diagnosed in 385 (48.7%) patients, while a total of 332 patients (41.9%) were diagnosed with atrophic gastritis. Table 1 shows the clinical characteristics of all the patients. Significant differences in gender, mean age, body mass index (BMI), alcohol drinking habits, smoking habits, diabetes, atrophic gastritis, and present Hp infection status were observed between the colorectal polyp and the control group. P < 0.05 for all the characteristics mentioned above, indicating statistically significant differences.
The relationship between atrophic gastritis and the incidences of colorectal polyps
The incidence of colorectal polyps in patients with atrophic and non-atrophic gastritis was analyzed. The number of colorectal polyps in patients with atrophic gastritis was significantly higher than in non-atrophic gastritis patients (P < 0.05) and significant difference was observed in the diagnosis of adenoma and the polyp diameter between the two groups (Table 2).
Correlation between the severity of atrophic gastritis and colorectal polyps
To understand the association between atrophic gastritis and colorectal polyps, the atrophic gastritis patients were further classified based on the severity of atrophy, and Kruskal-Wallis test was performed (Table 3). O-2 and O-3 type gastritis were excluded as only one patient was graded as O-1 in the current study. As shown in Table 3, the incidence and number of polyps positively correlated with the severity of atrophy (P < 0.05).
The relationship between atrophic gastritis and colorectal polyps
Previous studies have shown an association between Hp infection and colorectal polyps. This association focused on current Hp infections, whereas patients with the previous infection mainly were neglected. To further investigate the association between Hp infection and colorectal polyps, the patients were divided into three groups: patients with current Hp infection, patients with previous Hp infection, and Hp negative patients (Table 4).
Logistic regression analysis showed that gender, mean age, body mass index (BMI), alcohol drinking habits, smoking habits, diabetes, atrophic gastritis, and current Hp infection were associated with colorectal polyps. Multivariate analysis (Table 4) revealed that age (P<0.05, OR = 1.032), gender (P < 0.05, OR = 1.545) and atrophic gastritis (P < 0 0.05, OR = 2.294) were independent risk factors for colorectal polyps.
The predictive value of atrophic gastritis for colorectal polyps
The area under the curve (AUC) for the ROC curve analysis was performed to check the predictive value of atrophic gastritis and Hp infection for colorectal polyps. AUC for atrophic gastritis was 0.62 (P < 0.001) and for Hp infections was 0.57 (P < 0.001).
Although the value is low and should be combined with other indicators for a more accurate diagnosis, these results still reveal that atrophic gastritis was better than Hp infection in finding colorectal polyps in patients.
Discussion
Previous studies have established that colorectal polyps, especially adenomatous polyps, are risk factors for colon cancer [14, 15]. Colonoscopy is the gold standard for detecting colorectal polyps before they become clinically symptomatic. However, several issues, including difficulties associated with the operations, the invasive nature of the procedure, and the steps involved in preparing the patients for a colonoscopy, are complex, which hampers the rampant use of colonoscopy for colorectal polyp screening in the general population [16]. Therefore, a simple, less invasive, and accessible strategy for screening colorectal polyps is required.
Several studies have shown that Hp infection is a risk factor for colorectal polyps [12, 17]. Hp causes hyperplasia of the intestinal mucosa by increasing the levels of gastrin, which can lead to the development of intestinal polyps [11, 18, 19]. However, some studies contradict this hypothesis [20]. Reports also suggest Hp directly induces atypical hyperplasia of the intestinal mucosa [21]via CagA, a Hp virulence factor [22]. In this study, we report Hp infection was a risk factor for colorectal polyps. To further evaluate the predictive value of Hp infection status for colorectal polyps, Hp infection was divided into previous Hp infection, current Hp infection, and Hp-negative group. Further analysis revealed that previous Hp infections (P = 0.054. OR = 0.721) and current Hp infections (P = 0.131. OR = 0.696) were not independent risk factors for colorectal polyps.
Hp infection leads to chronic inflammation, which causes damage to the gastric mucosa.
Previous studies have also demonstrated that atrophic gastritis with Hp infection is a risk factor for colorectal polyps [23]. However, in the current study, Hp infection was not an independent risk factor for colorectal polyps. It is important to note that the previous studies did not account for atrophic gastritis caused by Hp infection [12, 24]. Therefore, in this study, patients with atrophic gastritis were further categorized based on the severity of atrophic gastritis, and we discovered that the number of polyps increased with the severity of atrophic gastritis (P < 0.05), but the severity of atrophic gastritis did not make a difference to the size of polyps. These results confirm that atrophic gastritis could hint the development of colorectal polyps. Further ROC analysis confirmed that atrophic gastritis and different grades of atrophic gastritis could better predict the status of colorectal polyps than Hp infection.
The mechanism of atrophic gastritis leading to colorectal polyps is not yet fully understood [23]. Several studies show the relationship between intestinal microbiota and colorectal carcinogenesis [25, 26]. Wong et al. used feces of colorectal cancer patients to feed the mice, and the results show that the experimental group had a significantly higher proportion of heterogeneous hyperplasia and fleshy polyps in the colon compared to the control group [27]. Studies have also shown that atrophic gastritis could cause alterations in the intestinal flora [28], causing neoplastic transformation of the intestine by altering various signaling pathways [29]. Besides, the growth of certain bacteria in the intestine may increase the production of secondary bile acids, which may also contribute to developing colon polyps and cancer [30]. Furthermore, patients with atrophic gastritis are often more inclined to take proton pump inhibitors, which may significantly increase the incidence of intestinal adenomas [31]. What’s more, hypochlorhydria caused by atrophic gastritis may impede protein assimilation, giving rise to some unabsorbed metabolites and nutrients [32]. Hypochlorhydria leads to colorectal disorders, which will eventually result in colorectal polyps and even colorectal carcinogenesis [33].
However, the study has some limitations. This was a single-center and retrospective study with relatively small sample size, leading to biased results. Although atrophic gastritis was an independent risk factor of colorectal polyps and was better than Hp infection in hinting colorectal polyps, the sensitivity and specificity of atrophic gastritis were low, indicating that additional studies are needed to validate the results.
Conclusion
Previous studies focus on the association between Hp infections and colorectal polyps [12, 17]. However, the relationship between atrophic gastritis and colorectal polyps has not yet been reported. In our study, we performed a subgroup analysis of different Hp infection statuses and found that atrophic gastritis was an independent risk factor for colorectal polyps. Thus, detecting atrophic gastritis using gastroscopy could effectively predict colonic polyps, indicating a brand-new screening strategy for colorectal polyps via gastroscopy.
Data Availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Bray F, Ferlay J, Soerjomataram I, Siegel R, Torre L, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Cancer J Clin. 2018;68(6):394–424.
Haumaier F, Sterlacci W, Vieth M. Histological and molecular classification of gastrointestinal polyps. Best Pract Res Clin Gastroenterol. 2017;31(4):369–79.
Wolf A, Fontham E, Church T, Flowers C, Guerra C, LaMonte S, et al. Colorectal cancer screening for average-risk adults: 2018 guideline update from the American Cancer Society. Cancer J Clin. 2018;68(4):250–81.
Khatoon J, Rai R, Prasad K. Role of Helicobacter pylori in gastric cancer: updates. World J Gastrointest Oncol. 2016;8(2):147–58.
Sugano K, Tack J, Kuipers E, Graham D, El-Omar E, Miura S, et al. Kyoto global consensus report on Helicobacter pylori gastritis. Gut. 2015;64(9):1353–67.
Hatakeyama M. Helicobacter pylori CagA and gastric cancer: a paradigm for hit-and-run carcinogenesis. Cell Host Microbe. 2014;15(3):306–16.
Talebi Bezmin Abadi A, Perez-Perez G. dupARole of in virulence of. World J Gastroenterol. 2016;22(46):10118–23.
Xie Y, Zhou J, Zhao Y, Zhang T, Mei L. H. pylori modifies methylation of global genomic DNA and the gastrin gene promoter in gastric mucosal cells and gastric cancer cells. Microb Pathog. 2017;108:129–36.
Waldum H, Fossmark R, Gastritis. Gastric Polyps and Gastric Cancer. Int J Mol Sci. 2021;22(12).
Siddheshwar R, Muhammad K, Gray J, Kelly S. Seroprevalence of Helicobacter pylori in patients with colorectal polyps and colorectal carcinoma. Am J Gastroenterol. 2001;96(1):84–8.
Lu D, Wang M, Ke X, Wang Q, Wang J, Li D, et al. H. pyloriAssociation between infection and colorectal polyps: a Meta-analysis of Observational Studies. Front Med. 2021;8:706036.
Huang L, Wu L, Qiao Q, Fang L. Correlation between Colon polyps and metabolic syndrome and HP infection status. Gastroenterol Res Pract. 2019;2019:3916154.
Murata M, Sugimoto M, Otsuka T, Nishida A, Inatomi O, Bamba S, et al. Successful Helicobacter pylori eradication therapy improves symptoms of chronic constipation. Helicobacter. 2018;23(6):e12543.
He X, Wu K, Ogino S, Giovannucci E, Chan A, Song M. Association between Risk factors for Colorectal Cancer and Risk of Serrated polyps and conventional adenomas. Gastroenterology. 2018;155(2):355–73e18.
Okamoto K, Kitamura S, Kimura T, Nakagawa T, Sogabe M, Miyamoto H, et al. Clinicopathological characteristics of serrated polyps as precursors to colorectal cancer: current status and management. J Gastroenterol Hepatol. 2017;32(2):358–67.
Rodrigues-Pinto E, Ferreira-Silva J, Macedo G, Rex D. (Technically) difficult colonoscope insertion - Tips and tricks. Dig endoscopy: official J Japan Gastroenterological Endoscopy Soc. 2019;31(5):583–7.
Sonnenberg A, Genta R. Helicobacter pylori is a risk factor for colonic neoplasms. Am J Gastroenterol. 2013;108(2):208–15.
Georgopoulos S, Polymeros D, Triantafyllou K, Spiliadi C, Mentis A, Karamanolis D, et al. Hypergastrinemia is associated with increased risk of distal colon adenomas. Digestion. 2006;74(1):42–6.
Watson SA, Smith AM. Hypergastrinemia promotes adenoma progression in the APC(Min-/+) mouse model of familial adenomatous polyposis. Cancer Res. 2001;61(2):625–31.
Robertson D, Sandler R, Ahnen D, Greenberg E, Mott L, Cole B, et al. Gastrin, Helicobacter pylori, and colorectal adenomas. Clin Gastroenterol hepatology: official Clin Pract J Am Gastroenterological Association. 2009;7(2):163–7.
Soylu A, Ozkara S, Alis H, Dolay K, Kalayci M, Yasar N, et al. Immunohistochemical testing for Helicobacter Pylori existence in neoplasms of the colon. BMC Gastroenterol. 2008;8:35.
Shimoda A, Ueda K, Nishiumi S, Murata-Kamiya N, Mukai S, Sawada S, et al. Exosomes as nanocarriers for systemic delivery of the Helicobacter pylori virulence factor CagA. Sci Rep. 2016;6:18346.
Kawahara Y, Kodama M, Mizukami K, Saito T, Hirashita Y, Sonoda A, et al. Endoscopic gastric mucosal atrophy as a predictor of colorectal polyps: a large scale case-control study. J Clin Biochem Nutr. 2019;65(2):153–9.
Kumar A, Kim M, Lukin D. Helicobacter pylori is associated with increased risk of serrated colonic polyps: analysis of serrated polyp risk factors. Indian J gastroenterology: official J Indian Soc Gastroenterol. 2018;37(3):235–42.
Garrett W. The gut microbiota and colon cancer. Sci (New York NY). 2019;364(6446):1133–5.
Chattopadhyay I, Dhar R, Pethusamy K, Seethy A, Srivastava T, Sah R, et al. Exploring the role of gut microbiome in Colon cancer. Appl Biochem Biotechnol. 2021;193(6):1780–99.
Wong S, Zhao L, Zhang X, Nakatsu G, Han J, Xu W, et al. Gavage of fecal samples from patients with Colorectal Cancer promotes intestinal carcinogenesis in germ-free and conventional mice. Gastroenterology. 2017;153(6):1621–33e6.
Iino C, Shimoyama T, Chinda D, Arai T, Chiba D, Nakaji S, et al. Helicobacter pyloriInfection of and Atrophic Gastritis Influence in Gut Microbiota in a japanese Population. Front Immunol. 2018;9:712.
Vacante M, Ciuni R, Basile F, Biondi A. Gut Microbiota and Colorectal Cancer Development: A Closer Look to the Adenoma-Carcinoma Sequence. Biomedicines. 2020;8(11).
Lee J, Park H, Choi J, Lee J, Koo J, Chung E, et al. Helicobacter pylori infection with atrophic gastritis is an independent risk factor for Advanced Colonic Neoplasm. Gut Liver. 2016;10(6):902–9.
Sonnenberg A, Turner K, Genta R. Associations between gastric histopathology and the occurrence of colonic polyps. Colorectal disease: the official journal of the Association of Coloproctology of Great Britain and Ireland. 2020;22(7):814–7.
Kanno T, Matsuki T, Oka M, Utsunomiya H, Inada K, Magari H, et al. Gastric acid reduction leads to an alteration in lower intestinal microflora. Biochem Biophys Res Commun. 2009;381(4):666–70.
Inoue I, Kato J, Tamai H, Iguchi M, Maekita T, Yoshimura N, et al. Helicobacter pylori-related chronic gastritis as a risk factor for colonic neoplasms. World J Gastroenterol. 2014;20(6):1485–92.
Acknowledgements
This work was supported by the National natural science foundation of China under Grant 81602056.
Funding
This work was supported by the National natural science foundation of China under Grant 81602056.
Author information
Authors and Affiliations
Contributions
Zhang Shaohua wrote the main manuscript text. Tang Yun-he and Su Shen prepared Tables 1, 2, 3 and 4. Tian Zi-bin and Mao Tao helped perform the analysis with constructive discussions. Ren Linlin and Liu Yi contributed significantly to the translation. All authors reviewed the manuscript. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Ethical approval
This research study was conducted retrospectively from data obtained for clinical purposes and was reviewed and approved by the Ethics Committee of the Affiliate Hospital of Qingdao University (Reference Number QYFYWZLL27003). The procedures used in this study adhere to the tenets of the Declaration of Helsinki. The need for written informed consent was waived by the Ethics Committee of the Affiliate Hospital of Qingdao University due to retrospective nature of the study.
Consent for publication
Not applicable.
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Shao-hua, Z., Lin-lin, R., Shen, S. et al. Atrophic gastritis rather than Helicobacter pylori infection can be an independent risk factor of colorectal polyps: a retrospective study in China. BMC Gastroenterol 23, 213 (2023). https://doi.org/10.1186/s12876-023-02764-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12876-023-02764-w