Abstract
Background
Colonic diverticulitis is a leading cause of abdominal pain. The monocyte distribution width (MDW) is a novel inflammatory biomarker with prognostic significance for coronavirus disease and pancreatitis; however, no study has assessed its correlation with the severity of colonic diverticulitis.
Methods
This single-center retrospective cohort study included patients older than 18 years who presented to the emergency department between November 1, 2020, and May 31, 2021, and received a diagnosis of acute colonic diverticulitis after abdominal computed tomography. The characteristics and laboratory parameters of patients with simple versus complicated diverticulitis were compared. The significance of categorical data was assessed using the chi-square or Fisher’s exact test. The Mann–Whitney U test was used for continuous variables. Multivariable regression analysis was performed to identify predictors of complicated colonic diverticulitis. Receiver operator characteristic (ROC) curves were used to test the efficacy of inflammatory biomarkers in distinguishing simple from complicated cases.
Results
Of the 160 patients enrolled, 21 (13.125%) had complicated diverticulitis. Although right-sided was more prevalent than left-sided colonic diverticulitis (70% versus 30%), complicated diverticulitis was more common in those with left-sided colonic diverticulitis (61.905%, p = 0.001). Age, white blood cell (WBC) count, neutrophil count, C-reactive protein (CRP) level, neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), and MDW were significantly higher in the complicated diverticulitis group (p < 0.05). Logistic regression analysis indicated that the left-sided location and the MDW were significant and independent predictors of complicated diverticulitis. The area under the ROC curve (AUC) was as follows: MDW, 0.870 (95% confidence interval [CI], 0.784–0.956); CRP, 0.800 (95% CI, 0.707–0.892); NLR, 0.724 (95% CI, 0.616–0.832); PLR, 0.662 (95% CI, 0.525–0.798); and WBC, 0.679 (95% CI, 0.563–0.795). When the MDW cutoff was 20.38, the sensitivity and specificity were maximized to 90.5% and 80.6%, respectively.
Conclusions
A large MDW was a significant and independent predictor of complicated diverticulitis. The optimal cutoff value for MDW is 20.38 as it exhibits maximum sensitivity and specificity for distinguishing between simple and complicated diverticulitis The MDW may aid in planning antibiotic therapy for patients with colonic diverticulitis in the emergency department.
Similar content being viewed by others
Background
A diverticulum is a herniation through a weak site of the bowel wall that produces a small outpouching [1]. When the diverticular wall is eroded by increased intraluminal pressure or inspissated food particles, diverticulitis may occur [2]. Colonic diverticulitis is one of the most common causes of abdominal pain and lower gastrointestinal bleeding in the emergency department (ED). The prevalence of colonic diverticulitis is increasing not only in Western countries but also in Asian countries [3]. It is predicted that approximately 50% of individuals aged 60 years or older have diverticulosis, whereas by the age of 80 years, this percentage is predicted to be approximately 70% [4]. Of those who developed diverticulosis, 10%–25% experienced an acute episode of diverticulitis [5]. Colonic diverticulitis is usually diagnosed in the ED through intravenous (IV) contrast computed tomography, which is the modality of choice for the diagnosis and staging of colonic diverticulitis with a sensitivity of 94% and a specificity of 99% [6]. Simple acute diverticulitis is a self-limiting and mild disease. It is defined as localized inflammation without any abscess or perforation [7]. The clinical symptoms include lower abdominal pain, fever, constipation, and diarrhea. Outpatient treatment is required for patients who have simple non-septic diverticulitis, are immunocompetent, and can tolerate oral intake. However, approximately 15% of diverticulitis cases have been reported to be complicated forms and were manifested with abscess, stricture, obstruction, fistulae to adjacent organs, or perforation [8,9,10]. As a consequence of bacterial translocation, fecal contamination, or phlegmon development, complicated diverticulitis may present with severe abdominal pain, bloating, dehydration, and signs of sepsis [11]. On physical examination, patients may exhibit peritonitis with rebound tenderness and guarding. Patients with complicated diverticulitis must receive treatment specific to their complications. The current therapeutic options for diverticulitis vary with disease severity, which can be determined based on clinical, radiological, and laboratory findings. When diffuse peritonitis is suspected given the findings of a physical examination, emergency surgery may be required even if imaging shows that the abscess is localized [12]. Therefore, early assessment of the severity of complicated diverticulitis and adequate resuscitation are important.
The monocyte distribution width (MDW) is a novel hematological parameter assessed as part of the complete blood count (CBC) with the differential count. It helps in determining the size distribution of circulating monocytes, which are the first immune cells to respond to pathogenic organisms [13]. In a multicenter international European study, the MDW in combination with the white blood cell (WBC) count was suggested to be a novel screening test for the early detection of sepsis in the ED [14]. Comparison of diagnostic performance according to the Sepsis-3 criteria revealed that the MDW was not inferior to the C-reactive protein (CRP) or procalcitonin level in terms of area under the receiver operator characteristic (ROC) curve (AUC) values [15]. Few studies have focused on the efficacy of the MDW in diagnosing diseases other than sepsis. To our knowledge, the MDW has been used for the detection of the novel coronavirus disease (COVID-19) [16,17,18] and pancreatitis [19]. However, there is a lack of evidence on the efficacy of the MDW in early prediction of the severity of other diseases.
In this retrospective cohort study, we aimed to investigate whether the MDW data preceding CT assessment is helpful in differentiating simple from complicated colonic diverticulitis in an ED.
Methods
Study design and setting
This retrospective cohort study was conducted at a university-affiliated medical center receiving approximately 150,000 ED visits annually. The study was approved by the Hospital Ethics Committee on Human Research. The study protocol was reviewed and qualified as exempt from the requirement to acquire informed consent.
Patient selection
Patients older than 18 years who presented to our ED between November 1, 2020, and May 31, 2021, and who received a diagnosis of acute colonic diverticulitis after abdominal CT was performed were included in this study. All the enrolled patients received the indicated blood examinations. Patients who had any other concomitant active inflammation or infection, were receiving any antibiotic course, had a final pathological diagnosis other than colonic diverticulitis, or had incomplete medical records or laboratory data were excluded from the study. Data were retrieved from the institutional electronic medical chart of the ED.
Methods and measurements
The collected variables included patient demographics and laboratory data. Blood tests were obtained at the same time inserting the IV line within an hour after the treating physicians’ examination of patients at the ED. Blood samples were acquired before antibiotic treatment and IV contrast CT scan. The patients’ age; sex; and comorbidities—such as diabetes mellitus, hypertension, ischemic heart disease, heart failure, liver cirrhosis, cholelithiasis, rheumatological disease, asthma, chronic obstructive pulmonary disease, chronic renal insufficiency, urolithiasis, cerebrovascular disease, and existing cancer—were recorded. Moreover, the body temperature upon triage and laboratory findings (including WBC, neutrophil, lymphocyte, and platelet counts; MDW; and hemoglobin, CRP, creatinine, alanine aminotransferase, blood sugar, sodium, and potassium levels) were recorded.
All the patients’ admission diagnoses coded as diverticulitis were reviewed, then IV contrast CT images were confirmed by two ED physicians and revalidated with the radiologists’ formal final reports whether there was a perforation, abscess, or fistula formation. Based on the IV contrast CT findings, the patients were divided into simple colonic diverticulitis and complicated colonic diverticulitis groups. The radiographic features of simple diverticulitis on CT are enhancement of the colonic wall with segmental thickening and pericolic fat stranding, often disproportionately prominent compared to the amount of bowel wall thickening. As for the complicated types, accumulation with fluid or/with gas suggested abscess formation. And extravasation of gas and fluid into the pelvis and peritoneal cavity are the characteristics of diverticular perforation [20, 21].
The MDW was measured by the UniCel DxH 900 analyzer (Beckman Coulter, Brea, CA, USA) from K3EDTA vacutainer tubes within 2 h of collection, as recommended by the manufacturer. The analyzer uses volume, conductivity, and scatter properties of leukocytes technology to characterize and separate WBCs into 5 different groups (neutrophils, eosinophils, monocytes, lymphocytes, and basophils). The system furthermore calculates the means and standard deviations of these groups’ cell morphometric parameters [22]. The manufacturer of the hematology analyzer did not offer the unit for MDW, as previously reported [23, 24].
Statistical analysis
Descriptive statistics were used to compare variables—baseline demographics, laboratory test results, and inflammatory biomarker measurements—between the two groups. Categorical variables are expressed as proportions, and continuous variables are expressed as medians with interquartile ranges (IQRs, quartile 1 through quartile 3). Univariate analysis was performed using the chi-square or Fisher’s exact test for categorical variables and the Mann–Whitney U test for continuous variables in order to identify predictors of complicated colonic diverticulitis. Variables with p-values < 0.10 in the univariate analysis were then subjected to backward stepwise logistic regression analysis. ROC curves were also used to assess the performance of inflammatory biomarkers— including the WBC count, neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), MDW, and CRP level—in order to distinguish simple from complicated colonic diverticulitis. Youden’s indices were calculated on ROC curves to find the best discriminatory cut-off values [25]. Test characteristics of MDW in terms of sensitivity, specificity, positive predictive value, and negative predictive value along with their 95% CIs for the optimal cut-off value were also computed. A p-value ≤ 0.05 was considered statistically significant. All data were analyzed using SAS (version 9.1; SAS Institute, Cary, NC, USA). Post-hoc power of the study was estimated using G*Power software (version 3.1.9.7; Heinrich Heine University Düsseldorf, Germany) with the α error probability set at 0.05.
This study was approved by the Institutional Review Board of the Ethics Committee of China Medical University and Hospital (CMUH110-REC3-106). The requirement of informed consent from the patients was waived by the Ethics Committee of China Medical University and Hospital.
Results
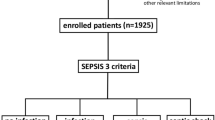
From November 1, 2020, to May 31, 2021, 84,173 ED visits were recorded. A total of 176 visits were retrieved from the institutional electronic medical chart during the study period. Of the 16 patients who were excluded from the study, 2 had other concomitant infections, 1 was a repeat patient who had revisited the ED and was taking oral antibiotics for colonic diverticulitis, 6 had an initial ambiguous diagnosis of colonic diverticulitis on CT scans but a final pathological diagnosis of colon cancer and not diverticulitis, 4 had no CRP data, and 3 had no MDW data because their monocyte count was less than 100/μL. Thus, a total of 160 patients were enrolled in the study: 139 in the simple colonic diverticulitis group and 21 in the complicated colonic diverticulitis group (Fig. 1).
Overall, the number of male patients was higher than that of female patients (n = 90/160, 56.25%). Most patients had right-sided colonic diverticulitis (n = 112, 70%): 14 in the cecum (8.75%), 91 in the ascending colon (56.875%), and 7 in the transverse colon (4.375%). Seventeen patients (10.63%) had other diverticula in different segments of the colon. Recurrent colonic diverticulitis was noted in 27 patients (16.88%). Hypertension was the most prevalent comorbidity (n = 30, 18.75%). Comparison of the variables for the two groups revealed that the patients with complicated colonic diverticulitis were older, with a median age of 58 years (IQR: 41–72 years). Conversely, the patients with simple colonic diverticulitis had a median age of 44 years (IQR: 30–59 years; p = 0.008). Complicated colonic diverticulitis was more frequently detected in the left colon than in the right colon (61.905% versus 38.095%). By contrast, simple colonic diverticulitis was more prevalent in the right colon than in the left colon (74.180% versus 25.180%). The location of colonic diverticulitis significantly differed between the two groups (p = 0.001). However, no significant difference was noted in comorbidities between the two groups. Laboratory values—WBC (p = 0.008) and neutrophil (p = 0.003) counts, MDW (p < 0.001), and CRP level (p < 0.001)—were significantly higher in the complicated colonic diverticulitis group. By contrast, lymphocyte count (p = 0.001) and sodium level (p < 0.001) were significantly higher in the simple colonic diverticulitis group (Table 1).
Univariate and multivariable binary logistic regression analyses (Table 2) revealed that left-sided location and the MDW were the only two variables that were significant predictors of complicated colonic diverticulitis after adjusting for the other variables. The adjusted odds ratio (OR) of complicated colonic diverticulitis was 5.197 (95% confidence interval [CI], 1.651–16.359; p = 0.005) for left-sided location and 1.552 (95% CI, 1.290–1.867; p < 0.001) for the MDW.
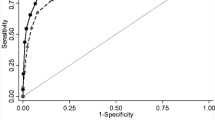
Further evaluation through ROC analysis was performed to determine the diagnostic value of the MDW for complicated colonic diverticulitis. The AUC values were as follows: MDW, 0.870 (95% CI, 0.784–0.956); WBC + MDW, 0.827 (95% CI, 0.725–0.928); CRP, 0.800 (95% CI, 0.707–0.892); NLR, 0.724 (95% CI, 0.616–0.832); WBC, 0.679 (95% CI, 0.563–0.795); and PLR, 0.662 (95% CI, 0.525–0.798) (Fig. 2 and Table 3). The largest AUC value was that for the MDW among all the inflammatory biomarkers for diagnosing patients with complicated colonic diverticulitis. When the MDW cutoff was 20.38, the sensitivity and specificity were maximized to 90.5% and 80.6%, respectively, with a low positive predictive value of 41.3% but a high negative predictive value of 98.3% (Table 4). A post-hoc power analysis was performed through G*power, which revealed that the sample size was adequate to achieve 100% power (1–β) when the MDW cut-off value was 20.38. The power exceeded 80% when the total sample size was greater than 20 (Fig. 3).
Discussion
From the results of the study, it was concluded that the increase in age, WBC count, neutrophil count, CRP level, NLR, PLR, and MDW were related to the severity of colonic diverticulitis.
The gold standard diagnostic tool for acute diverticulitis is CT, in which complications can also be visualized. However, the schedule of CT at an ED may be delayed because of the high number of patients. The optimal use of CT for patients in whom complicated diverticulitis is suspected should be based on clinical and laboratory findings to minimize treatment costs and radiation hazards [26]. Therefore, recognizing the risk factors of complicated diverticulitis and providing the right treatment before CT imaging are crucial.
Some findings of the present study are in accordance with previous findings. In particular, right-sided diverticulitis was more prevalent than left-sided diverticulitis (70% versus 30%); this finding is compatible with reports from other Asian countries [27,28,29]. However, a higher number of patients with complicated diverticulitis had left-sided diverticulitis than right-sided diverticulitis (61.905% versus 38.095%); this finding is similar to the findings of previous Japanese and Korean studies [3, 27]. We also found that patients with complicated diverticulitis were older than those with simple diverticulitis (median age, 58 versus 44 years); this finding is also compatible with previous findings. For instance, in a Japanese retrospective multicenter study involving 1,112 patients, although right-sided colonic diverticulitis was more prevalent among the study population (70.1%), left-sided colonic diverticulitis was significantly more common among elderly patients (61.0%) [30]. Right-sided diverticulitis differs from left-sided diverticulitis in many respects. While right-sided diverticulitis is usually congenital and solitary [31, 32], left-sided diverticulitis is usually associated with secondary causes, including dietary factors, constipation, increased colonic pressure, defecation habits, and an irritable bowel. Consequently, left-sided diverticulitis more commonly occurs in older patients [33].
In our study, the WBC and neutrophil count, MDW, and CRP levels were higher in the complicated colonic diverticulitis group (p < 0.05, Table 1); however, only the MDW was found to have a statistically significant association with complicated diverticulitis in the multivariable binary logistic regression analysis (p < 0.001, Table 2). The WBC count and CRP level are the most common indicators of the severity of intra-abdominal inflammation in the ED. A higher WBC count or CRP level usually indicates a higher level of inflammation. Several studies [26, 34,35,36] have attempted to calculate the optimal threshold for the WBC count and CRP level in distinguishing complicated diverticulitis from simple diverticulitis; however, so far, no consensus has been reached.
In addition to the WBC count and CRP level, two easily accessible hemogram-derived parameters, namely the NLR and PLR, have been used to predict complicated diverticulitis [37,38,39]. One study reported that the NLR could predict the need for surgical intervention more accurately than the CRP level and WBC count [37]. Palacios Huatuco et al. recently found the NLR cutoff of 4.2 to be the best diagnostic approach, with a sensitivity of 80% and specificity of 64%, for detecting complicated diverticulitis [38]. Mari et al. found that the PLR had lower diagnostic accuracy than the NLR (AUC values, 0.67%, and 0.75%, respectively) [39].
Circulating neutrophils and monocytes are the first response to pathogenic organisms. The MDW is a parameter that describes the size distribution of circulating monocytes. Several studies have reported that the MDW can be used for the early diagnosis of sepsis in the ED [13, 14, 40, 41]. Similarly, Şenlikci et al. found that the MDW can be used to differentiate mild pancreatitis from nonmild pancreatitis [19]. However, little known is about the efficacy of the MDW in detecting acute complicated diverticulitis. In our cohort, the MDW cutoff of 20.38 had a sensitivity of 90.5% and a specificity of up to 80.6%. Moreover, it had the largest AUC value (0.870) for the diagnosis of acute complicated diverticulitis. The AUC value of the MDW for complicated diverticulitis was higher than those of other inflammatory biomarkers—CRP (0.800), NLR (0.724), WBC (0.679), and PLR (0.662; Table 3 and Fig. 2).
The diagnostic accuracy of the MDW for complicated diverticulitis noted in our study was comparable with that of procalcitonin. In a previous study, the AUC of procalcitonin for complicated diverticulitis was 0.867, with a sensitivity of 81% and specificity of 91% [42]. However, procalcitonin is not routinely used as a biomarker in EDs. In Taiwan, the national health insurance reimbursement price for procalcitonin tests is 1,000 New Taiwan dollars (NT$) [43], which is approximately four times the price for CBC determination (NT$270, including differential WBC count and MDW) [44, 45]. Therefore, procalcitonin testing is preserved as an auxiliary test for patients with ambiguous diagnoses of sepsis or bacterial infection, which cannot be verified on the basis of the WBC count, NLR, or CRP level.
In our study, the MDW was the only inflammatory biomarker that was found to be a significant predictor of complicated colonic diverticulitis after adjusting for other covariables in multivariable binary logistic regression analysis (p < 0.001, Table 2). In previous studies, the MDW was found to have some advantages over other biomarkers. In particular, the MDW can be easily measured from the CBC through a blood test in the ED. In addition, the results are obtained faster than those of a biochemistry panel. Use of the MDW has been reported to improve both the clinical and economic outcomes of patients with sepsis in the ED, with the estimated time to antibiotic administration being reduced from 3.98 h to 2.07 h and US$3,460 being saved per hospitalization (US$23,466 versus US$26,926) [46]. By using a combination of the MDW and advanced imaging (CT), ED physicians will be able to diagnose complicated diverticulitis more accurately and in a timely manner, to initiate antibiotic therapy, and to convince surgeons regarding early intervention. Recent guidelines have recommended avoiding the use of antibiotics for otherwise healthy patients with simple diverticulitis [47]. The high negative predictive value (98.3%) of MDW could enhance physicians’ diagnosis and decision-making. Patients with colonic diverticulitis and normal MDW values are unlikely to be complicated. Hence, an early and accurate diagnosis of simple diverticulitis by using the MDW will help reduce the use of antibiotics.
Limitations
To our knowledge, this is the first study to evaluate the utility of the MDW for diagnosing colonic diverticulitis in the ED. However, our study has some limitations. First, the MDW cannot be measured when the peripheral blood sample for a patient has a monocyte count < 100/μL. In our study, three patients' MDW data were unavailable; these patients had simple diverticulitis. Second, because this was a retrospective study, medical records were not designed for research purposes and did not contain all parameters of interest to the investigators. For instance, the procalcitonin level was not measured for comparison with the MDW. Third, our classification of colonic diverticulitis was based on CT findings. CT has an accuracy of 98% in diagnosing acute diverticulitis; thus, misdiagnosis may occur in 2% of cases [48]. Nevertheless, abdominal CT imaging is still considered the gold standard for diagnosing acute diverticulitis and its complications [49]. Finally, this was a single-center study conducted in only one ED in East Asia; therefore, our finding that diverticulitis was more prevalent in the right colon may not be generalizable to all EDs and other populations. Further prospective studies with larger numbers of patients from multiple centers are needed to more accurately assess the role of the MDW in differentiating simple from complicated colonic diverticulitis.
Conclusions
Our study revealed that acute colonic diverticulitis was more prevalent in the right colon than in the left colon in Taiwanese patients. Patients with complicated diverticulitis were significantly older and predominantly had left-sided diverticulitis. In addition, a large MDW was found to be a significant and independent predictor of complicated diverticulitis preceding CT assessment in the ED. The optimal cutoff value for MDW is 20.38 as it exhibits maximum sensitivity and specificity for distinguishing between simple and complicated diverticulitis. The MDW may aid in initiating early antibiotic therapy for patients with complicated diverticulitis and in decreasing antibiotic use in patients with simple diverticulitis.
Availability of data and materials
The datasets generated and analyzed during the current study are not publicly available due to the non-disclosure agreement in IRB restrictions. However, they are available on reasonable request. Please contact Dr. Tai-Yi Hsu for details.
Abbreviations
- AUC:
-
Area under the ROC curve
- CBC:
-
Complete blood count
- CI:
-
Confidence interval
- CT:
-
Computed tomography
- ED:
-
Emergency department
- IV:
-
Intravenous
- IQR:
-
Interquartile range
- MDW:
-
Monocyte distribution width
- NLR:
-
Neutrophil-to-lymphocyte ratio
- OR:
-
Odds ratio
- PLR:
-
Platelet-to-lymphocyte ratio
- ROC:
-
Receiver operator characteristic
- WBC:
-
White blood cell
References
Simpson J, Scholefield JH, Spiller RC. Pathogenesis of colonic diverticula. Br J Surg. 2002;89(5):546–54.
Rege RV, Nahrwold DL. Diverticular disease. Curr Probl Surg. 1989;26(3):133–89.
Imaeda H, Hibi T. The Burden of diverticular disease and its complications: West versus East. Inflamm Intest Dis. 2018;3(2):61–8.
Feuerstein JD, Falchuk KR. Diverticulosis and Diverticulitis. Mayo Clin Proc. 2016;91(8):1094–104.
Klarenbeek BR, de Korte N, van der Peet DL, Cuesta MA. Review of current classifications for diverticular disease and a translation into clinical practice. Int J Colorectal Dis. 2012;27(2):207–14.
You H, Sweeny A, Cooper ML, Von Papen M, Innes J. The management of diverticulitis: a review of the guidelines. Med J Aust. 2019;211(9):421–7.
Sartelli M, Chichom-Mefire A, Labricciosa FM, et al. The management of intra-abdominal infections from a global perspective: 2017 WSES guidelines for management of intra-abdominal infections. World J Emerg Surg. 2017;12:29.
Bharucha AE, Parthasarathy G, Ditah I, et al. Temporal trends in the incidence and natural history of diverticulitis: A population-based study. Am J Gastroenterol. 2015;110(11):1589–96.
Stollman N, Smalley W, Hirano I. AGA institute clinical guidelines committee. american gastroenterological association institute guideline on the management of acute diverticulitis. Gastroenterology. 2015;149(7):1944–9.
Sugi MD, Sun DC, Menias CO, Prabhu V, Choi HH. Acute diverticulitis: Key features for guiding clinical management. Eur J Radiol. 2020;128:109026.
Morris AM, Regenbogen SE, Hardiman KM, Hendren S. Sigmoid diverticulitis: a systematic review. JAMA. 2014;311(3):287–97.
Tochigi T, Kosugi C, Shuto K, Mori M, Hirano A, Koda K. Management of complicated diverticulitis of the colon. Ann Gastroenterol Surg. 2017;2(1):22–7.
Crouser ED, Parrillo JE, Seymour C, et al. Improved Early Detection of Sepsis in the ED With a Novel Monocyte Distribution Width Biomarker. Chest. 2017;152(3):518–26.
Hausfater P, Robert Boter N, Morales Indiano C, et al. Monocyte distribution width (MDW) performance as an early sepsis indicator in the emergency department: comparison with CRP and procalcitonin in a multicenter international European prospective study. Crit Care. 2021;25(1):227.
la Woo A, Oh DK, Park CJ, Hong SB. Monocyte distribution width compared with C-reactive protein and procalcitonin for early sepsis detection in the emergency department. PLoS ONE. 2021;16(4):e0250101.
Riva G, Castellano S, Nasillo V, et al. Monocyte Distribution Width (MDW) as novel inflammatory marker with prognostic significance in COVID-19 patients. Sci Rep. 2021;11(1):12716.
Lorubbio M, Tacconi D, Iannelli G, et al. The role of Monocyte Distribution Width (MDW) in the prognosis and monitoring of COVID-19 patients. Clin Biochem. 2022;103:29–31.
Lin HA, Lin SF, Chang HW, Lee YJ, Chen RJ, Hou SK. Clinical impact of monocyte distribution width and neutrophil-to-lymphocyte ratio for distinguishing COVID-19 and influenza from other upper respiratory tract infections: A pilot study. PLoS ONE. 2020;15(11):e0241262.
Şenli̇Kci̇ A, Ergüder E, Süleyman M, et al. Does Monocyte Distribution Width (MDW) have prognostic value in acute pancreatitis? J Contemporary Med. 2021;11(3):335–9.
Horton KM, Corl FM, Fishman EK. CT evaluation of the colon: inflammatory disease. Radiographics. 2000;20(2):399–418.
Pereira JM, Sirlin CB, Pinto PS, Jeffrey RB, Stella DL, Casola G. Disproportionate fat stranding: A helpful CT sign in patients with acute abdominal pain. Radiographics. 2004;24(3):703–15.
Ahmed Bentahar. What is Monocyte Distribution Width (MDW) and What Role Does It Play in the Early Detection of Sepsis? Published September 1, 2022. https://www.beckmancoulter.com/blog/diagnostics/monocyte-distribution-width. Accessed 27 Jan 2023.
Lee AJ, Kim SG. Mean cell volumes of neutrophils and monocytes are promising markers of sepsis in elderly patients. Blood Res. 2013;48(3):193–7.
Polilli E, Frattari A, Esposito JE, et al. Monocyte distribution width (MDW) as a new tool for the prediction of sepsis in critically ill patients: a preliminary investigation in an intensive care unit. BMC Emerg Med. 2021;21(1):147.
Fluss R, Faraggi D, Reiser B. Estimation of the Youden Index and its associated cutoff point. Biom J. 2005;47(4):458–72.
Mäkelä JT, Klintrup K, Takala H, Rautio T. The role of C-reactive protein in prediction of the severity of acute diverticulitis in an emergency unit. Scand J Gastroenterol. 2015;50(5):536–41.
Kim JH, Cheon JH, Park S, et al. Relationship between disease location and age, obesity, and complications in Korean patients with acute diverticulitis: a comparison of clinical patterns with those of Western populations. Hepatogastroenterology. 2008;55(84):983–6.
Wong SK, Ho YH, Leong AP, Seow-Choen F. Clinical behavior of complicated right-sided and left-sided diverticulosis. Dis Colon Rectum. 1997;40(3):344–8.
Yamamichi N, Shimamoto T, Takahashi Y, et al. Trend and risk factors of diverticulosis in japan: age, gender, and lifestyle/metabolic-related factors may cooperatively affect on the colorectal diverticula formation. PLoS ONE. 2015;10(4):e0123688.
Manabe N, Haruma K, Nakajima A, et al. Characteristics of colonic diverticulitis and factors associated with complications: A Japanese multicenter, retrospective. Cross-Sectional Study Dis Colon Rectum. 2015;58(12):1174–81.
Puylaert JBCM. Ultrasound of colon diverticulitis. Dig Dis. 2012;30(1):56–9.
Radhi JM, Ramsay JA, Boutross-Tadross O. Diverticular disease of the right colon. BMC Res Notes. 2011;4(1):383.
Oh HK, Han EC, Ha HK, et al. Surgical management of colonic diverticular disease: Discrepancy between right- and left-sided diseases. World J Gastroenterol. 2014;20(29):10115–20.
Nizri E, Spring S, Ben-Yehuda A, Khatib M, Klausner J, Greenberg R. C-reactive protein as a marker of complicated diverticulitis in patients on anti-inflammatory medications. Tech Coloproctol. 2014;18(2):145–9.
Tursi A, Brandimarte G, Giorgetti G, Elisei W, Maiorano M, Aiello F. The clinical picture of uncomplicated versus complicated diverticulitis of the colon. Dig Dis Sci. 2008;53(9):2474–9.
van de Wall BJM, Draaisma WA, van der Kaaij RT, Consten ECJ, Wiezer MJ, Broeders IAMJ. The value of inflammation markers and body temperature in acute diverticulitis. Colorectal Dis. 2013;15(5):621–6.
Reynolds IS, Heaney RM, Khan W, Khan IZ, Waldron R, Barry K. The utility of neutrophil to lymphocyte ratio as a predictor of intervention in acute diverticulitis. Dig Surg. 2017;34(3):227–32.
Palacios Huatuco RM, Pantoja Pachajoa DA, Bruera N, et al. Neutrophil-to-lymphocyte ratio as a predictor of complicated acute diverticulitis: A retrospective cohort study. Ann Med Surg (Lond). 2021;63:102128.
Mari A, Khoury T, Lubany A, et al. Neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios are correlated with complicated diverticulitis and hinchey classification: a simple tool to assess disease severity in the emergency department. Emerg Med Int. 2019;2019:6321060.
Li CH, Seak CJ, Chaou CH, et al. Comparison of the diagnostic accuracy of monocyte distribution width and procalcitonin in sepsis cases in the emergency department: a prospective cohort study. BMC Infect Dis. 2022;22(1):26.
Agnello L, Bivona G, Vidali M, et al. Monocyte distribution width (MDW) as a screening tool for sepsis in the emergency department. Clin Chem Lab Med. 2020;58(11):1951–7.
Jeger V, Pop R, Forudastan F, Barras JP, Zuber M, Piso RJ. Is there a role for procalcitonin in differentiating uncomplicated and complicated diverticulitis in order to reduce antibiotic therapy? A prospective diagnostic cohort study. Swiss Med Wkly. 2017;147:w14555.
Medical service payment items and payment standard inquiry: Procalcitonin. https://www.nhi.gov.tw/query/Query2_Detail.aspx?Ser_id=3685. Accessed 27 Jan 2023.
Medical service payment items and payment standard inquiry: CBC. https://www.nhi.gov.tw/query/Query2_Detail.aspx?Ser_id=2570. Accessed 27 Jan 2023.
Medical service payment items and payment standard inquiry: WBC differential count. https://www.nhi.gov.tw/query/Query2_Detail.aspx?Ser_id=2573. Accessed 27 Jan 2023.
Paoli CJ, Reynolds MA, Coles C, Gitlin M, Crouser E. Predicted economic benefits of a novel biomarker for earlier sepsis identification and treatment: A counterfactual analysis. Crit Care Explor. 2019;1(8):e0029.
Schultz JK, Azhar N, Binda GA, et al. European Society of Coloproctology: guidelines for the management of diverticular disease of the colon. Colorectal Dis. 2020;22(Suppl 2):5–28.
Kandagatla PG, Stefanou AJ. Current status of the radiologic assessment of diverticular disease. Clin Colon Rectal Surg. 2018;31(4):217–20.
Tursi A. A critical appraisal of advances in the diagnosis of diverticular disease. Expert Rev Gastroenterol Hepatol. 2018;12(8):791–6.
Acknowledgements
This manuscript was edited by Wallace Academic Editing.
Funding
This work was supported by China Medical University Hospital, Taiwan (grant number DMR-109–226).
Author information
Authors and Affiliations
Contributions
CYC and TYH contributed equally to the work. CYC: data curation, writing the original draft, review, and editing. TYH: conceptualization, investigation, data curation, formal analysis, funding acquisition, and editing. GYH: writing, review, and editing. HMS: project administration, review, and editing. SHW: review and editing. FWH: methodology, formal analysis, and project administration. PCC and WCT: review and supervision. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All methods were carried out in accordance with the relevant guidelines and regulations in accordance with the Declaration of Helsinki. The need to obtain informed consent from participants was waived because this is a purely retrospective study that does not affect patient care. This waiver was approved by the China Medical University Institutional Review Board in conjunction with the China Medical University Ethics Committee. This study was approved by the research ethics committee of China Medical University and Hospital (Institutional Review Board No. CMUH110-REC3-106).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Chang, CY., Hsu, TY., He, GY. et al. Utility of monocyte distribution width in the differential diagnosis between simple and complicated diverticulitis: a retrospective cohort study. BMC Gastroenterol 23, 96 (2023). https://doi.org/10.1186/s12876-023-02736-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12876-023-02736-0