Abstract
Objective
We aim to evaluate the relationship between the use of metformin and the risk of pancreatic cancer in type 2 diabetes patients.
Method
We systematically searched the observational studies on PubMed, Embase, Web of Science, Cochrane Library, clinicalrials.gov, and CNKI databases, extracted relevant data, combined the OR value and 95% CI using the random effect model, and conducted a sensitivity analysis, subgroup analysis, and meta-regression to evaluate the size and stability of this relationship.
Result
Twenty-nine studies from twenty-four articles met our inclusion criteria, including more than 2 million subjects. Overall analysis showed that compared with no use of metformin, the use of metformin could reduce the risk of pancreatic cancer in patients with type 2 diabetes (OR = 0.82, 95% CI (0.69, 0.98)). Subgroup analysis showed that compared with the use of hypoglycemic drugs, the use of metformin could reduce the risk of pancreatic cancer in patients with type 2 diabetes (OR = 0.79, 95% CI (0.66, 0.94)). However, compared with no drugs or only diet therapy, metformin users might increase the risk of pancreatic cancer (OR = 2.19, 95% CI (1.08, 4.44)). Sensitivity analysis confirmed the stability of the study, and there was no significant publication bias.
Conclusion
Compared with the no-use of metformin, metformin users with diabetes can reduce the risk of pancreatic cancer. More research is needed to prove it works.
Similar content being viewed by others
Background
According to GLOBOCAN 2020 statistics, pancreatic cancer ranks 14th in the global cancer incidence rate and 7th in the global cancer mortality [1]. Approximately 495,733 new cases of pancreatic cancer are diagnosed each year worldwide and 466,003 deaths [1]. The incidence rate is almost the same as the death rate, which profoundly reflects the malignancy of pancreatic cancer. With the development of medical technology, there are many treatments for pancreatic cancer (PC), such as surgery, chemotherapy, immunotherapy, targeted therapy, radio frequency, HAIFU, and microbial therapy. However, the overall survival rate is only 9% [2]. Surgical treatment is considered to be the only way to cure PC., but the 5-year survival rate of patients receiving surgical treatment is only 15–25% [3]. Early identification of pancreatic cancer risk factors for intervention has become an essential means to reduce the incidence rate of pancreatic cancer. Current research shows that smoking, drinking, obesity, diabetes, pancreatitis, and pancreatic cancer family history are high-risk factors for pancreatic cancer [4].
The relationship between diabetes and pancreatic cancer is particularly complex. Although there is disagreement on the relationship between the duration of diabetes and the risk of pancreatic cancer, almost all studies show that the risk of pancreatic cancer in diabetes patients is significantly higher [5,6,7]. Clarifying the relationship between antidiabetic drugs and the incidence rate of pancreatic cancer has become a hot spot in clinical practice.
Metformin is the first-line drug of type 2 diabetes mellitus (DM), and its role in reducing the mortality of patients with pancreatic cancer is widely recognized [8, 9]. Specifically, compared with other drugs or no use of metformin, the overall survival period and 5-year survival rate of patients with pancreatic cancer treated with metformin significantly increased [10, 11]. However, its relationship with the incidence rate of pancreatic cancer has not yet been unified. Therefore, we conducted a more detailed and rigorous meta-analysis to clarify the relationship between the use of metformin in diabetes patients and the risk of pancreatic cancer.
Materials and methods
Guidelines
This paper is based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA). The agreement of this overview has been published in PROSPERO (Registration No: CRD42022359987).
Retrieval strategy
From the beginning of the database construction to August 31, 2022, We performed an electronic search on PubMed, Embase, Web of Science, Cochrane Library, clinicalrials.gov, and China National Knowledge Infrastructure (CNKI) databases, using the keywords "metformin" OR "biguanide" OR "dimethyl biguanide" AND "pancreatic cancer" OR "pancreatic tumor" in "Title/Abstract", with no language restriction. All the studies retrieved were independently screened by two authors (Jian Hu and Hong-Dan Fan). We will consult with a third person(Qing-Song Mao) if there are different opinions in the literature screening process. To include sufficiently accurate literature, we also searched and screened the references included in the literature.
Inclusion and exclusion criteria
The inclusive criteria were as follows: (1) case–control or cohort study; (3) reporting or including studies on the association between metformin use and pancreatic cancer risk; (4) reporting the Relative Risk (RR), Hazard Ratio (HR) or Odds Ratio (OR) and 95% confidence interval (CI) of pancreatic cancer, or providing data that we can calculate them.
The exclusion criteria were as follows: (1) cross-sectional studies; (2) duplicated studies; (3) preclinical studies (such as in vivo studies, primary studies, and animal studies); (4) abstracts, case reports, reviews, conferences, letters, and books; (5) only showing the relationship between metformin and pancreatic cancer mortality; (6) no full-text studies; (7) contrast agent containing metformin; (8) lacking necessary data.
Data collection
Two investigators (Jian Hu and Hong-Dan Fan) independently extracted and then checked the extracted data by a third party (Qing-Song Mao). For each study, we recorded the following information: the first author, publication year, publication region/country, study design, basic characteristics (including baseline age, average age, and male proportion), the time of diagnosis of diabetes in the study population, sample size, study period, outcome indicators (including adjusted OR value and 95% CI), adjusted confounding factors and contrast agent. If there is no adjusted OR value and 95% CI, the crude OR value and 95% CI will be extracted. Suppose there are multiple groups (multiple control groups or test groups) in the literature that all meet the inclusion criteria. In that case, we extract or calculate the sample size data of each group and use the method of merging multiple groups of sample size into a new group to calculate the OR value and 95% CI [12]. Since the incidence rate of pancreatic cancer is low (less than 5%), the RR and HR values can be equated with OR values.
Quality evaluation
This analysis uses the Newcastle Ottawa Scale (NOS) [13] to evaluate the method quality of the included studies. The score of NOS ranges from 0 to 9. We define studies with ≥ 7 points as high-quality studies in this analysis.
Statistical methods
STATA MP 17.0 is adopted for all statistical analyses in this paper. The heterogeneity between studies was investigated by the Q test and measured by I2 statistics. If the I2 values exceeded 25%, 50%, and 75% respectively, it represented low, medium, and high heterogeneity [14]. When the I2 value is greater than 50%, the random effect model is used; otherwise, the fixed effect model is used. We conducted sensitivity analysis by excluding each study or some studies that may affect the stability of the study results and conducted subgroup analysis and single factor meta-regression analysis on some characteristics of the included studies. We assessed publication bias by visual funnel plots and the Egger regression asymmetry test. Unless otherwise stated, the statistical significance level was set at P < 0.05 under a double-sided test.
Results
Search process and results
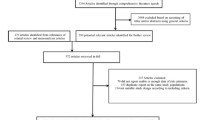
Through the search of the above databases, we have preliminarily obtained 1477 articles that may be relevant. After importing the received articles into Note-Express, we found 199 duplicate articles. After reading the title and abstract, we excluded 1218 articles irrelevant to the study. Then, the remaining 60 articles were reviewed in full text, and 36 studies were excluded again. Among them, 21 studies had no available data, 9 were conferences or abstracts, three were unable to obtain the full text, 2 were meta-analyses or reviews, and one was treated with metformin combined with dipeptidyl peptidase-4 inhibitors (DPP-4i) as the contrast agent. Finally, the remaining 24 studies that met the inclusion criteria were analyzed. The retrieval and filtering process is shown in Fig. 1.
Research characteristics
We included a total of 24 articles [15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38] (29 studies are included because some studies have multiple control groups or test groups), including 18 cohort studies and six case–control studies involving more than 2.28 million people. Their basic characteristics are shown in Table 1. Among the 24 articles, ten were conducted in Asia (seven [19, 20, 24,25,26, 31, 35] in China and three [22, 30, 36] in South Korea), and the remaining 14 were conducted in no-Asia (six [16, 18, 27, 29, 32, 37] in Britain, four [15, 23, 34, 38] in the United States, two [17, 28] in the Netherlands, 1 [33] in Italy and 1 [21] in Europe). Only two studies [24, 26] are of low quality. Four articles [19, 27, 35, 37] reported that many studies met the inclusion criteria, and the above methods were used to merge the study groups. All selected studies reported the results between the use of metformin and the risk of pancreatic cancer, but the reference group drugs they designed were not identical. The results of 13 studies [15, 18, 20,21,22,23,24,25,26, 28, 33,34,35] were not statistically significant. Eight studies [17, 19, 26, 27, 30, 31, 37, 38] reported that metformin significantly reduced the risk of pancreatic cancer, and three studies [29, 32, 36] reported an increase in the risk of pancreatic cancer.
Overall analysis
An overall analysis of 24 articles using the random effect model showed that compared with no use of metformin, the use of metformin could reduce the risk of pancreatic cancer in patients with type 2 diabetes (OR = 0.82, 95% CI (0.69, 0.98)), with significant heterogeneity (Q = 198.67, df = 14, pQ = 0.000; I2 = 88.4%) (Fig. 2).
Sensitivity analysis, subgroup analysis, and meta-regression
To estimate the accuracy and robustness of the combined effect amount, we conducted a sensitivity analysis by excluding each study one by one and excluding some studies that may affect the research results (Table 2). There were four studies whose effect values came from the combination of multiple groups, but after all of them were excluded, the study showed no statistical significance (OR = 0.95, 95% CI (0.80, 1.12)). The sensitivity analysis result shows that the stability of the conclusion is acceptable. To further clarify the source of research heterogeneity, we selected the random effect model to conduct subgroup analysis and single-factor meta-regression analysis on the characteristics that may cause research heterogeneity, such as study area, study type, contrast agent, research quality, and diabetes status of study subjects. When the analysis is limited to a cohort study, high-quality study, no-newly-diagnosed diabetes population, and contrast agent, the research results are statistically significant (Fig. 3). Single factor meta-regression analysis found that the contrast agent may be one of the sources of heterogeneity (Table 3), which can explain 13.01% of the heterogeneity sources (p = 0.047, Adj R-square = 13.01%).
Publication bias
Finally, to evaluate the publication bias of the included studies, we intuitively evaluated the publication bias through the funnel chart (Fig. 4) and quantified it through the Egger regression. No significant publication bias was found (p = 0.445) (Fig. 5).
Discussion
The epidemiology of cancer is constantly changing. As research showed [39], several aspects related to the epidemiology of liver cancer (such as etiology, clinical manifestations, treatment and treatment results) have changed dramatically from the previous ones, and the use of drugs may play an essential role in it. Meta-analysis has shown that statins have a specific chemopreventive effect on hepatocellular carcinoma [40]. A similar relationship may exist between some drugs and pancreatic cancer.
The mechanism and clinical research of diabetes increasing the risk of liver cancer have been studied in detail [41], but its relationship with pancreatic cancer still needs further investigation. Diabetes is a high-risk factor for pancreatic cancer and a possible consequence of pancreatic cancer [42]. To a certain extent, controlling diabetes mellitus can reduce the risk of developing pancreatic cancer. Metformin is one of the most commonly used oral hypoglycemic drugs in clinical practice, and its relationship with cancer has been widely studied. A study [43] investigating the impact of the use of metformin on the incidence rate or survival outcome of cancer showed that the use of metformin is related to reducing the incidence rate of pancreatic cancer and improving the overall survival of colorectal cancer, but there is no obvious evidence to show its correlation in other aspects. Some studies even believe that metformin is the first choice for the treatment of cancer patients with type 2 diabetes, because compared with other hypoglycemic drugs, the use of metformin can reduce the risk of death of cancer patients, especially in patients with pancreatic cancer, colorectal cancer and other cancers (except lung cancer, breast cancer cancer and prostate cancer [44]. Among them, studies on the survival rate or overall survival period of patients with metformin and pancreatic cancer are more frequent. Almost all studies show that patients with pancreatic cancer and diabetes can benefit from metformin [45, 46]. The anticancer effect of metformin is closely related to its powerful hypoglycemic effect. The effect of metformin on lowering blood glucose is carried out through the following ways: ① hepatic effect: improving hepatic insulin resistance, thus reducing hepatic glucose output, mainly reducing gluconeogenesis [47]; ② muscle effect: acting on skeletal muscle to increase insulin-stimulating glucose uptake and increase muscle AMPK activity and phosphorylation [48, 49]; ③ intestinal effects: changing intestinal microbial composition, changing hormone secretion (mainly growth and differentiation factor 15 and glucagon-like peptide-1), changing enterocyte glucose metabolism and delaying gastric emptying [50, 51].
This efficient hypoglycemic effect of metformin may contribute to reducing pancreatic carcinogenesis.
The current preclinical studies also confirmed the potential preventive effect of metformin on pancreatic cancer to some extent, although the evidence remains in animal (mouse) experiments. Metformin added in drinking water can prevent the pancreatic carcinogenesis induced by N-nitrosobis—(2-oxopropyl) amine in hamsters fed a high-fat diet [52]. In obese/pre-diabetes mice induced by diet, metformin reduced pancreatic tumor growth and mammalian target of rapamycin (mTOR) related signal transduction [53] (mTOR is a crucial complex involved in protein translation regulation). Metformin can prevent weight gain, liver steatosis, hyperlipoproteinemia, and hyperinsulinemia in KC (LSL-KrasG12D/ + ;p48-Cre) mice induced by high-fat and high-calorie diet. And it also can effectively prevent the progress of late PanINs and the development of KRAS( Kirsten Rat Sarcoma Viral Oncogene) driven pancreatic ductal adenocarcinoma promoted by diet-induced obesity [54]. Dong TS's [55] study showed that oral metformin could significantly change the regional microbiome of the duodenum and inhibit the development of PanIN lesions in the diet-induced obesity model of pancreatic cancer. Chen K [56] team found that the intake of metformin could delay the occurrence of pancreatic tumors through the study of KC mouse models, which showed that the percentage of early lesions and late mPanIN lesions (mPanIN2 and mPanIN3) decreased. In addition, metformin inhibits the tumorigenesis induced by chronic pancreatitis and may play a relevant role in reducing the pancreatic fibrosis induced by chronic pancreatitis. The combination of metformin and some drugs also reflects its role in cancer prevention to a certain extent. Metformin and rapamycin can inhibit pancreatic tumor growth in obese and pre-diabetes mice through common and different mechanisms [53]. It was proved that the combination of metformin and aspirin significantly inhibited tumor growth and downregulated the protein expression of Mcl-1 and Bcl-2 in tumors in the xenotransplantation mouse model [57], which has preventive significance for the occurrence of pancreatic cancer. The emergence of these mechanisms seems to indicate that metformin does play a role in reducing the incidence of pancreatic cancer.
However, as far as the published meta-analysis is concerned, its role is still uncertain. Wang Z [58], Yu X [59], and Zhang P [60] all showed that metformin is a protective factor for pancreatic cancer, which can reduce the incidence of pancreatic cancer by 37%, 36%, and 46%. However, Singh S [61] suggested no significant correlation between metformin and pancreatic cancer (OR = 0.76, 95% CI 0.57–1.03). A recent meta-analysis [62] on the relationship between metformin and the incidence of total cancer also showed that using metformin could reduce the risk of pancreatic cancer. According to the difference in the control group, the study was divided into the group that has never used metformin and the group that has used other anti-diabetes drugs (OR = 0.62, 95% CI (0.45,0.84)); OR = 0.57, 95%CI (0.35,0.93)).
Since there is no consensus on the role of metformin in the existing meta-analysis results, we conducted this meta-analysis involving 24 articles. In this analysis, more than 2.28 million people participated. The overall analysis of the study showed that the use of metformin was negatively correlated with the occurrence of pancreatic cancer (OR = 0.82, 95% CI (0.69, 0.98)), which was consistent with most previous studies. When subgroup analysis is conducted according to study quality, only the subgroup of the high-quality study shows that metformin is negatively related to the risk of pancreatic cancer, which may be due to the deviation of the research methodology of the low-quality study. When the subgroup analysis was carried out according to the status of diabetes of the study subject, only the subgroup of non-newly diagnosed diabetes suggested that metformin was negatively related to the risk of pancreatic cancer, which may be because the protective effect of metformin on pancreatic cancer needs a certain delay. When subgroup analysis is conducted according to the study design, metformin can reduce the risk of pancreatic cancer only in the cohort study subgroup, which may be caused by the relatively small sample size of the case–control study and the low statistical efficiency in the study. It is worth noting that when the contrast agent was sub-analyzed, the opposite results were obtained. Single-factor meta-regression showed that the contrast agent was one of the heterogeneities of the study. The overall sensitivity analysis indicated that the study was stable, and no significant publication bias was found through the funnel plot and Egger test.
Compared with the previous meta-analysis, our research has some advantages. Firstly, this paper has included 24 articles from many countries, including more than 2 million participants, with high study quality, enhancing the statistical power of the data analysis and providing more reliable estimates. Secondly, we explored the research heterogeneity through subgroup analysis and single-factor meta-regression. Fortunately, we found the source of some research heterogeneity. Finally, since the existing evidence shows a relationship between the duration of diabetes and the occurrence of pancreatic cancer, we conducted a subgroup analysis on whether the study subjects were newly diagnosed with diabetes and obtained inconsistent results. As far as we know, this is the first meta-analysis of this subgroup analysis. When researchers later conduct relevant research, it can remind them to consider the diabetes status of the subjects.
However, we must admit that this study has some limitations. First of all, the heterogeneity of the study is remarkable. Although we have carried out some subgroup analysis, sensitivity analysis, and meta-regression, we only found partial sources of heterogeneity. The rest of the heterogeneity may be attributed to retrospective studies, inconsistent adjustment of confounding factors, or inconsistent follow-up time. Second, although we think that the flushing period and lag period will significantly impact the research results due to the inability to extract relevant data in some studies, no further analysis can be conducted. Third, the contrast agents of all the studies included in the analysis differ. Most appear as "no metformin users", but the specific drugs they contain are unclear. Although we have conducted subgroup analysis, whether "no metformin users" includes "no drug users" is ambiguous, which may lead to errors and bias in the results. Fourth, part of the literature contains several studies. We calculated and combined the sample size to obtain data for analysis, which may be biased from the actual situation. Fifth, we have extracted risk estimates that reflect the maximum control of potential confounding factors. However, the results of adjustments based on specific confounding factors may be different from those based on standards.
Conclusion
Metformin can reduce the risk of pancreatic cancer in patients with diabetes. Prospective research is needed to confirm our view in the future further.
Availability of data and materials
All data and materials from this study are presented within the manuscript.
Abbreviations
- PC:
-
Pancreatic cancer
- DM:
-
Diabetes mellitus
- PRISMA:
-
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- CNKI:
-
China National Knowledge Infrastructure
- NOS:
-
Newcastle Ottawa Scale
- DPP-4i:
-
Dipeptidyl peptidase-4 inhibitors
- mTOR:
-
Mammalian target of rapamycin
- KRAS:
-
Kirsten Rat Sarcoma Viral Oncogene
- KC:
-
LSL-KrasG12D/ + ;p48-Cre
- RR:
-
Relative Risk
- HR:
-
Hazard Ratio
- OR:
-
Odds Ratio
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA-Cancer J Clin. 2021;71(3):209–49.
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA-Cancer J Clin. 2020;70(1):7–30.
He J, Ahuja N, Makary MA, Cameron JL, Eckhauser FE, Choti MA, Hruban RH, Pawlik TM, Wolfgang CL. 2564 resected periampullary adenocarcinomas at a single institution: trends over three decades. HPB. 2014;16(1):83–90.
Klein AP. Pancreatic cancer epidemiology: understanding the role of lifestyle and inherited risk factors. Nat Rev Gastro Hepat. 2021;18(7):493–502.
Li D, Tang H, Hassan MM, Holly EA, Bracci PM, Silverman DT. Diabetes and risk of pancreatic cancer: a pooled analysis of three large case-control studies. Cancer Cause Control. 2011;22(2):189–97.
Elena JW, Steplowski E, Yu K, Hartge P, Tobias GS, Brotzman MJ, Chanock SJ, Stolzenberg-Solomon RZ, Arslan AA, Bueno-de-Mesquita HB, et al. Diabetes and risk of pancreatic cancer: a pooled analysis from the pancreatic cancer cohort consortium. Cancer Cause Control. 2013;24(1):13–25.
Bosetti C, Rosato V, Li D, Silverman D, Petersen GM, Bracci PM, Neale RE, Muscat J, Anderson K, Gallinger S, et al. Diabetes, antidiabetic medications, and pancreatic cancer risk: an analysis from the International Pancreatic Cancer Case-Control Consortium. Ann Oncol. 2014;25(10):2065–72.
Wan G, Sun X, Li F, Wang X, Li C, Li H, Yu X, Cao F. Survival benefit of metformin adjuvant treatment for pancreatic cancer patients: a systematic review and meta-analysis. Cell Physiol Biochem. 2018;49(3):837–47.
Jian-Yu E, Graber JM, Lu SE, Lin Y, Lu-Yao G, Tan XL. Effect of metformin and statin use on survival in pancreatic cancer patients: a systematic literature review and meta-analysis. Curr Med Chem. 2018;25(22):2595–607.
Amin S, Mhango G, Lin J, Aronson A, Wisnivesky J, Boffetta P, Lucas AL. Metformin improves survival in patients with pancreatic ductal adenocarcinoma and pre-existing diabetes: a propensity score analysis. Am J Gastroenterol. 2016;111(9):1350–7.
Terasaki F, Sugiura T, Okamura Y, Ito T, Yamamoto Y, Ashida R, Ohgi K, Uesaka K. Oncological benefit of metformin in patients with pancreatic ductal adenocarcinoma and comorbid diabetes mellitus. Langenbeck Arch Surg. 2020;405(3):313–24.
Higgins JPT TJCJ: Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). In.: Cochrane, 2022; 2022.
Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–5.
Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ-BRIT Med J. 2003;327(7414):557–60.
Oliveria SA, Koro CE, Ulcickas Yood M, Sowell M. Cancer incidence among patients treated with antidiabetic pharmacotherapy. Diabetes Metab Syndr. 2008;2(1):47–57.
Tsilidis KK, Capothanassi D, Allen NE, Rizos EC, Lopez DS, Van Veldhoven K, Sacerdote C, Ashby D, Vineis P, Tzoulaki I, et al. Metformin does not affect cancer risk: a cohort study in the U.K. clinical practice research datalink analyzed like an intention-to-treat trial. Diabetes Care. 2014;37(9):2522–32.
Ruiter R, Visser LE, van Herk-Sukel MP, Coebergh JW, Haak HR, Geelhoed-Duijvestijn PH, Straus SM, Herings RM, Stricker BH. Lower risk of cancer in patients on metformin in comparison with those on sulfonylurea derivatives: results from a large population-based follow-up study. Diabetes Care. 2012;35(1):119–24.
Bodmer M, Becker C, Meier C, Jick SS, Meier CR. Use of antidiabetic agents and the risk of pancreatic cancer: a case-control analysis. Am J Gastroenterol. 2012;107(4):620–6.
Sung JJ, Ho JM, Lam AS, Yau ST, Tsoi KK. Use of metformin and aspirin is associated with delayed cancer incidence. Cancer Epidemiol. 2020;69: 101808.
Zhao H, Liu Z, Zhuo L, Shen P, Lin H, Sun Y, Zhan S. Sulfonylurea and cancer risk among patients with Type 2 diabetes: a population-based cohort study. Front Endocrinol. 2022;13: 874344.
Valente R, Hayes AJ, Haugvik SP, Hedenstrom P, Siuka D, Korsaeth E, Kammerer D, Robinson SM, Maisonneuve P, Delle FG, et al. Risk and protective factors for the occurrence of sporadic pancreatic endocrine neoplasms. Endocr-Relat Cancer. 2017;24(8):405–14.
Oh TK, Song IA. Metformin use and the risk of cancer in patients with diabetes: a nationwide sample cohort study. Cancer Prev Res. 2020;13(2):195–202.
Murff HJ, Roumie CL, Greevy RA, Hackstadt AJ, McGowan L, Hung AM, Grijalva CG, Griffin MR. Metformin use and incidence cancer risk: evidence for a selective protective effect against liver cancer. Cancer Cause Control. 2018;29(9):823–32.
Wang SY, Chuang CS, Muo CH, Tu ST, Lin MC, Sung FC, Kao CH. Metformin and the incidence of cancer in patients with diabetes: a nested case-control study. Diabetes Care. 2013;36(9):e155–6.
Liao KF, Lai SW, Li CI, Chen WC. Diabetes mellitus correlates with increased risk of pancreatic cancer: a population-based cohort study in Taiwan. J Gastroen Hepatol. 2012;27(4):709–13.
Tseng CH. Metformin and pancreatic cancer risk in patients with type 2 diabetes. Pancreas. 2018;47(9):e57–9.
Currie CJ, Poole CD, Gale EA. The influence of glucose-lowering therapies on cancer risk in type 2 diabetes. Diabetologia. 2009;52(9):1766–77.
de Jong RG, Burden AM, de Kort S, van Herk-Sukel MP, Vissers PA, Janssen PK, Haak HR, Masclee AA, de Vries F, Janssen-Heijnen ML. No decreased risk of gastrointestinal cancers in users of metformin in the netherlands; a time-varying analysis of metformin exposure. Cancer Prev Res. 2017;10(5):290–7.
Farmer RE, Ford D, Mathur R, Chaturvedi N, Kaplan R, Smeeth L, Bhaskaran K. Metformin use and risk of cancer in patients with type 2 diabetes: a cohort study of primary care records using inverse probability weighting of marginal structural models. Int J Epidemiol. 2019;48(2):527–37.
Lee DY, Yu JH, Park S, Han K, Kim NH, Yoo HJ, Choi KM, Baik SH, Kim NH, Seo JA. The influence of diabetes and antidiabetic medications on the risk of pancreatic cancer: a nationwide population-based study in Korea. Sci Rep-UK. 2018;8(1):9719.
Lee MS, Hsu CC, Wahlqvist ML, Tsai HN, Chang YH, Huang YC. Type 2 diabetes increases and metformin reduces total, colorectal, liver and pancreatic cancer incidences in Taiwanese: a representative population prospective cohort study of 800,000 individuals. BMC Cancer. 2011;11:20.
Lu Y, Garcia RL, Malgerud L, Gonzalez-Perez A, Martin-Perez M, Lagergren J, Bexelius TS. New-onset type 2 diabetes, elevated HbA1c, antidiabetic medications, and risk of pancreatic cancer. Brit J Cancer. 2015;113(11):1607–14.
Vicentini M, Ballotari P, Giorgi RP, Venturelli F, Sacchettini C, Greci M, Mangone L, Pezzarossi A, Manicardi V. Effect of different glucose-lowering therapies on cancer incidence in type 2 diabetes: An observational population-based study. Diabetes Res Clin Pr. 2018;143:398–408.
Walker EJ, Ko AH, Holly EA, Bracci PM. Metformin use among type 2 diabetics and risk of pancreatic cancer in a clinic-based case-control study. Int J Cancer. 2015;136(6):E646–53.
Hsieh MC, Lee TC, Cheng SM, Tu ST, Yen MH, Tseng CH. The influence of type 2 diabetes and glucose-lowering therapies on cancer risk in the Taiwanese. Exp Diabetes Res. 2012;2012: 413782.
You JH, Song SO, Kang MJ, Cho YY, Kim SW, Suh SH, Lee S, Lee YH, Lee BW: Metformin and Gastrointestinal Cancer Development in Newly Diagnosed Type 2 Diabetes: A Population-Based Study in Korea. Clin Transl Gastroen 2020, 11(11):e254
van Staa TP, Patel D, Gallagher AM, de Bruin ML. Glucose-lowering agents and the patterns of risk for cancer: a study with the General Practice Research Database and secondary care data. Diabetologia. 2012;55(3):654–65.
Li D, Yeung SC, Hassan MM, Konopleva M, Abbruzzese JL. Antidiabetic therapies affect risk of pancreatic cancer. Gastroenterology. 2009;137(2):482–8.
Garuti F, Neri A, Avanzato F, Gramenzi A, Rampoldi D, Rucci P, Farinati F, Giannini EG, Piscaglia F, Rapaccini GL, et al. The changing scenario of hepatocellular carcinoma in Italy: an update. Liver Int. 2021;41(3):585–97.
Facciorusso A, Abd EAM, Singh S, Pusceddu S, Milione M, Giacomelli L, Sacco R: Statin use decreases the incidence of hepatocellular carcinoma: an updated meta-analysis. Cancers 2020, 12(4).
Facciorusso A. The influence of diabetes in the pathogenesis and the clinical course of hepatocellular carcinoma: recent findings and new perspectives. Curr Diabetes Rev. 2013;9(5):382–6.
Gong J, Robbins LA, Lugea A, Waldron RT, Jeon CY, Pandol SJ. Diabetes, pancreatic cancer, and metformin therapy. Front Physiol. 2014;5:426.
Yu H, Zhong X, Gao P, Shi J, Wu Z, Guo Z, Wang Z, Song Y. The potential effect of metformin on cancer: an umbrella review. FRONT ENDOCRINOL. 2019;10:617.
Yin M, Zhou J, Gorak EJ, Quddus F. Metformin is associated with survival benefit in cancer patients with concurrent type 2 diabetes: a systematic review and meta-analysis. Oncologist. 2013;18(12):1248–55.
Shi YQ, Zhou XC, Du P, Yin MY, Xu L, Chen WJ, Xu CF. Relationships are between metformin use and survival in pancreatic cancer patients concurrent with diabetes: a systematic review and meta-analysis. Medicine. 2020;99(37): e21687.
Zhou PT, Li B, Liu FR, Zhang MC, Wang Q, Li YY, Xu C, Liu YH, Yao Y, Li D. Metformin is associated with survival benefit in pancreatic cancer patients with diabetes: a systematic review and meta-analysis. Oncotarget. 2017;8(15):25242–50.
Scarpello JH, Howlett HC. Metformin therapy and clinical uses. Diabetes Vasc Dis Re. 2008;5(3):157–67.
Widen EI, Eriksson JG, Groop LC. Metformin normalizes nonoxidative glucose metabolism in insulin-resistant normoglycemic first-degree relatives of patients with NIDDM. Diabetes. 1992;41(3):354–8.
Musi N, Hirshman MF, Nygren J, Svanfeldt M, Bavenholm P, Rooyackers O, Zhou G, Williamson JM, Ljunqvist O, Efendic S, et al. Metformin increases AMP-activated protein kinase activity in skeletal muscle of subjects with type 2 diabetes. Diabetes. 2002;51(7):2074–81.
Wu H, Esteve E, Tremaroli V, Khan MT, Caesar R, Manneras-Holm L, Stahlman M, Olsson LM, Serino M, Planas-Felix M, et al. Metformin alters the gut microbiome of individuals with treatment-naive type 2 diabetes, contributing to the therapeutic effects of the drug. Nat Med. 2017;23(7):850–8.
Gontier E, Fourme E, Wartski M, Blondet C, Bonardel G, Le Stanc E, Mantzarides M, Foehrenbach H, Pecking AP, Alberini JL. High and typical 18F-FDG bowel uptake in patients treated with metformin. Eur J Nucl Med Mol I. 2008;35(1):95–9.
Schneider MB, Matsuzaki H, Haorah J, Ulrich A, Standop J, Ding XZ, Adrian TE, Pour PM. Prevention of pancreatic cancer induction in hamsters by metformin. Gastroenterology. 2001;120(5):1263–70.
Cifarelli V, Lashinger LM, Devlin KL, Dunlap SM, Huang J, Kaaks R, Pollak MN, Hursting SD. Metformin and rapamycin reduce pancreatic cancer growth in obese prediabetic mice by distinct microrna-regulated mechanisms. Diabetes. 2015;64(5):1632–42.
Chang HH, Moro A, Chou C, Dawson DW, French S, Schmidt AI, Sinnett-Smith J, Hao F, Hines OJ, Eibl G, et al. Metformin decreases the incidence of pancreatic ductal adenocarcinoma promoted by diet-induced obesity in the conditional Krasg12d mouse model. Sci Rep-UK. 2018;8(1):5899.
Dong TS, Chang HH, Hauer M, Lagishetty V, Katzka W, Rozengurt E, Jacobs JP, Eibl G. Metformin alters the duodenal microbiome and decreases the incidence of pancreatic ductal adenocarcinoma promoted by diet-induced obesity. Am J Physiol-Gastr L. 2019;317(6):G763–72.
Chen K, Qian W, Jiang Z, Cheng L, Li J, Sun L, Zhou C, Gao L, Lei M, Yan B, et al. Metformin suppresses cancer initiation and progression in genetic mouse models of pancreatic cancer. Mol Cancer. 2017;16(1):131.
Yue W, Zheng X, Lin Y, Yang CS, Xu Q, Carpizo D, Huang H, DiPaola RS, Tan XL. Metformin combined with aspirin significantly inhibit pancreatic cancer cell growth in vitro and in vivo by suppressing anti-apoptotic proteins Mcl-1 and Bcl-2. Oncotarget. 2015;6(25):21208–24.
Wang Z, Lai ST, Xie L, Zhao JD, Ma NY, Zhu J, Ren ZG, Jiang GL. Metformin is associated with reduced risk of pancreatic cancer in patients with type 2 diabetes mellitus: a systematic review and meta-analysis. Diabetes Res Clin PR. 2014;106(1):19–26.
Yu X, Qy H, Tang S. Relation between metformin and the risk of pancreatic cancer in type 2 diabetes patients: a meta - analysis. Chin Gen Pract. 2016;2(19):195–8.
Zhang P, Li H, Tan X, Chen L, Wang S. Association of metformin use with cancer incidence and mortality: a meta-analysis. Cancer Epidemiol. 2013;37(3):207–18.
Singh S, Singh PP, Singh AG, Murad MH, McWilliams RR, Chari ST. Antidiabetic medications and risk of pancreatic cancer in patients with diabetes mellitus: a systematic review and meta-analysis. Am J Gastroenterol. 2013;108(4):510–9.
Zhang K, Bai P, Dai H, Deng Z. Metformin and risk of cancer among patients with type 2 diabetes mellitus: A systematic review and meta-analysis. Prim Care Diabetes. 2021;15(1):52–8.
Acknowledgements
None.
Funding
None.
Author information
Authors and Affiliations
Contributions
Jian Hu is responsible for the design and writing of this article. Jian Hu, Hong-Dan Fan and Qing-Song Mao have extracted, checked, and analyzed the data. Qing-Song Mao and Jian-Ping Gong were mainly responsible for reviewing and modifying the article. All the authors contributed to the writing, and they all approved the final manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate.
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors deny any competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Hu, J., Fan, HD., Gong, JP. et al. The relationship between the use of metformin and the risk of pancreatic cancer in patients with diabetes: a systematic review and meta-analysis. BMC Gastroenterol 23, 50 (2023). https://doi.org/10.1186/s12876-023-02671-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12876-023-02671-0