Abstract
Background
Primary gastric lymphoma (PGL) is the most common extranodal non-Hodgkin lymphoma (NHL). Due to the rarity of the disease, it is important to create a predictive model that provides treatment and prognosis for patients with PGL and physicians.
Methods
A total of 8898 and 127 patients diagnosed with PGL were obtained from the SEER database and from our Cancer Center as training and validation cohorts, respectively. Univariate and multivariate Cox proportional hazards models were used to investigate independent risk factors for the construction of predictive survival nomograms, and a web nomogram was developed for the dynamic prediction of survival of patients with PGL. The concordance index (C-index), calibration plot, and receiver operating characteristics (ROC) curve were used to evaluate and validate the nomogram models.
Results
There were 8898 PGL patients in the SEER cohort, most of whom were married men over the age of 60, 16.1% of the primary tumors were localized in the antrum and pylori of the stomach, which was similar to the composition of 127 patients in the Chinese cohort, making both groups comparable. The Nomogram of overall survival (OS) was compiled based on eight variables, including age at diagnosis, sex, race, marital status, histology, stage, radiotherapy and chemotherapy. Cancer-specific survival (CSS) nomogram was developed with eight variables, including age at diagnosis, sex, marital status, primary tumor site, histology, stage, radiotherapy and chemotherapy. The C-index of OS prediction nomogram was 0.948 (95% CI: 0.901–0.995) in the validation cohort, the calibration plots showed an optimal match and a high area below the ROC curve (AUC) was observed in both training and validation sets. Also, we established the first web-based PGL survival rate calculator (https://yangjinru.shinyapps.io/DynNomapp/).
Conclusion
The web dynamic nomogram provided an insightful and applicable tool for evaluating PGL prognosis in OS and CSS, and can effectively guide individual treatment and monitoring.
Similar content being viewed by others
Background
Primary gastric lymphoma (PGL) is a malignant extranodal lymphoma originating from gastric submucous lymphoid tissue [1,2,3]. PGL represents almost 30-40% of extranodal lymphomas and approximately 5% of all gastric malignancies [1,2,3]. The two predominant histological subtypes are extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue (MALT) and diffuse large B cell lymphoma (DLBCL), constituting around 90% of all cases [1,2,3]. So far, there is still no consensus on the optimal treatment, especially for H. pylori negative MALT, DLBCL, and advanced PGL [4, 5]. Given the rareness of this disease, treatment recommendations are mostly based on case-series data, rather than large randomized clinical trials [6]. The Ann-Arbor stage system is a tool commonly used by oncologists to predict disease progression and design therapeutic strategies for lymphoma, taking the location of lymph node spread as the basis for staging. However, it does not include other factors that may affect survival, such as personal and cancer treatment information [1, 3]. Considering various factors affecting the etiology of lymphoma, the prognosis based on the Ann-Arbor staging system alone is unreliable [7]. Nomogram is a reliable and convenient prognostic tool, which has been widely used to predict the overall probability of specific outcomes in clinical oncology by incorporating a number of prognostic factors [8,9,10,11,12,13]. It can use known and important prognostic factors to quantitatively predict the prognosis in certain patients and explain the numerical probability of clinical outcomes [8, 9]. A prognostic tool [14] has recently been proposed for primary gastric DLBCL. However, a complete nomogram for predicting survival in patients with PGL has not been reported. The purpose of this study is to develop comprehensive and effective nomogram based on the data retrieved from the SEER and Chinese cohorts to better predict the survival rate of PGL patients.
Methods
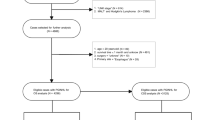
Study population
The Surveillance, Epidemiology, and End Results (SEER) project sponsored by the National Cancer Institute (NCI) is a free public database (https://seer.cancer.gov/). It consists of 18 registered cancer centers and currently covering approximately 30% of the United States population. We got access to the SEER Research Data. SEER*Stat software (Version 8.3.9.2; NCI; Bethesda, MD) was used to extract clinical data of patients with PGL from the SEER in 2000-2018 (November 2020 Submission). Patients with PGL were identified by the International Classification of Diseases for Oncology, the third edition (ICD-O-3) histologic codes 9590-9599, 9650-9669, 9670-9699, 9700-9719, 9720-9729 for lymphoma and primary site codes C16.0-C16.9 for gastric. Patients with more than one primary tumor site, without pathological confirmation, race records, marital status, stage records, survival months and active follow-up were excluded from the extracted patients. The demographic and clinical characteristics obtained in the database are as follows: age at diagnosis, sex, race, marital status, primary tumor site, histology, cancer stage, surgery therapy, radiotherapy, chemotherapy and survival months until death or last follow-up. The cancer stages were according to the Ann Arbor staging system, which can be found in the American Joint Committee on Cancer (AJCC) Cancer Staging Manual (7th edition) or Union for International Cancer Control (UICC) staging manual. The overall survival (OS) was calculated from the date of diagnosis to the date of death or the date of censoring, regardless of whether the patients survived at the last follow-up. The cancer-specific survival (CSS) was calculated from the diagnosis date to death date only from PGL or censorship date if patients were alive or dead from another cause.
For clinical data, patients initially diagnosed with PGL from May 2015 to September 2021 in our Cancer Center were included in this retrospective study, the last follow-up was in November 2021. The inclusion and exclusion criteria were in line with the SEER cohort above. However, cancer-specific deaths have not been observed in the Chinese cohort. This study was approved by the Ethical Committees of Union Hospital, Tongji Medical College, Huazhong University of Science and Technology and in accordance with the Helsinki Declaration.
Statistical analysis
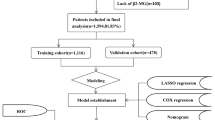
In the SEER database, we identified 8898 eligible PGL patients in order to compile the effective OS and CSS nomograms. One hundred and twenty-seven patients initially diagnosed with PGL were assigned to the validation cohort. Cox proportional hazards model was used for univariate and multivariate analyses to identify the variables that significantly influenced OS and CSS in the training cohort. Then, OS and CSS nomograms based on independent prognostic factors were constructed by training cohort. The OS nomogram was validated in both the training and the validation cohort. The predictive performance of nomograms was evaluated with discrimination and calibration tests. Concordance index (C-index) and receiver operating characteristic curve (ROC) were used for assessing the discrimination, and calibration curve was used to compare actual results and survival probability predicted by nomogram. For this purpose, bootstraps with 1000 resamples were used [15]. The web-based OS and CSS probability calculators were built using packages “DynNom” and “shiny” in R software. Statistical analysis was performed using the SPSS Statistics software (version 23.0; IBM Corporation; Armonk, NY) and the R software (version 4.0.3). Hazards ratios and 95% confidence intervals (95% CI) were calculated. Two-sided P value less than 0.05 was considered statistically significant.
Results
Demographic and clinical characteristics
A total of 8898 patients in the SEER cohort and 127 patients in the Chinese cohort met the inclusion criteria. Table 1 summarizes the demographic and clinical characteristics of patients in the training and validation cohorts. In SEER training cohort, most patients aged between 60 and 79 years old (48.57%). Over half of eligible patients were male (54.57%) and married (58.65%). The majority of patients were white (80.22%), followed by black (9.53%). The primary site of the patients' tumor was 12.13% in the cardiac and gastric fundus, 13.49% in the gastric body, 16.10% in antrum and pylorus, and 9.59% in the lesser and greater curvature. DLBCL and MALT histologic subtype accounted for 42.23% and 44.83%, respectively. About 61.77% of patients were diagnosed with Ann Arbor phase I disease. Of the PGL patients, 50.24% received chemotherapy, only 10.29% underwent surgery and 24.53% performed radiotherapy.
Identifying independent prognostic factors for OS and CSS
Univariate and multivariate Cox proportional hazards analysis of OS and CSS in patients with PGL in the training cohort were listed in Tables 2 and 3. Univariate analyses showed that age at diagnosis, sex, race, marital status, histology, stage, radiotherapy and chemotherapy were associated with OS. Multivariate analyses identified eight variables, including age at diagnosis, sex, race, marital status, histology, stage, radiotherapy and chemotherapy, to be significantly associated with OS. Age at diagnosis, sex, marital status, primary tumor site, histology, stage, surgery, radiotherapy and chemotherapy were closely related to CSS in univariate analyses. Age at diagnosis, sex, marital status, primary tumor site, histology, stage, radiotherapy and chemotherapy were independent risk factors for CSS in multivariate analyses.
Nomogram construction
The prognostic nomograms based on sorted significant independent factors from multivariate analysis for predicting 3-year or 5-year OS and CSS in the training cohort were shown in Fig. 1. Scores were given at each level of each variable, and the total score was obtained by adding a score on the point scale of each selected variable, which subsequently helped correlate with the probability of the event for each patient.
Nomogram validation
The OS nomograms were validated externally. In the training cohort, the prognostic nomogram C-index predictied by OS and CSS was 0.716 (95% CI: 0.708–0.794) and 0.767 (95% CI: 0.757–0.777), respectively. While, in the validation cohort, the C-index was 0.948 (95% CI: 0.901–0.995). However, due to the lack of cancer-specific survival information, it was not possible to obtain the C-index of the validation cohort. The calibration plots of the training and validation cohorts for 3-year or 5-year OS showed an optimal match between nomogram prediction and actual observed outcomes (Fig. 2). A high area below the ROC curve (AUC) was also observed in both training and validation sets, respectively (Fig. 3).
Web-based probability calculators
According to the multivariate results above, a dynamic nomogram was created for prediction of OS probability in patients with PGL, which was of great convenient and intuitive to individually prognosis prediction based on the personal characteristics of PGL patients (https://yangjinru.shinyapps.io/DynNomapp/). For instance, the 5-year survival rate of DLBCL patients was approximately 86.0% (95% CI: 84.0%-87.0%) in 40-59 years old, male, white, married, stage I patients with radiotherapy, chemotherapy and without surgery, see details in Fig. 4, and in patients with MALT more than 80 years old, female, black, separated/divorced, stage IV, with chemotherapy, without surgery and radiotherapy, 33.0% (95% CI: 27.6–40.0%) of them would survive within 60 months (Additional File 1: Fig. S1).
A web-based PGL probability calculator. The 5-year survival probability of PGL with DLBCL, 40–59 years old, male, white, married, stage I patients with radiotherapy, chemotherapy and without surgery showed in the dynamical nomogram. A The estimated survival probability. B Numerical summary showed the probability and its 95% CI.
Discussion
PGL is the most common extranodal NHL and ranks as the second most common tumour of the stomach [1,2,3]. PGL is a relatively rare cancer and is easily misdiagnosed due to its non-specific symptoms [16,17,18]. There are many treatment options include gastrectomy, radiotherapy, chemotherapy, immunotherapy, and observation, while gastrectomy remains controversial due to a considerably favorable prognosis versus quality of life [19,20,21,22,23,24,25]. A previous study by Wang et al [2] showed that advanced stage and malignant pathological type are significantly associated with poor overall survival. Most studies demonstrated that female gender, low-grade histology, good PS, and surgical resection were associated with better overall survival [2, 18, 21]. Our study corresponds to these previous reports except for gastrectomy. The balance between efficacy of gastrectomy and quality of life requires further studies.
Although the Ann-Arbor staging system is widely used and recognized for PGL forecasting, it neglects some significant risk factors such as age, race and marital status [26, 27]. In addition, the International Prognostic Index (IPI) and related indices can also divide the prognosis of lymphoma into risk groups. The evaluation criteria included age, stage, ECOG score, extranodal lesions and LDH level, but do not include cancer-related treatment information, therefore it is necessary to develop a more systematic model for predicting PGL risk and to make better therapeutic strategies for individual patients. It is known that nomograms were used to predict the survival status of various diseases [28]. Therefore, we constructed a comprehensive nomogram based on different risk factors to better predict the prognosis of PGL patients. Although the clinical treatment of inert lymphoma, such as MALT and invasive lymphoma is different, the nomogram can well distinguish PGL by pathological type, and better guide the treatment and predict prognosis. These nomograms were able to perform more accurate evaluation and predictions in patients with PGL in both training and validation cohorts, and the results of C-index and calibration curves showed that the models were repeatable and reliable. As far as we know, this is a comprehensive and in-depth large population study aimed at building nomograms for PGL patients, and a web-based dynamic nomogram can directly help clinicians quantify the probability to provide personalized and accurate treatment for patients and to determine the best follow-up time according to disease progression and recurrence rate. CSS is an epidemiological statistical method that can rule out non-cancer deaths. It is an ideal prediction model, which differs from the actual situation, but the index can better predict the mortality attributable to the cancer, and better design precise treatment strategies for patients.
However, there are several limitations in our study. First, our nomogram has been externally validated in a single cancer center, which requires multi-center and large samples verification. Second, some potentially vital information related to the prognosis, such as surgical details, the surgical margin status, vascular invasion, hematological indicators, molecular pathologic characteristics, cell of origin (COO), immunohistochemical results and Helicobacter pylori infection status, family history were not included in the SEER database, which could improve predictive ability if incorporated. Third, the majority race of SEER cohort was white and all patients in the Chinese cohort were yellow, therefore the study could be with potential racial heterogeneity. Finally, due to lack of data, we excluded patients from the study, which may lead to potential selection bias.
Conclusion
To sum up, we constructed a web dynamic nomogram based on the SEER database to better predict the prognosis of PGL patients, and pre-validated it in the Chinese cohort. The nomogram only needs basic information, and has a wider range of application.
Availability of data and materials
The datasets generated and analysed during the current study are available in the SEER research database (https://seer.cancer.gov/). SEER*Stat software (Version 8.3.9.2; NCI; Bethesda, MD) was used to extract clinical data for patients with PGL from the SEER for the period 2000–2018 (November 2020 Submission).
Abbreviations
- GC:
-
gastric cancer
- PGL:
-
primary gastric lymphoma
- C-index:
-
concordance index
- ROC:
-
receiver operating characteristics
- OS:
-
overall survival
- CSS:
-
cancer-specific survival
- SEER:
-
The surveillance, epidemiology, and end results
- HL:
-
Hodgkin lymphoma
- NA:
-
Not available
References
Huang H, Lu ZW, Jiang CG, Li J, Xu K, Xu ZJ. Clinical and prognostic characteristics of pulmonary mucosa-associated lymphoid tissue lymphoma: a retrospective analysis of 23 cases in a Chinese population. Chin Med J (Engl). 2011;124(7):1026–30.
Wang YG, Zhao LY, Liu CQ, Pan SC, Chen XL, Liu K, et al. Clinical characteristics and prognostic factors of primary gastric lymphoma: a retrospective study with 165 cases. Medicine. 2016;95(31):e4250.
Ghimire P, Wu GY, Zhu L. Primary gastrointestinal lymphoma. World J Gastroenterol. 2011;17(6):697–707.
Asano N, Iijima K, Koike T, Imatani A, Shimosegawa T. Helicobacter pylori-negative gastric mucosa-associated lymphoid tissue lymphomas: a review. World J Gastroenterol. 2015;21(26):8014–20.
Schmelz R, Miehlke S, Thiede C, Brueckner S, Dawel M, Kuhn M, et al. Sequential H. pylori eradication and radiation therapy with reduced dose compared to standard dose for gastric MALT lymphoma stages IE & II1E: a prospective randomized trial. J Gastroenterol. 2019;54(5):388–95.
Castro FA, Jansen L, Krilaviciute A, Katalinic A, Pulte D, Sirri E, et al. Survival of patients with gastric lymphoma in Germany and in the United States. J Gastroenterol Hepatol. 2015;30(10):1485–91.
Matysiak-Budnik T, Fabiani B, Hennequin C, Thieblemont C, Malamut G, Cadiot G, et al. Gastrointestinal lymphomas: French Intergroup clinical practice recommendations for diagnosis, treatment and follow-up. Dig Liver Dis. 2018;50(2):124–31.
Balachandran VP, Gonen M, Smith JJ, DeMatteo RP. Nomograms in oncology: more than meets the eye. Lancet Oncol. 2015;16(4):e173-80.
Chen S, Liu Y, Yang J, Liu Q, You H, Dong Y, et al. Development and validation of a nomogram for predicting survival in male patients with breast cancer. Front Oncol. 2019;9:361.
Ceci F, Bianchi L, Borghesi M, Polverari G, Farolfi A, Briganti A, et al. Prediction nomogram for Ga-PSMA-11 PET/CT in different clinical settings of PSA failure after radical treatment for prostate cancer. Eur J Nucl Med Mol Imag. 2020;47(1):136–46.
Martini A, Cumarasamy S, Beksac AT, Abaza R, Eun DD, Bhandari A, et al. A nomogram to predict significant estimated glomerular filtration rate reduction after robotic partial nephrectomy. Eur Urol. 2018;74(6):833–9.
Fakhry C, Zhang Q, Nguyen-Tân PF, Rosenthal DI, Weber RS, Lambert L, et al. Development and validation of nomograms predictive of overall and progression-free survival in patients with oropharyngeal cancer. J Clin Oncol. 2017;35(36):4057–65.
Wang Y, Li J, Xia Y, Gong R, Wang K, Yan Z, et al. Prognostic nomogram for intrahepatic cholangiocarcinoma after partial hepatectomy. J Clin Oncol. 2013;31(9):1188–95.
Lin JL, Lin JX, Li P, Xie JW, Wang JB, Lu J, et al. Dynamic prediction of long-term survival in patients with primary gastric diffuse large B-cell lymphoma: a SEER population-based study. BMC cancer. 2019;19(1):873.
Wang C, Yang C, Wang W, Xia B, Li K, Sun F, et al. A Prognostic nomogram for cervical cancer after surgery from SEER database. J Cancer. 2018;9(21):3923–8.
Chang YT, Huang MY, Shih HH, Wu CC, Lu TY, Lin PC. Gastric MALT lymphoma presented with primary perforation in an adolescent: a case report. BMC Pediatr. 2019;19(1):63.
Zucca E, Copie-Bergman C, Ricardi U, Thieblemont C, Raderer M, Ladetto M. Gastric marginal zone lymphoma of MALT type: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24(Suppl 6):144–8.
Ge Z, Liu Z, Hu X. Anatomic distribution, clinical features, and survival data of 87 cases primary gastrointestinal lymphoma. World J Surg Oncol. 2016;14:85.
Ikoma N, Badgwell BD, Mansfield PF. Multimodality treatment of gastric lymphoma. Surg Clin North Am. 2017;97(2):405–20.
Amiot A, Lévy M, Copie-Bergman C, Dupuis J, Szablewski V, Le Baleur Y, et al. Rituximab, alkylating agents or combination therapy for gastric mucosa-associated lymphoid tissue lymphoma: a monocentric non-randomised observational study. Aliment Pharmacol Ther. 2014;39(6):619–28.
Yang H, Wu M, Shen Y, Lei T, Mi L, Leng X, et al. Treatment strategies and prognostic factors of primary gastric diffuse large B cell lymphoma: a retrospective multicenter study of 272 cases from the china lymphoma patient registry. Int J Med Sci. 2019;16(7):1023–31.
Merchionne F, Iacopino P, Minoia C, Iacobazzi A, Rana A, Serrati S, et al. Targeted strategies in the treatment of primary gastric lymphomas: from rituximab to recent insights into potential new drugs. Curr Med Chem. 2014;21(8):1005–16.
Ohkubo Y, Saito Y, Ushijima H, Onishi M, Kazumoto T, Saitoh JI, et al. Radiotherapy for localized gastric mucosa-associated lymphoid tissue lymphoma: long-term outcomes over 10 years. J Radiat Res. 2017;58(4):537–42.
Yoon SS, Coit DG, Portlock CS, Karpeh MS. The diminishing role of surgery in the treatment of gastric lymphoma. Ann Surg. 2004;240(1):28–37.
Avilés A, Nambo MJ, Neri N, Huerta-Guzmán J, Cuadra I, et al. The role of surgery in primary gastric lymphoma: results of a controlled clinical trial. Ann Surg. 2004;240(1):44–50.
Yin X, Xu A, Fan F, Huang Z, Cheng Q, Zhang L, et al. Incidence and mortality trends and risk prediction nomogram for extranodal diffuse large B-cell lymphoma: an analysis of the surveillance, epidemiology, and end results database. Front Oncol. 2019;9:1198.
Yang Y, Zhang YJ, Zhu Y, Cao JZ, Yuan ZY, Xu LM, et al. Prognostic nomogram for overall survival in previously untreated patients with extranodal NK/T-cell lymphoma, nasal-type: a multicenter study. Leukemia. 2015;29(7):1571–7.
Zhu Y, Fang X, Wang L, Zhang T, Yu D. A predictive nomogram for early death of metastatic gastric cancer: a retrospective study in the SEER database and China. J Cancer. 2020;11(18):5527–35.
Acknowledgements
The authors would like to thank SEER program for providing clinical survival data.
Funding
This work was supported by the National Natural Science Foundation of China (Dandan Yu, No.81872429). Funders played a role in data collection.
Author information
Authors and Affiliations
Contributions
(I) Conception and design: DDY and LLZ; (II) Administrative support: DDY; (III) Provision of study materials or patients: JRY and TL; (IV) Collection and assembly of data: JRY, TL and YZ; (V) Data analysis and interpretation: FYZ and MLZ; (VI) Manuscript writing: All authors; (VII) All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study was approved and consented by the Ethical Committees of Union Hospital, Tongji Medical College, Huazhong University of Science and Technology and in accordance with the Helsinki Declaration. The data used in this study are anonymous.
Consent for publication
Not applicable.
Competing interests
All authors have completed the ICMJE uniform disclosure form. The authors have no conflicts of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Figure S1
. A web-based PGL probability calculator. The 5-year survival probability of PGL with MALT, ≥80 years old, female, black, separated/divorced, stage IV patients with chemotherapy, without surgery and radiotherapy showed in the dynamical nomogram. A The estimated survival probability. B Numerical summary showed the probability and its 95% CI.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Yang, J., Liu, T., Zhu, Y. et al. A dynamic predictive nomogram of long-term survival in primary gastric lymphoma: a retrospective study. BMC Gastroenterol 22, 347 (2022). https://doi.org/10.1186/s12876-022-02419-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12876-022-02419-2