Abstract
Backgrounds
Clinical evidence of the preventive effectiveness of medium-class topical corticosteroids for capecitabine-induced hand foot syndrome (HFS) is limited. Although the pathogenesis and mechanism of HFS are unclear, inflammatory reactions are thought to be involved in HFS development. This study aimed to evaluate the preventive effect of medium-class topical corticosteroids (hydrocortisone butyrate 0.1% topical therapy) for capecitabine-induced HFS in patients with colorectal cancer receiving adjuvant chemotherapy with capecitabine plus oxaliplatin.
Methods
This is a single-center, single-arm, phase 2 study. Patients with colorectal cancer scheduled to receive adjuvant chemotherapy with capecitabine plus oxaliplatin are enrolled, and topical hydrocortisone butyrate 0.1% is applied prophylactically in addition to standard moisturizing therapy. The primary endpoint is the incidence of grade ≥ 2 HFS within three months. The secondary endpoints are the time to onset of HFS, rates of dose reduction, schedule delay, discontinuation caused by capecitabine-induced HFS, and other adverse events. All adverse events are evaluated by clinical pharmacists and attending physicians.
Discussion
This study is expected to contribute to the establishment of new supportive care for preventing HFS, not only for colorectal cancer patients receiving adjuvant chemotherapy, but also for various cancer patients receiving capecitabine-based chemotherapy.
Trial registration: This trial was registered in the Japan Registry of Clinical Trials (jRCT) as jRCTs031220002. Registered 5 April 2022, https://jrct.niph.go.jp/search
Protocol version V.1.0, 16 February 2022.
Similar content being viewed by others
Background
Hand-foot syndrome (HFS) is a major side effect of capecitabine and often impairs the patient's quality of life (QOL) [1]. In randomized controlled trials (RCT) of chemotherapy for colorectal cancer, the incidence of capecitabine-induced HFS was reported to be approximately 30–80% in all grades [2,3,4], but 90% or more in the real world [5]. Severe HFS presents as swelling, blisters, desquamation, and ulcers, which cause interruptions, schedule delays, dose reductions, and discontinuation of capecitabine.
Two treatment options are recommended for adjuvant chemotherapy of colorectal cancer [6]: oral capecitabine plus intravenous oxaliplatin (CAPEOX regimen) and intravenous 5-fluorouracil and leucovorin plus oxaliplatin (FOLFOX regimen). Although the CAPEOX regimen has a shorter infusion time, its use in clinical practice is limited by the fear of HFS. In addition, HFS affects self-adherence [7]. In particular, the dose intensity of capecitabine affects the outcomes of adjuvant chemotherapy for colon cancer [8]. Thus, HFS management is important not only for the maintenance of patients’ QOL but also for ensuring the treatment efficacy of chemotherapy using capecitabine [9].
Moisturizing and avoiding local pressure [10,11,12] are generally recognized as standard management methods for preventing capecitabine-induced HFS. Moreover, the effectiveness of exfoliating agents [13, 14], celecoxib [15, 16], and pyridoxine [17] for HFS has been reported. However, there are no established methods for the prevention of capecitabine-induced HFS based on high-level evidence, expect for treatment modification (interruption and/or dose reduction) [18].
The mechanism of HFS involves inhibition of the proliferation of skin basal cells, secretion of drugs from the eccrine sweat glands, involvement of drug degradation products [9, 19], and an inflammatory response caused by IL-1a, IL-1b, IL-6, and reactive oxygen species [20]. Corticosteroids exert anti-inflammatory effects by inhibiting the release of chemical mediators. Oral dexamethasone (8 mg/day, followed by tapering) was administered to patients with pegylated liposomal doxorubicin-induced palmar-plantar erythrodysesthesia in a prospective study [21], which reported that patients receiving dexamethasone could receive treatment without dose modification, but those without dexamethasone administration required schedule delays or dose reductions. Other case reports have suggested that corticosteroids might be effective against cytarabine-and vinorelbine-induced HFS [22, 23]. Similarly, the mechanism of epidermal growth factor receptor (EGFR) inhibitor-related skin rash is assumed to be associated with inflammatory reactions [24,25,26]. The preventive effect of topical corticosteroids on EGFR inhibitor-related skin rashes has been reported [27, 28]. Based on this evidence, it is expected that topical corticosteroids may prevent the reduction in dose intensity of chemotherapy induced by HFS and improve patients’ quality of life during the adjuvant chemotherapy period. However, no study has clearly suggested the preventive or therapeutic effects of topical corticosteroids for capecitabine-related HFS.
Hence, we conducted a single-center, single-arm, interventional study to evaluate the preventive effect of add-on therapy with medium-class topical corticosteroids (hydrocortisone butyrate 0.1% topical therapy) for capecitabine-induced HFS in the adjuvant chemotherapy of colorectal cancer patients.
We evaluate the preventive effect of 0.1% topical hydrocortisone butyrate therapy for capecitabine-induced HFS in colorectal cancer patients receiving adjuvant chemotherapy with capecitabine and oxaliplatin.
Methods
Study design
This trial is a single-center, single-arm, phase 2 study. This protocol was reviewed and approved by the certified Clinical Research Review Board of the University of Tokyo (approval number: 2021512SP). This clinical trial was conducted in compliance with the Clinical Trials Act in Japan and registered in the Japan Registry of Clinical Trials (jRCT) as jRCTs031220002.
Subjects
The inclusion criteria are: (i) curatively resected and histologically confirmed colorectal adenocarcinoma, (ii) pathological stage II or III, (iii) scheduled to receive adjuvant chemotherapy with capecitabine plus oxaliplatin, (iv) age ≥ 18 years old, (v) ECOG performance status of 0 to 1, (vi) no prior chemotherapy or radiation therapy, (vii) adequate organ function, and (viii) provision of written informed consent. In contrast, the exclusion criteria are: (i) bacterial/fungal/spirochete/virus skin infections, (ii) eczema otitis externa with perforations in the eardrum, (iii) ulcers (excluding Behçet’s disease), second-degree or deeper burns/frostbite, (iv) other skin disease, and (v) a history of hypersensitivity to local hydrocortisone.
Adjuvant chemotherapy
Adjuvant chemotherapy with capecitabine plus oxaliplatin comprise a 2-h intravenous infusion of oxaliplatin (130 mg/m2) on day 1 and oral administration of capecitabine (1000 mg/m2, twice daily) from the evening of day 1 to the morning of day 15, repeat every 3 weeks (21 days) for eight or four cycles according to the physician’s discretion and patient’s consent. Dose interruption, delay, reduction, and discontinuation are allowed, if necessary, at the attending physician’s discretion. In principle, capecitabine dose can be reduced by 20% up to 2 levels. In a previous study, the control group was administered celecoxib as the study drug, and the number of cycles to the development of grade 1 and 2 HFS was 4.336 [16]. In addition, the cumulative dose of capecitabine, which causes grade 1 or higher HFS in more than 80% of patients, is 100,000 mg/m2 [5]. Therefore, the minimum cumulative dosage of capecitabine in the present study, considering the dose reduction of the drug, should, in principle, be 100,000 mg/m2 to complete 4 cycles or more.
Intervention
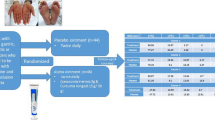
Topical hydrocortisone butyrate 0.1% and standard moisturizing therapy are applied to the hands and feet daily in the morning and evening. One fingertip unit (1 fingertip unit provides 0.5 g) of topical hydrocortisone butyrate 0.1% is applied to both palms and feet for each application, only once in the morning and once in the evening. This preventive treatment is started on day 1 and continued until the end of adjuvant chemotherapy (Fig. 1). All patients received standard self-care education (ex. keeping clean, moisturizing, wearing gloves, avoiding strenuous exercise, avoiding pressure, and sun protection) at the start of the chemotherapy, and is confirmed the amount of topical hydrocortisone butyrate 0.1% used regularly by clinical pharmacists. Clinical pharmacists regularly educate the patients to improve self-adherence to intervention protocols.
Study scheme. HFS hand foot syndrome. Topical hydrocortisone butyrate 0.1% and standard moisturizing therapy are applied to the hands and feet daily in the morning and evening, started on day 1 and continued until the end of adjuvant chemotherapy. To keep patients’ self-adherence, all patients received standard self-care education at the start of the chemotherapy, and is confirmed the amount of topical hydrocortisone butyrate 0.1% used regularly by clinical pharmacists. Clinical pharmacists regularly educate the patients to improve self-adherence to intervention protocols
Criteria for discontinuing interventions
Interventions for the following patients will be discontinued. (i) Withdrawal of consent, (ii) Discontinuation of CAPEOX regimen, (iii) Occurrence of serious adverse events induced by topical hydrocortisone butyrate 0.1%, (iv) Administration of prohibited therapy and drugs. For participants who discontinue or deviate from intervention protocols, optimal medical care without study drugs is guaranteed.
Prohibited therapy and drugs
The following treatments or concomitant use of drugs are prohibited. (i) Cancer treatment other than CAPEOX regimen, (ii) Administration of other topical corticosteroids for hands or feet, (iii) Administration of celecoxib, (iv) Administration of pyridoxine.
Evaluation
Patient symptoms and laboratory tests are checked at every visit (Table 1), and the severity of HFS and other adverse events are assessed by the clinical pharmacist and the attending physician, referring to the self-report of adverse events (Tables 2, 3). Adverse events are evaluated based on laboratory parameters and according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 5.0. Evaluators will be provided regular training to promote data quality.
Endpoints
The primary endpoint is the incidence of grade ≥ 2 HFS within 3 months of the start of adjuvant chemotherapy. The secondary endpoints are: i) incidence of grade ≥ 2 HFS within 6 months of the start of adjuvant chemotherapy, ii) time to onset of grade ≥ 2 HFS, iii) time to onset of any grade HFS, and iv) incidence of dose reduction, schedule delay, and discontinuation rate of capecitabine caused by any grade of HFS.
Sample size calculation
For the primary endpoint, the incidence of grade ≥ 2 HFS is calculated among the enrolled patients, excluding those whose adjuvant chemotherapy would be discontinued within 3 months due to recurrence or adverse events other than grade ≥ 2 HFS. In previous RCTs investigating the preventive effect of HFS, the incidence of grade ≥ 2 HFS in the control group without preventive measures was reported to be approximately 40% [15, 16, 29, 30]. It is assumed that hydrocortisone butyrate 0.1% (external application) would suppress grade ≥ 2 HFS within 3 months by 15%, corresponding to the expected incidence of 25% and threshold of 40%. The power was set at 70%, considering feasibility. The power is the probability that the 90% WALD upper confidence limit is below the threshold incidence rate with continuity correction, and the minimum number of patients exceeding 70% is 39 (one-sided α = 0.1). Therefore, considering that some patients were excluded from the primary analysis, the target number of enrolled patients was planned to be 50. The above calculations were performed using SAS Studio 3.8.
Implementation
NB will generate the allocation sequence, enrol participants, and assign participants to interventions. Clinical pharmacists will support them.
Discussion
At present, there are no established methods for preventing capecitabine-induced HFS. Moisturizing and avoiding local pressure are widely used to prevent capecitabine-induced HFS in clinical practice because of their low risk of adverse events, although their efficacy has not been proven in clinical trials. Based on the pathogenic mechanism of HFS and previous one prospective study [21] and two case reports [22, 23], we believe that medium-class topical corticosteroids is effective for preventing HFS. This single-center, single-arm, intervention study evaluate the effect of medium-class topical corticosteroids (hydrocortisone butyrate 0.1% topical therapy) added to the present standard care to prevent capecitabine-induced HFS in adjuvant chemotherapy.
The prevention of capecitabine-induced HFS using prophylactic agents, such as exfoliating agents [13, 14], celecoxib [15, 16], and pyridoxine [17], has been reported. Exfoliating agents, which are often used to avoid local pressure for hand-foot reactions induced by other anti-tumor agents, such as sunitinib, sorafenib, and regorafenib, are sometimes irritating and may enhance the symptoms of HFS. In two studies using celecoxib as a preventive drug [15, 16], the frequency of grade ≥ 2 HFS was reduced by 20% or more. However, nonsteroidal anti-inflammatory drugs, including celecoxib, can induce cardiovascular adverse events [31, 32], and long-term administration should be avoided. Therefore, celecoxib is not recommended as a preventive option for HFS in clinical practice. The prophylactic effect of pyridoxine for HFS has been reported [33, 34]. However, the efficacy of pyridoxine is controversial and has been proven to be negative in multiple meta-analyses and RCTs [17, 35,36,37,38]. It is important to use agents with a low risk of adverse events to prevent capecitabine-induced HFS.
Topical corticosteroids have some advantages over oral agents owing to their low risk of adverse events. Continued oral steroids can induce infections and should not be administered to patients on chemotherapy. Topical steroids have a low risk of infection because they do not act systemically, and it is needless to consider drug-drug interactions with cytochrome P450 3A4. In addition, topical corticosteroids can be applied at the same time as the moisturizer, which ensures self-adherence.
In the present study, patients are administered medium-class corticosteroids. Stronger topical corticosteroids may increase the risk of local fungal infections and skin atrophy. Therefore, medium-class corticosteroids with low risk of infection can be used safely to prevent HFS.
The preventive effect of hydrocortisone on HFS is expected to improve the QOL for patients on CAPEOX regimen and broaden the choice of adjuvant chemotherapy. This study is expected to contribute to the establishment of new supportive care for preventing HFS, not only for colorectal cancer patients on adjuvant chemotherapy, but also for various cancer patients receiving capecitabine-based chemotherapy.
This study has several limitations. First, it is a single-arm study and not a comparative study. Second, because the study participants receive adjuvant chemotherapy and the evaluation period is as short as 3 months, long-term efficacy and adverse events of topical corticosteroids cannot be evaluated. Based on this study, we will plan an RCT that verifies the preventive effect of topical corticosteroids for HFS, regardless of cancer type, combined agents, or treatment settings.
Conclusions
We believe that these findings will contribute more evidence on supportive care that is useful not only for adjuvant chemotherapy but also for various cancer treatments that induce HFS. This protocol was established based on the pathogenic mechanism of HFS and previous one prospective study [21] and two case reports [22, 23].
Availability of data and materials
The data that support the findings of this study are available from the corresponding author, YI, upon reasonable request.
Abbreviations
- CAPEOX:
-
Capecitabine plus intravenous oxaliplatin
- EGFR:
-
Epidermal growth factor receptor
- FOLFOX:
-
5-Fluorouracil and leucovorin plus oxaliplatin
- HFS:
-
Hand-foot syndrome
- jRCT:
-
Japan Registry of Clinical Trials
- QOL:
-
Quality of life
- RCT:
-
Randomized controlled trial
References
Sibaud V, Dalenc F, Chevreau C, Roché H, Delord JP, Mourey L, et al. HFS-14, a specific quality of life scale developed for patients suffering from hand-foot syndrome. Oncologist. 2011;16:1469–78.
Grothey A, Sobrero AF, Shields AF, Yoshino T, Paul J, Taieb J, et al. Duration of adjuvant chemotherapy for Stage III colon cancer. N Engl J Med. 2018;378:1177–88.
Yamazaki K, Yamanaka T, Shiozawa M, Manaka D, Kotaka M, Gamoh M, et al. Oxaliplatin-based adjuvant chemotherapy duration (3 versus 6 months) for high-risk stage II colon cancer: the randomized phase III ACHIEVE-2 trial. Ann Oncol. 2021;32:77–84.
Hamaguchi T, Shimada Y, Mizusawa J, Kinugasa Y, Kanemitsu Y, Ohue M, et al. Capecitabine versus S-1 as adjuvant chemotherapy for patients with stage III colorectal cancer (JCOG0910): an open-label, non-inferiority, randomised, phase 3, multicentre trial. Lancet Gastroenterol Hepatol. 2018;3:47–56.
Yokokawa T, Kawakami K, Mae Y, Sugita K, Watanabe H, Suzuki K, et al. Risk factors exacerbating hand-foot skin reaction induced by capecitabine plus oxaliplatin with or without bevacizumab therapy. Ann Pharmacother. 2015;49:1120–4.
NCCN guidelines colon cancer. https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1428.
Kawakami K, Nakamoto E, Yokokawa T, Sugita K, Mae Y, Hagino A, et al. Patients’ self-reported adherence to capecitabine on XELOX treatment in metastatic colorectal cancer: findings from a retrospective cohort analysis. Patient Prefer Adherence. 2015;9:561–7.
Lakkunarajah S, Breadner DA, Zhang H, Yamanaka E, Warner A, Welch S. The influence of adjuvant chemotherapy dose intensity on five-year outcomes in resected colon cancer: A single centre retrospective analysis. Curr Oncol. 2021;9(28):4031–41. https://doi.org/10.3390/curroncol28050342.
Degen A, Alter M, Schenck F, Satzger I, Völker B, Kapp A, et al. The hand-foot-syndrome associated with medical tumor therapy—classification and management. J Dtsch Dermatol Ges. 2010;8:652–61.
Son HS, Lee WY, Lee WS, Yun SH, Chun HK. Compliance and effective management of the hand-foot syndrome in colon cancer patients receiving capecitabine as adjuvant chemotherapy. Yonsei Med J. 2009;50:796–802.
Van Cutsem E, Twelves C, Cassidy J, Allman D, Bajetta E, Boyer M, et al. Oral capecitabine compared with intravenous fluorouracil plus leucovorin in patients with metastatic colorectal cancer: results of a large phase III study. J Clin Oncol. 2001;19:4097–106.
Blum JL, Jones SE, Buzdar AU, LoRusso PM, Kuter I, Vogel C, et al. Multicenter phase II study of capecitabine in paclitaxel-refractory metastatic breast cancer. J Clin Oncol. 1999;17:485–93.
Lu W, Huang Z, Chen S, Lv H, Chen X, Lei J, et al. The effectiveness of EVOSKIN®Palm and sole moisturizing cream in treating capecitabine-associated hand-foot syndrome: a randomized double-blind clinical trial. Ann Palliat Med. 2021;10:3009–17.
Wolf SL, Qin R, Menon SP, Rowland KM, Thomas S, Delaune R, et al. Placebo-controlled trial to determine the effectiveness of a urea/lactic acid-based topical keratolytic agent for prevention of capecitabine-induced hand-foot syndrome: North Central Cancer Treatment Group Study N05C5. J Clin Oncol. 2010;28:5182–7.
Zhang RX, Wu XJ, Lu SX, Pan ZZ, Wan DS, Chen G. The effect of COX-2 inhibitor on capecitabine-induced hand-foot syndrome in patients with stage II/III colorectal cancer: a phase II randomized prospective study. J Cancer Res Clin Oncol. 2011;137:953–7.
Zhang RX, Wu XJ, Wan DS, Lu ZH, Kong LH, Pan ZZ, et al. Celecoxib can prevent capecitabine-related hand-foot syndrome in stage II and III colorectal cancer patients: result of a single-center, prospective randomized phase III trial. Ann Oncol. 2012;23:1348–53.
Jo SJ, Shin H, Jo S, Kwon O, Myung SK. Prophylactic and therapeutic efficacy of pyridoxine supplements in the management of hand-foot syndrome during chemotherapy: a meta-analysis. Clin Exp Dermatol. 2015;40:260–70.
Scheithauer W, Blum J. Coming to grips with hand-foot syndrome. Insights from clinical trials evaluating capecitabine. Oncology (Williston Park). 2004 Aug;18:1161–8, 1173; discussion 1173–6, 1181–4.
Diasio RB. Oral DPD-inhibitory fluoropyrimidine drugs. Oncology (Williston Park). 2000;14(Suppl 9):19–23.
Yokomichi N, Nagasawa T, Coler-Reilly A, Suzuki H, Kubota Y, Yoshioka R, et al. Pathogenesis of Hand-Foot Syndrome induced by PEG-modified liposomal doxorubicin. Hum Cell. 2013;26:8–18.
Drake RD, Lin WM, King M, Farrar D, Miller DS, Coleman RL. Oral dexamethasone attenuates Doxil-induced palmar-plantar erythrodysesthesias in patients with recurrent gynecologic malignancies. Gynecol Oncol. 2004;94:320–4.
Brown J, Burck K, Black D, Collins C. Treatment of cytarabine acral erythema with corticosteroids. J Am Acad Dermatol. 1991;24:1023–5.
Hoff PM, Valero V, Ibrahim N, Willey J, Hortobagyi GN. Hand-foot syndrome following prolonged infusion of high doses of vinorelbine. Cancer. 1998;82:965–9.
Van Doorn R, Kirtschig G, Scheffer E, Stoof TJ, Giaccone G. Follicular and epidermal alterations in patients treated with ZD1839 (Iressa), an inhibitor of the epidermal growth factor receptor. Br J Dermatol. 2002;147:598–601.
Busam KJ, Capodieci P, Motzer R, Kiehn T, Phelan D, Halpern AC. Cutaneous side-effects in cancer patients treated with the antiepidermal growth factor receptor antibody C225. Br J Dermatol. 2001;144:1169–76.
Pascual JC, Belinchón I, Sivera F, Yuste A. Severe cutaneous toxicity following treatment with gefitinib (ZD1839). Br J Dermatol. 2005;153:1222–3.
Lacouture ME, Mitchell EP, Piperdi B, Pillai MV, Shearer H, Iannotti N, et al. Skin toxicity evaluation protocol with panitumumab (STEPP), a phase II, open-label, randomized trial evaluating the impact of a pre-emptive Skin treatment regimen on skin toxicities and quality of life in patients with metastatic colorectal cancer. J Clin Oncol. 2010;28:1351–7.
Kobayashi Y, Komatsu Y, Yuki S, Fukushima H, Sasaki T, Iwanaga I, et al. Randomized controlled trial on the skin toxicity of panitumumab in Japanese patients with metastatic colorectal cancer: HGCSG1001 study. J-STEPP Future Oncol. 2015;11:617–27.
Watanabe K, Ishibe A, Watanabe J, Ota M, Fujii S, Ichikawa Y, et al. The effect of TJ-28 (Eppikajutsuto) on the prevention of hand-foot syndrome using capecitabine for colorectal cancer: the Yokohama Clinical Oncology Group Study (YCOG1102). Indian J Gastroenterol. 2020;39:204–10.
Ostwal V, Kapoor A, Mandavkar S, et al. Effect of a structured teaching module including intensive prophylactic measures on reducing the incidence of capecitabine-induced hand-foot syndrome: results of a prospective randomized Phase III study. Oncologist. 2020 Dec;25:e1886–92-e1892. doi: https://doi.org/10.1634/theoncologist.2020-0698.
Trelle S, Reichenbach S, Wandel S, Hildebrand P, Tschannen B, Villiger PM, et al. Cardiovascular safety of non-steroidal anti-inflammatory drugs: network meta-analysis. BMJ. 2011;11(342): c7086. https://doi.org/10.1136/bmj.c7086.
Arfè A, Scotti L, Varas-Lorenzo C, Nicotra F, Zambon A, Kollhorst B, et al. Non-steroidal anti-inflammatory drugs and risk of heart failure in four European countries: nested case-control study. BMJ. 2016;28(354): i4857. https://doi.org/10.1136/bmj.i4857.
Chalermchai T, Tantiphlachiva K, Suwanrusme H, Voravud N, Sriuranpong V. Randomized trial of two different doses of pyridoxine in the prevention of capecitabine-associated palmar-plantar erythrodysesthesia. Asia Pac J Clin Oncol. 2010;6:155–60. https://doi.org/10.1111/j.1743-7563.2010.01311.x.
Yoshimoto N, Yamashita T, Fujita T, Hayashi H, Tsunoda N, Kimura M, et al. Impact of prophylactic pyridoxine on occurrence of hand-foot syndrome in patients receiving capecitabine for advanced or metastatic breast cancer. Breast Cancer. 2010;17:298–302. https://doi.org/10.1007/s12282-009-0171-3.
Lian S, Zhang X, Zhang Y, Zhao Q. Pyridoxine for prevention of hand–foot syndrome caused by chemotherapy agents: a meta-analysis. Clin Exp Dermatol. 2021;46:629–35. https://doi.org/10.1111/ced.14486.
Zhou Y, Peng L, Li Y, Chen L. Prophylactic pyridoxine was not able to reduce the incidence of capecitabine-induced hand-foot syndrome: a meta-analysis. Biomed Rep. 2013;1:873–8. https://doi.org/10.3892/br.2013.161.
Kang YK, Lee SS, Yoon DH, Lee SY, Chun YJ, Kim MS, et al. Pyridoxine is not effective to prevent hand-foot syndrome associated with capecitabine therapy: results of a randomized, double-blind, placebo-controlled study. J Clin Oncol. 2010;20(28):3824–9. https://doi.org/10.1200/JCO.2010.29.1807.
Yap YS, Kwok LL, Syn N, Chay WY, Chia JWK, Tham CK, et al. Predictors of hand-foot syndrome and pyridoxine for prevention of capecitabine-induced hand-foot syndrome: a randomized clinical trial. JAMA Oncol. 2017;1(3):1538–45. https://doi.org/10.1001/jamaoncol.2017.1269.
Acknowledgements
We would like to thank Editage (www.editage.com) for English language editing.
Trial status
The study is ongoing, and patients are being enrolled. Enrolment started in May 2022. As of May 2022, 5 of patients have participated. We thus expect to complete the recruitment by February 2027.
Funding
This trial did not receive any specific grants from funding agencies in the public, commercial, or not-for-profit sectors. This trial is carried out with the operating expenses grant of our hospital.
Author information
Authors and Affiliations
Contributions
YI conceived the idea presented in this study, and NB. revised the study design. MN created the statistical settings for this study. NF, MI, YA, TT, SA, DS, MN, SK, NB developed the theory. All authors discussed the results and contributed to the final manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This protocol was reviewed and approved by the certified Clinical Research Review Board of the University of Tokyo (approval number: 2021512SP) prior to conducting the trial. This clinical trial was conducted in compliance with the Clinical Trials Act in Japan and was registered in the Japan Registry of Clinical Trials (jRCT) as jRCTs031220002. All patients will be required to provide written informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Iimura, Y., Furukawa, N., Ishibashi, M. et al. Study protocol of a single-arm phase 2 study evaluating the preventive effect of topical hydrocortisone for capecitabine-induced hand-foot syndrome in colorectal cancer patients receiving adjuvant chemotherapy with capecitabine plus oxaliplatin (T-CRACC study). BMC Gastroenterol 22, 341 (2022). https://doi.org/10.1186/s12876-022-02411-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12876-022-02411-w