Abstract
Background
Patients can present for a wide variety of etiologies for dysphagia, and it is important to consider less common causes once common etiologies have been ruled out. Extrapulmonary Mycobacterium tuberculosis (TB) presentations are rare to see in the western populations due to relative lack of TB exposure and overall less immunocompromised populations, but should be considered for at-risk patients. Gastrointestinal (GI) TB is rare, and the GI tract is considered only the sixth most frequent site of extrapulmonary TB (EPTB).
Case presentation
This is a case report of a 35-year-old Ethiopian male presenting with dysphagia and retrosternal odynophagia who was found to have infiltration of mediastinal lymphadenopathy into the esophageal wall secondary to TB. This patient underwent an upper endoscopy, which revealed a linear 2 cm full thickness mucosal defect in the middle esophagus concerning for an infiltrative process with full thickness tear. Computed tomography (CT) of the chest demonstrated a subcarinal soft tissue mass that was inseparable from the esophagus. He was referred to thoracic surgery and underwent an exploratory mediastinal dissection. A mediastinoscopy scope was inserted and the mediastinal dissection was made until the subcarinal nodes were identified and removed. Biopsy results showed necrotizing and non-necrotizing granulomas, and acid-fast bacilli (AFB) culture from the surgically removed lymph node showed Mycobacterium TB complex growth. He had no known TB exposures and did not have any TB risk factors. He then followed up in infectious disease clinic and was managed with anti-tuberculosis treatment (ATT) with complete resolution of symptoms.
Conclusions
Our patient was ultimately found to have esophageal TB secondary to mediastinal invasion into the esophageal wall from lymphadenopathy associated with TB. This is an extremely rare presentation in western populations due to diminished exposure rates and overall less immunocompromised populations compared to impoverished countries with increased TB exposure and human immunodeficiency virus (HIV) infection rates. Although TB is not as commonly seen in western populations, it should be considered on the differential for any atypical presentations of GI diseases for patients with clinical or geographic risk factors.
Similar content being viewed by others
Background
Mycobacterium tuberculosis infection affects nearly 10 million people a year and causes 1.5 million deaths [1]. This number is significantly lower in the United States as seen in 2019, at 2.7 cases per 100,000 [2]. TB is common in the immunosuppressed population with 12% of all new diagnoses occurring in human immune deficiency virus (HIV) positive patients. Extrapulmonary TB (EPTB) occurs in 12% of patients with active TB infection of which 3.5% is hepatobiliary and 6–38% is intra-abdominal [3].
Case presentation
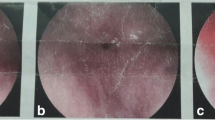
A 35-year HIV-negative, Ethiopian male presented with two months of progressive dysphagia and odynophagia. He underwent an upper endoscopy that showed a mid-esophageal ulcer and was referred to our tertiary care center. He subsequently underwent an upper endoscopy that revealed a linear 2 cm full thickness defect in the middle esophagus (Figs. 1, 2, 3). Nodular tissue was visualized at the defect site, and biopsied with cold forceps.
Histopathologic results demonstrated gastroesophageal mucosa with ulceration, granulation tissue formation, and marked reactive changes with negative Cytomegalovirus (CMV) and Herpes simplex virus (HSV) immunostains. The patient then underwent a CT chest that showed a large subcarinal soft tissue mass inseparable from the esophagus with enlarged mediastinal and hilar lymph nodes and thickening of the right suprahilar region (Fig. 4).
Based on these findings, the patient was referred to thoracic surgery. Mediastinal dissection and mediastinoscopy was then performed with visualization and resection of several enlarged subcarinal lymph nodes. Histopathology of the resected lymph nodes were notable for necrotizing and non-necrotizing granulomas (Fig. 5), with negative fungal and AFB stains. Infectious disease was consulted based on these findings, with recommendations for empiric antifungal therapy with itraconazole and urine antigen assays for Blastomyces dermatitidis and Histoplasma capsulatum, which were negative.
Three weeks later, the AFB culture from the surgically removed lymph node resulted with Mycobacterium TB complex growth. Other than prior travel to Ethiopia, he denied any known TB exposures and did not have any other TB risk factors. He was then started on anti-tuberculosis treatment (ATT) with complete resolution of symptoms. At a 6-month follow-up, he reported complete resolution of his previous dysphagia and odynophagia.
Discussion and conclusions
Gastrointestinal manifestations of TB can include involvement of the gastrointestinal tract, peritoneum, lymph nodes, and/or solid organs [4]. Lymph nodes are the most common site of involvement of EPTB. Uncommon forms of abdominal TB include involvement of the esophagus (2.8% of all cases of GI TB), stomach, duodenum, pancreas, and spleen [5].
Symptoms of Esophageal TB (ET) include dysphagia, odynophagia, chest pain, low‐grade fever, and weight loss. ET usually occurs at the middle third of the esophagus at the level of the carina [6]. Dysphagia is the most common presenting manifestation of gastrointestinal (GI) TB, although rare and occurs either due to primary involvement of the esophagus by TB or secondary to direct extension from adjacent structures [7]. Primary ET is very rare because of various protective mechanisms including the presence of stratified squamous epithelial cells covered with mucus in the esophagus [8]. In a case study that looked at 2176 patients with persistent dysphagia, 12 patients were found to have ET, and 10 of these patients had likely primary ET with no other identifiable sources of TB [9]. Primary ET should be suspected in any patient presenting with dysphagia, the typical finding of a linear ulcer, or hypertrophic growth on endoscopy [9]. Most cases of ET are secondary to direct extension from adjacent structures, such as mediastinal lymph nodes or pulmonary sites [10]. TB penetrates the mucosa and localizes in the submucosal lymphoid tissue, where it initiates an inflammatory reaction with subsequent lymphangitis, endarteritis, granuloma formation, caseation necrosis, mucosal ulceration, and scarring.
The clinical, radiological, and endoscopic features of ET are not well defined because of its rarity [11]. CT scans typically demonstrate more lesions than are visible on chest radiographs and are particularly helpful in detecting hilar or mediastinal lymphadenopathy. When there is a concern for esophageal involvement of TB, deep endoscopic biopsies are recommended to be taken from the margins of any visualized ulceration, as TB granulomas are often submucosal compared to the mucosal granulomas typically seen in Crohn’s disease [12]. Given the rarity of esophageal related TB in western populations, other differential diagnoses should be considered, including Crohn’s disease and primary esophageal malignancy [13].
Histopathology and TB‐polymerase chain reaction (PCR) are the mainstay for confirming the diagnosis of ET [14]. The presence of caseating granulomas is suggestive of tuberculosis but is not pathognomonic [15]. Studies show PCR sensitivities ranging from 74 to 100% for smear-positive specimens [16]. The sensitivity of the AFB smear and mycobacterial culture for biopsy specimens is low (< 50%) [15]. Histology shows epithelioid granulomas with Langhans cells and central caseous necrosis. Classical granulomas are seen only in 50% of cases, whereas AFB is demonstrated in < 25% of cases. Endoscopic mucosal biopsy has a sensitivity of 22% as reported by Mokoena et al. [17].
Early diagnosis and initiation of antituberculosis therapy and surgical treatment are essential to prevent morbidity and mortality [4]. Most patients respond well with ATT and patients with ET complicated with esophagotracheal and esophagomediastinal fistulas can be safely treated with ATT alone [14]. Patients with GI related TB should undergo multidisciplinary management with infectious disease consultation for the guidance of pharmacologic treatment.
The reported complications of ET include aspiration pneumonia, fatal hematemesis, esophagotracheal fistula, esophagomediastinal fistula, traction diverticula, esophageal strictures, and amyloidosis [17]. Surgical treatment is reserved for complications such as perforation, abscess formation, high-grade obstructions, and fistulas (esophageal, tracheoesophageal, and aorto-esophageal) [18].
Our patient was ultimately found to have esophageal TB secondary to mediastinal invasion into the esophageal wall from lymphadenopathy associated with TB. This is an extremely rare presentation in western populations due to diminished exposure rates and overall less immunocompromised populations compared to impoverished countries with increased TB exposure and HIV infection rates. Although TB is not as commonly seen in western populations, it should be considered on the differential for any atypical presentations of GI diseases for patients with clinical or geographic risk factors.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- TB:
-
Mycobacterium tuberculosis
- GI:
-
Gastrointestinal
- EPTB:
-
Extrapulmonary tuberculosis
- cm:
-
Centimeter
- CT:
-
Computed tomography
- AFB:
-
Acid Fast Bacillus
- ATT:
-
Anti-tuberculosis treatment
- HIV:
-
Human immunodeficiency virus
- CMV:
-
Cytomegalovirus
- HSV:
-
Herpes simplex virus
- ET:
-
Esophageal TB
- PCR:
-
Polymerase chain reaction
References
Houben RM, Dodd PJ. The global burden of latent tuberculosis infection: a re-estimation using mathematical modelling. PLoS Med. 2016;13(10):e1002152.
Schwartz NG, Price SF, Pratt RH, Langer AJ. Tuberculosis—United States, 2019. MMWR Morb Mortal Wkly Rep. 2020;69(11):286–9.
Evans RP, Mourad MM, Dvorkin L, Bramhall SR. Hepatic and intra-abdominal tuberculosis: 2016 update. Curr Infect Dis Rep. 2016;18(12):45.
Debi U, Ravisankar V, Prasad KK, Sinha SK, Sharma AK. Abdominal tuberculosis of the gastrointestinal tract: revisited. World J Gastroenterol. 2014;20(40):14831–40.
Rathi P, Gambhire P. Abdominal tuberculosis. J Assoc Physicians India. 2016;64(2):38–47.
Huang YK, Wu YC, Liu YH, Liu HP. Esophageal tuberculosis mimicking submucosal tumor. Interact Cardiovasc Thorac Surg. 2004;3(2):274–6.
Rana SS, Bhasin DK, Rao C, Srinivasan R, Singh K. Tuberculosis presenting as dysphagia: clinical, endoscopic, radiological and endosonographic features. Endosc Ultrasound. 2013;2(2):92–5.
Leung VKS, Chan WH, Chow TL, Luk ISC, Chau TN, Loke TKL. Oesophageal tuberculosis mimicking oesophageal carcinoma. Hong Kong Med J. 2006;12(6):473–6.
Jain SK, Jain S, Jain M, Yaduvanshi A. Esophageal tuberculosis: is it so rare? Report of 12 cases and review of the literature. Am J Gastroenterol. 2002;97(2):287–91.
Welzel TM, Kawan T, Bohle W, Richter GM, Bosse A, Zoller WG. An unusual cause of dysphagia: esophageal tuberculosis. J Gastrointestin Liver Dis. 2010;19(3):321–4.
Patel N, Amarapurkar D, Agal S, Baijal R, Kulshrestha P, Pramanik S, et al. Gastrointestinal luminal tuberculosis: establishing the diagnosis. J Gastroenterol Hepatol. 2004;19(11):1240–6.
Kirsch R, Pentecost M, Hall Pde M, Epstein DP, Watermeyer G, Friederich PW. Role of colonoscopic biopsy in distinguishing between Crohn’s disease and intestinal tuberculosis. J Clin Pathol. 2006;59(8):840–4.
Griga T, Duchna HW, Orth M, Nicolas V, Müller KM, Schultze-Werninghaus G, et al. Tuberculous involvement of the oesophagus with oesophagobroncheal fistula. Dig Liver Dis. 2002;34(7):528–31.
Baijal R, Agal S, Amarapurkar D, Kumar P, Kotli N, Jain M, editors. Esophageal tuberculosis: an analysis of fourteen cases. Singapore: Springer; 2013.
Hickey AJ, Gounder L, Moosa MY, Drain PK. A systematic review of hepatic tuberculosis with considerations in human immunodeficiency virus co-infection. BMC Infect Dis. 2015;15:209.
Kaul KL. Molecular detection of Mycobacterium tuberculosis: impact on patient care. Clin Chem. 2001;47(8):1553–8.
Mokoena T, Shama DM, Ngakane H, Bryer JV. Oesophageal tuberculosis: a review of eleven cases. Postgrad Med J. 1992;68(796):110–5.
Reddy NPK, Jeyasekharan SS, Nithila C, Kishore AS. Rare presentation of TB oesophagus: a case report. Int Surg J. 2018;5(9):3180.
Acknowledgements
Not applicable.
Funding
None to disclose.
Author information
Authors and Affiliations
Contributions
DO, KL, ET, AA. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor of this journal.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor of this journal.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Olson, D., Liu, K.C., Merza, A.P. et al. Esophageal tuberculosis induced dysphagia: a case report. BMC Gastroenterol 22, 131 (2022). https://doi.org/10.1186/s12876-022-02211-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12876-022-02211-2