Abstract
Aims and objectives
Acute-on-chronic hepatitis B liver failure (ACHBLF) is a critical clinical syndrome with a high short-term mortality evolved from chronic hepatitis B (CHB)-related liver disease. Prediction of mortality risk and early intervention can improve the prognosis of patients. This study aimed to develop and validate the nomogram for short-time mortality estimation in ACHBLF patients defined according to Asian Pacific Association for the Study of the Liver (APASL).
Methods
A study of 105 ACHBLF patients with 90-day follow up was performed to develop the nomogram. Patients were randomly assigned to derivation cohort (n = 75) and validation cohort (n = 35) according to 7:3. Concordance index (C-index), calibration curve and decision curve analysis (DCA) were used to evaluate the nomogram. We also compared the nomogram with APASL ACLF research consortium (AARC) score, model for end-stage liver disease (MELD) score, MELD with serum sodium (MELD-Na) score and albumin-bilirubin (ALBI) score. The nomogram was validated using an external cohort including 40 patients.
Results
The 28-day and 90-day mortality of 105 patients were respectively 49.52% and 55.24%. Albumin (ALB), international normalized ratio (INR) and estimated glomerular filtration rate (eGFR) were independent predictors for 28-day mortality; INR and eGFR were independent predictors for 90-day mortality. C-index of Nomogram-1 for 28-day mortality and Nomogram-2 for 90-day mortality were respectively 0.82 and 0.81. Calibration curve and Hosmer–Lemeshow test (Nomogram-1, 0.323; Nomogram-2, 0.231) showed optimal agreement between observed and predicted death. Areas under receiver operator characteristic curve(AUROC) of Nomogram-1(0.772) and Nomogram-2(0.771) were larger compared with AARC, MELD, MELD-Na and ALBI score. The results were well estimated in the external validation cohort.
Conclusions
This study highlighted the predictive value of eGFR, and the nomogram based on INR and eGFR could effectively estimate individualized risk for short-term mortality of ACHBLF patients defined according to APASL.
Similar content being viewed by others
Introduction
Acute-on-chronic liver failure (ACLF) is a critical clinical syndrome with acute decompensation of liver function based on chronic liver disease, characterized by systemic inflammation and high short-term mortality [1,2,3]. It can be accompanied by multi-organ dysfunction and progressed very rapidly. The main causes of ACLF are diverse between East and West. In China, acute-on-chronic hepatitis B liver failure (ACHBLF) accounts for more than 80% of all causes [4]. Thus, it is necessary to stratify ACHBLF patients for clinical decision- making depending on prognosis.
Until now model for end-stage liver disease (MELD) score and MELD with serum sodium (MELD-Na) score were most widely used in clinical to evaluate prognosis of patients with severe liver disease, however, with limited prognostic value. Recently APASL ACLF research consortium (AARC) derived and internally validated AARC score, which enrolled total bilirubin (TBIL), hepatic encephalopathy (HE) grades, international normalized ratio (INR), lactate and creatinine (Cr), showed fairly good predictive power [5]. However, AARC score is more complex and enrolls subjective factors, such as HE grades, which brings inconvenience to the wide promotion of clinical practice. Both AARC score and MELD score took into consideration extrahepatic organ failure, such as acute renal injury(AKI), and enrolled Cr as an evaluation index. However, Cr was easily affected by external factors and couldn't accurately represent AKI, so we aimed to enroll eGFR as a predictor, which could evaluate AKI more accurately.
In recent years, a variety of prognostic indices and models have been established with technological improvements in the field of molecular biology, genomics and transcriptomics, such as Neutrophil–lymphocyte ratio (NLR) [6], Th17/Treg [7], nomogram [8] and so on. Among them, nomogram has been proved to provide an individualized and highly accurate risk assessment [9, 10]. In this study, we aimed to develop and validate a nomogram to predict 28-day and 90-day mortality of ACHBLF patients defined according to Asian Pacific Association for the Study of the Liver (APASL). In addition, albumin-bilirubin (ALBI) score, which used to assess the severity of liver dysfunction in hepatocellular carcinoma (HCC) patients, was developed to evaluate the prognosis of ACLF patients lately [11,12,13]. In this study, we also aimed to estimate the prognostic value of ALBI score for ACHBLF patients.
Materials and methods
Study design and subjects
A cohort of ACHBLF patients who had been hospitalized in Tianjin Second People’s Hospital between January 2015 and December 2020 was enrolled. We developed a prognostic nomogram based on the above cohort, which was randomly assigned to derivation cohort (n = 75) and validation cohort (n = 35) according to 7:3 as described in previous studies [8]. Nomogram discrimination was assessed by concordance index (C-index), and calibration was conducted with calibration curve and Hosmer–Lemeshow test. Decision curve analysis (DCA) was proposed to assess the clinical validity of the predictive model. The predictive value between the nomogram with AARC, MELD, MELD-Na and ALBI score was performed by receiver operating characteristic (ROC) curve. In addition, we used an external cohort with identical characteristics for validation.
Clinical data was collected from medical records within 24 h of established diagnosis, including age, gender, pathologic basis, total bilirubin (TBIL), albumin(ALB), cholinesterase (CHE), Cr, blood urea nitrogen (BUN), estimated glomerular filtration rate (eGFR), international normalized ratio (INR), serum sodium (Na), lactate, HBV-DNA, mean arterial pressure(MAP), hepatic encephalopathy (HE), ascite and so on. All patients were followed up by telephone and lasted for 28 days, then 90 days if survived at 28 days. The outcome was recorded as survival or death. Patients who underwent liver transplantation from medical records were excluded. This study was approved by the Ethics Committee of the Tianjin Second People’s Hospital (018-18). Informed consent was waived by Ethics Committee of the Tianjin Second People’s Hospital due to the study’s retrospective nature.
Inclusion and exclusion criteria
All patients enrolled were diagnosed according to ACLF criteria of Asian Pacific Association for the Study of the Liver (APASL) [14]. ACLF was defined as acute decompensation of liver function, presented as severe gastrointestinal symptoms, hyperbilirubinemia and abnormal blood coagulation coagulopathy, with or without hepatic encephalopathy, which was developed from chronic hepatitis B (CHB) characterized by serum hepatitis B surface positive ≥ 6 months and histological evidence of chronic hepatitis. Exclusion criteria included: (1) coinfection with other hepatitis viruses or human immunodeficiency virus; (2) acute, subacute and chronic liver failure; (3) liver failure caused by other factors, such as autoimmune hepatitis, alcoholic liver disease and drug-induced hepatitis; (4) received liver transplantation; (5) renal dysfunction caused by underlying renal disease; (6) complicated with liver cancer, other malignant tumor or severe diseases in other organs.
Statistical analysis
Continuous variables were expressed as mean ± standard deviation, and categorical variables were represented as absolute and relative frequencies. Differences in continuous variables were analyzed using Student’s t test or Mann–Whitney U test, while Chi-square test or Fisher’s exact test was used for categorical variables. Logistic regression analysis was used to identify the independent prognostic predictors. Variables with P < 0.20 in the univariate logistic regression analysis were progressed to a multivariate analysis using stepwise regressions. All statistical tests were two-sided, and P < 0.05 were considered to be statistically significant. Odds ratio (OR) and 95% confidence interval (CI) were calculated. Nomogram was developed via rms package in R version 4.0.2.
Formulas
-
MELD score [15] = 3.8 × ln (TBIL mg/dL) + 9.6 × ln (Creatinine mg/dL) + 11.2 × ln (INR) + 6.4;
-
MELD-Na score [16] = MELD + 1.59 × (135 − Na);
-
ALBI score [12] = − 0.085 × (Albumin g/L) + 0.66 × log10 (TBIL µmol/L);
-
CKD-EPI eGFR [17] = 141×min (Cr/κ, 1)α×max(Cr/κ,1)−1.209×0.993Age×1.018 (if female)×1.159 (if black);
-
AARC score was referenced according to [5].
Results
Patient characteristics
We collected clinical data of 168 ACLF patients, and 105 patients were finally enrolled in accordance with the criteria (Fig. 1). The 105 patients comprised 80 men and 25 women, aged 23–74 years old (47.87 ± 12.07 years old), including 71 CHB (67.62%) and 34 hepatitis B cirrhosis (HBC) (32.38%) patients. The baseline characteristics were no statistical difference between derivation cohort and validation cohort (Table 1).
The overall 28-day mortality was 49.52% (52/105), and 90-day mortality was 55.24% (58/105). In 71 CHB patients, 28-day mortality was 53.52% (38/71) and 90-day mortality was 60.56% (43/71); In 34 HBC patients, 28-day mortality was 41.18% (14/34) and 90-day mortality was 44.12% (15/34), with statistically significant difference (P = 0.000). Age, pathologic basis, ALB, CHE, eGFR, INR, and HE were independent risk predictors by univariate logistic regression analysis. ALB, eGFR, INR were independent risk predictors for 28-day mortality, while eGFR and INR as independent risk predictors for 90-day mortality by multivariate logistic regression analysis. The results were showed in Table 2.
Development and validation of the prognostic nomogram
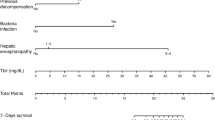
Two prognostic nomograms were developed incorporating independent risk predictors for 28-day mortality (Nomogram-1) and 90-day mortality (Nomogram-2). By calculating the total points through a vertical line from the variable to the point axis, we could estimate the probability of short-time mortality. The nomograms were showed in Fig. 2.
Prognostic nomograms of the training cohort. A Prognostic nomogram for 28-day mortality. B Prognostic nomogram for 90-day mortality. To use the nomogram, a line is drawn upward to determine the number of points received for each variable value. The sum of these scores is located on the total points axis and draw a line straight down to determine the probability of mortality. eGFR, estimated glomerular filtration rate; INR, international normalized ratio
In derivation cohort, C-index of Nomogram-1 was 0.82 (95% CI 0.72–0.91) and C-index of Nomogram-2 was 0.81 (95% CI 0.71–0.91), which revealed excellent discriminative abilities (Table 3). Calibration curve and Hosmer–Lemeshow test (Nomogram-1, 0.323; Nomogram-2, 0.231) indicated excellent calibration between nomogram predictions and actual observations. In validation cohort, C-index also showed good discriminative abilities with 0.73 (95% CI 0.54–0.92) for Nomogram-1 and 0.74 (95% CI 0.56–0.91) for Nomogram-2. Calibration curve and Hosmer–Lemeshow test (Nomogram-1, 0.382; Nomogram-2, 0.491) also indicated a good calibration. DCA confirmed the improved clinical utility of the nomogram in predicting mortality of ACHBLF patients both in derivation cohort and validation cohort. Calibration curve and DCA were showed in Figs. 3, 4. When subjected to the external validation, C-index was 0.78(95% CI 0.70–0.87) for Nomogram-1 and 0.88(95% CI 0.75–1.02) for Nomogram-2. Hosmer–Lemeshow test for Nomogram-1and Nomogram-2 were 0.362 and 0.178. C-index and calibration curve showed excellent discrimination and calibration ability. DCA confirmed the improvement of the nomogram on clinical decision-making. DCA of the external cohort were showed in Additional file 1: Figure S1. C-index and Hosmer–Lemeshow test were also showed in Table 3.
The calibration curves of the nomogram for 28-day and 90-day mortality in the training cohort and validation cohort. A The calibration curve in the training cohort at 28-day. B The calibration curve in the validation cohort at 28-day. C The calibration curve in the training cohort at 90-day. D The calibration curve in the validation cohort at 90-day
Decision curve analysis of the nomogram for 28-day and 90-day mortality in the training cohort and validation cohort. A Decision curve analysis in the training cohort at 28-day. B Decision curve analysis in the validation cohort at 28-day. C Decision curve analysis in the training cohort at 90-day. D Decision curve analysis in the validation cohort at 90-day
Comparison of predictive value between nomograms and clinical predictive models
The AUROC of Nomogram-1and Nomogram-2 for 28-day mortality were respectively 0.772 and 0.771, which showed similar predictive accuracy (Additional file 2: Figure S2).
AUROC of Nomogram-1 (0.772) was larger than AARC (0.759), MELD (0.712), MELD-Na (0.710) and ALBI (0.646) score. AUROC of Nomogram-2 (0.771) was also larger than AARC (0.732), MELD (0.718), MELD-Na (0.746) and ALBI (0.641) score (Fig. 5). The two nomograms both showed superior predictive accuracy.
Receiver operating characteristic curves of the nomogram, AARC, MELD, MELD-Na and ALBI score for predicting mortality of ACHBLF. A Receiver operating characteristic curves of Nomogram 1, AARC, MELD, MELD-Na and ALBI score. B Receiver operating characteristic curves of Nomogram 2, AARC, MELD, MELD-Na and ALBI score.
Discussion
ACLF is one of the most challenging health problems worldwide, characterized by acute onset, rapid emergence and extremely high short-term mortality [18, 19]. Prediction of mortality risk and early intervention can improve the prognosis of patients. Nowadays, the development of nomogram allows clinicians to standardize clinical decision through an evidence-based and fully-individualized tool. However, as we know, there was few study on nomogram specifically for ACHBLF patients. In this study, we developed and validated a nomogram based on INR and eGFR for short-term mortality prediction in ACHBLF patients defined according to APASL. Internal and external validations showed the nomogram with relatively high C-index and well-fitted calibration curves, which estimated the reliability and generalizability of the nomogram. Additionally, the performance of nomograms was, in turn, validated by ROC curve which showed better predictive value than AARC, MELD, MELD-Na and ALBI score.
In terms of clinical indicators, independent predictors for short-time mortality were ALB, INR and eGFR. ALB was included in Child–Pugh score to evaluate liver function, and lower ALB might represent poor liver function, which tended to aggravate disease progress. Consistent with previous studies [20, 21], INR, as the most commonly used index for coagulation assessment, was proved to be a high risk factor for prognosis of ACHBLF patients in our study. It was important to stress that, eGFR, but not Cr, was a powerful predictor of mortality in our data, which probably estimated its better prognostic value than Cr [22, 23]. Different with previous studies [5, 24], TBIL, MAP, lactate and HBV-DNA were not associated with short-time mortality in ACHBLF patients, which probably could be explained by the homogeneity of study population. In addition, HE, a well-established prognostic factor in AARC score, was also not identified as a prognostic factor in our data, but with a marginal statistical difference. Moreover, regardless of cirrhosis, ACLF patients developed from CHB also had a high mortality rate, and there was no significant difference between non-cirrhotic and cirrhotic HBV populations in short-time mortality, which further estimated the viewpoint of World Gastroenterology Organization [25].
Generally speaking, the nomogram would improve prognostic capabilities for mortality of ACHBLF patients. Parameters in the nomogram could be easily obtained and it would provide a user-friendly interface without computer software. More importantly, we highlighted the predictive value of eGFR. Nevertheless, our study had two main limitations. Firstly, the nomogram was developed based on a retrospective study from a single center, which might not represent the entire locally ACHBLF patients. Secondly, the nomogram was developed based on a relatively small group of patients, and was validated in only one external cohort, so a prospective multicenter clinical research might be needed to further improve and validate the nomogram.
Conclusions
In conclusion, we developed and validated a nomogram based on INR and eGFR for short-time mortality estimation in ACHBLF patients defined according to APASL. This study highlighted the predictive value of eGFR and could strengthen prognosis-based decision-making.
Availability of data and materials
All data generated or analyzed during this study are included in this article.
Abbreviations
- ACHBLF:
-
Acute-on-chronic hepatitis B liver failure
- CHB:
-
Chronic hepatitis B
- C-index:
-
Concordance index
- DCA:
-
Decision curve analysis
- ALBI:
-
Albumin-bilirubin
- ROC:
-
Receiver operating characteristics curve
- ACLF:
-
Acute-on-chronic liver failure
- AARC:
-
APASL ACLF research consortium
- MELD:
-
Model for end-stage liver disease
- MELD-Na:
-
MELD with serum sodium
- NLR:
-
Neutrophil–lymphocyte ratio
- HCC:
-
Hepatocellular carcinoma
- APASL:
-
Asian Pacific Association for the Study of the Liver
- OR:
-
Odds ratio
- CI:
-
Confidence interval
- HBC:
-
Hepatitis B cirrhosis
- TBIL:
-
Total bilirubin
- ALB:
-
Albumin
- Na:
-
Serum sodium
- Cr:
-
Creatinine
- BUN:
-
Blood urea nitrogen
- CHE:
-
Cholinesterase
- eGFR:
-
Estimated glomerular filtration rate
- INR:
-
International normalized ratio
- MAP:
-
Mean arterial pressure
- HE:
-
Hepatic encephalopathy
References
Wu T, Li J, Shao L, et al. Development of diagnostic criteria and a prognostic score for hepatitis B virus-related acute-on-chronic liver failure. Gut. 2018;67:2181–91.
Hernaez R, Solà E, Moreau R, Ginès P. Acute-on-chronic liver failure: an update. Gut. 2017;66:541–53.
Trovato F M, Zia R, Napoli S, et al. Dysregulation of the LPC-ATX-LPA axis in ACLF is associated with mortality and systemic inflammation via LPA-dependent monocyte activation. Hepatology. 2021.
You S, Rong Y, Bing Z, et al. Changing etiology of liver failure in 3,916 patients from northern China: a 10-year survey. Hep Intl. 2013;7:714–20.
Sarin SK, Kedarisetty CK, Abbas Z, et al. Acute-on-chronic liver failure: consensus recommendations of the Asian Pacific Association for the Study of the Liver (APASL) 2014. Hepatol Int. 2014;8(4):453–71.
Liu H, Zhang H, Wan G, et al. Neutrophil-lymphocyte ratio: a novel predictorfor short-term prognosis in acute-on-chronic hepatitis B liver failure. J Viral Hepatitis. 2014;21:499–507.
Liang X-S, Li C-Z, Zhou Y, et al. Changes in circulating Foxp3(+) regulatory T cells and interleukin-17-producing T helper cells during HBV-related acute-on-chronic liver failure. World J Gastroenterol. 2014;20:8558–71.
Zhiliang N, Pengcheng Z, Yishan S, et al. Nomograms to predict the prognosis in locally advanced oral squamous cell carcinoma after curative resection. BMC Cancer. 2021;21:372.
Fangyuan G, Qianqian Z, Yao L, et al. Nomogram prediction of individual prognosis of patients with acute-on-chronic hepatitis B liver failure. Dig Liver Dis. 2019;51:425–33.
Keqing S, Yijing C, Zhuo L, et al. Development and validation of a prognostic nomogram for acute-on-chronic hepatitis B liver failure. J Gastroenterol Hepatol. 2017;32:497–505.
Wang J, Zhang Z, Yan X, et al. Albumin-Bilirubin (ALBI) as an accurate and simple prognostic score for chronic hepatitis B-related liver cirrhosis. Dig Liver Dis. 2019;51:1172–78.
Pinato DJ, Sharma R, Allara E, Yen C, Arizumi T, Kubota K. The ALBI gradeprovides objective hepatic reserve estimation across each BCLC stage of hepatocellular carcinoma. J Hepatol. 2017;66:338–46.
Johnson PJ, Berhane S, Kagebayashi C, Satomura S, Teng M, Reeves HL. Assessment of liver function in patients with hepatocellular carcinoma: a newevidence-based approach-the ALBI grade. J Clin Oncol. 2015;33:550–8.
Sarin SK, Kedarisetty CK, Abbas Z, et al. Acute-on-chronic liver failure:consensus recommendations of the Asian Pacific Association for the Study of the liver (APASL) 2014. Hepatol Int. 2014;8:453–71.
Kamath PS, Kim WR. The model for end-stage liver disease (MELD). Hepatology. 2007;45:797–805.
Biggins SW, Kim WR, Terrault NA, Saab S, Balan V, Schiano T, et al. Evidence-based incorporation of serum sodium concentration into MELD. Gastroenterology. 2006;130:1652–60.
Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–12.
Katoonizadeh A, Laleman W, Verslype C, Wilmer A, Maleux G, Roskams T, et al. Early features of acute-on-chronic alcoholic liver failure: a prospective cohortstudy. Gut. 2010;59:1561–9.
Hernaez R, Liu Y, Kramer JR, et al. Model for end-stage liver disease-sodium underestimates 90-day mortality risk in patients with acute-on-chronic liver failure. J Hepatol. 2020;73:1425–33.
Cen Li, You S, Liu H, et al. The value of the baseline MELD scores, MELD-Na scores and iMELD scores in short-term prognosis in hepatitis B virus related acute-on-chronic liver failure patients. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2014;26:539–43.
Sundaram V, Shah P, Mahmud N, et al. Patients with severe acute-on-chronic liver failure are disadvantaged by model for end-stage liver disease-based organ allocation policy. Aliment Pharmacol Ther. 2020;52:1204–13.
DiMagnoeugene P, DiMagno Matthew J. National Institute of Diabetes and Digestive and Kidney Diseases workshop on EUS and related technologies: history of EUS. Gastrointest Endosc. 2018;88:205–6.
Ewa D-S, Aleksandra K-L, Jerzy C, et al. Assessment of renal function in geriatric palliative care patients-comparison of creatinine-based estimation equations. Clin Interv Aging. 2017;12:977–83.
Bernal W. Lactate is important in determining prognosis in acute liver failure. J Heptol. 2010;53:209–10.
William B, Rajiv J, Alberto Q, et al. Acute-on-chronic liver failure. Lancet. 2015;386:1576–87.
Acknowledgements
I would like to thank everyone who participates in this study, especially my teacher, for their support and help.
Funding
This study was supported by a grant from the National Science and Technology Major Project of China (2018ZX10725506-002).
Author information
Authors and Affiliations
Contributions
SL and XZ conceived the study and performed statistical analyses; BL, YZ and XY collected the data; QL, JJ and WL edited and checked the manuscript. All of the authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Ethics Committee of the Tianjin Second People’s Hospital (2018-18). Informed consent was waived by Ethics Committee of the Tianjin Second People’s Hospital due to the study’s retrospective nature. All methods were performed in accordance with the relevant guidelines and regulations.
Consent for publication
Not applicable.
Competing interests
All authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1. Figure. S1
Decision curve analysis of the external cohort at 28-day and 90-day. A Decision curve analysis at 28-day. B Decision curve analysis at 90-day.
Additional file 2.
Figure. S2 Receiver operating characteristic curves of Nomogram 1 and Nomogram 2.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Li, S., Zhang, X., Li, Q. et al. Development and validation of the nomogram based on INR and eGFR for estimation of mortality in patients with acute-on-chronic hepatitis B liver failure. BMC Gastroenterol 21, 474 (2021). https://doi.org/10.1186/s12876-021-02054-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12876-021-02054-3