Abstract
Background
Hereditary hemochromatosis is a heterogenous group of inherited iron-overload conditions that is characterized by increased intestinal absorption and deposition in vital organs. Hepcidin is a soluble regulator that acts to attenuate both intestinal iron absorption and iron release from reticuloendothelial macrophages through internalization of ferroportin-1, an iron exporter. Ferroportin disease is hereditary hemochromatosis which is affected by SLC40A1, a gene coding ferroportin-1, and phenotypically classified into two forms (classical and nonclassical). In nonclassical form, ferroportin mutations are responsible for a gain of function with full iron export capability but insensitivity to downregulation by hepcidin. Here, we report a case of nonclassical ferroportin disease.
Case presentation
A 46-year-old Japanese man showed elevated serum iron (284 μg/dl), ferritin (1722 ng/ml), transferrin saturation ratio (91.3%), and hepcidin-25 level (139.6 ng/ml). Magnetic resonance imaging (MRI) demonstrated a marked reduction in the signal intensity of the liver in T1- and T2-weighted images. The liver histology exhibited a large amount of iron that had accumulated predominantly in hepatocytes. We identified a heterozygous 1520A > G (p.H507R) mutation in the SLC40A1 gene. Phlebotomy (400 ml at a time) was monthly performed for 3 years in this patient. Importantly, the serum hepcidin level (1.0 ng/ml) was normal when the serum ferritin level was normal and hepatic iron accumulation was remarkably reduced after 3 years of phlebotomy.
Conclusions
The present case demonstrated for the first time that there was a correlation between hepatic iron levels as measured by MRI and serum hepcidin levels through long-term phlebotomy in a patient with ferroportin disease with the p.H507R mutation of in SLC40A1.
Similar content being viewed by others
Background
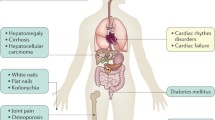
Hereditary hemochromatosis is a heterogeneous group of inherited iron overload conditions that is characterized by increased intestinal absorption and deposition in vital organs. Hereditary hemochromatosis has been clinically classified into two phenotypes. The classical form induces mainly cirrhosis, diabetes mellitus (DM), and/or skin pigmentation in middle-aged patients, while the other form, juvenile hemochromatosis, results in cardiac failure and hypogonadism before patients reach the age of 30 [1]. On the other hand, four types (types 1, 2, 3, and 4) of hemochromatosis have been genetically classified on the basis of mutations in five genes (HFE, human antimicrobial peptide [HAMP], hemojuvelin [HJV], transferrin receptor 2 [TFR2], and SLC40A1). The molecular mechanism common to all types of hereditary hemochromatosis, except type 4, fails to regulate hepcidin expression in response to cellular iron levels [2]. Hepcidin is a 25 amino-acid peptide hormone exclusively synthesized in the liver and a soluble regulator that acts to attenuate both intestinal iron absorption and iron release from reticuloendothelial macrophages [3, 4]. Hepcidin acts by triggering internalization of ferroportin-1, an iron exporter, which results in its degradation, and the trapping iron in absorptive enterocytes, macrophages, and hepatocytes [5].
Type 4 hemochromatosis, which is affected by mutations in SLC40A1, a gene coding ferroportin-1, mutations, is known as ferroportin disease [6]. The inheritance pattern is autosomal dominant. This disease is phenotypically heterogeneous with two forms (classical and nonclassical). In the classical form, the loss-of-function mutants of ferroportin prevent iron export from cells, resulting in hyperferritinemia, a normal to low transferrin saturation, and iron accumulation predominantly in reticuloendothelial cells [6]. In the nonclassical form, ferroportin mutations are responsible for a gain of function with full iron export capability but insensitivity to downregulation by hepcidin, leading to increased transferrin saturation and iron accumulation in hepatocytes in addition to hyperferritinemia [6, 7].
In this report, we present a Japanese patient with nonclassical ferroportin disease who showed a successful response to long-term phlebotomy based on the remarkable decrease in hepatic iron accumulation.
Case presentation
A 46-year-old Japanese man was referred to Kawasaki Medical School Hospital for further examination of liver dysfunction. The patient had been diagnosed with DM at approximately 35 years of age and treated with anti-DM drugs for the last 5 years. He also had continuous mild elevation of alanine aminotransferase (ALT) of unknown origin during the last 5 years. When the patient met his younger brother several months before his first visit to our department, he found out that his younger brother had been diagnosed with hemochromatosis in another hospital, and was advised to have the etiology of his liver dysfunction examined.
This patient did not have a history of alcohol abuse, blood transfusion, or medication except for anti-DM drugs. His parents had no history of chronic liver diseases, except for his mother, who has DM. The degree of his ALT elevation did not change significantly after the commencement of treatment with anti-DM drugs. His physical examination did not show skin pigmentation, arrhythmia, or hepatosplenomegaly.
Hematological examination revealed no abnormalities. Biochemical examination demonstrated mild elevation of ALT (43 U/l) and moderate elevation of fasting blood glucose (123 mg/dl) and hemoglobin A1c (6.9%). The patient was negative for hepatitis B surface antigen and anti-hepatitis C virus antibody. Importantly, the serum iron level was moderately elevated (284 μg/dl, reference range 40–188 μg/dl), and the serum ferritin (1722 ng/ml, reference range 10–240 ng/ml) and transferrin saturation ratio (91.3%) were remarkably elevated. Serum copper and ceruloplasmin levels were within the normal range. The serum hepcidin-25 level, which was measured using liquid chromatography coupled with tandem mass spectrometry, was remarkably increased (139.6 ng/ml, reference range 7.8 ± 7.0 ng/ml).
Ultrasonography demonstrated the mild hepatorenal contrast, suggesting the presence of fatty liver. Magnetic resonance imaging (MRI) showed a marked reduction in the signal intensity of the liver in T1- and T2-weighted images. Furthermore, an in-phase image of the T1-weighted image showed a greater reduction in the signal intensity than an out-of-phase image (Fig. 1a–c), suggesting hepatic iron accumulation, as evidenced in a previous report [8]. Because we suspected that liver dysfunction resulted from hereditary hemochromatosis based on the family history, elevated iron, ferritin and transferrin saturation ratio in serum, and MRI findings, we performed a liver biopsy in this patient after obtaining written consent. The liver histology exhibited mild hepatic steatosis, mild mononuclear cell infiltration (Fig. 2a), and mild to moderate periportal fibrosis (Fig. 2b). Notably, we observed a large amount of iron that had accumulated predominantly in hepatocytes in the liver biopsy specimen (Fig. 2c, d).
Magnetic resonance imaging of the liver before (2017/01) and after (2020/01) phlebotomy. a T1-weighted in-phase image before phlebotomy. b T1-weighted out-of-phase image before phlebotomy. c T2-weighted image before phlebotomy. d T1-weighted in-phase image after phlebotomy. e T1-weighted out-of-phase image after phlebotomy. f T2-weighted image after phlebotomy
In hereditary hemochromatosis (except for ferroportin disease), loss of any one of the hepcidin regulator genes (HFE, HAMP, HJV, and TFR2) attenuates or abrogates intracellular hepcidin signal transduction and hepcidin secretion [6, 9]. In contrast, hepcidin is appropriately synthesized and released in response to increased serum iron levels in ferroportin disease. We speculated that this patient might have ferroportin disease, since the serum hepcidin level (139.6 ng/ml) was increased in response to elevated serum iron levels. We scanned all the cording regions and the splicing junctions of SLC40A1, a gene coding ferroportin-1, after obtaining informed consent and having our genetic examination approved by the Kawasaki Medical School Ethics Committee. This patient had a heterozygous A > G transition at c.1520 in exon 8 (p.H507R) in the SLC40A1 gene (Fig. 3). Thus, taken together with increased hepcidin-25 level, high transferrin saturation ratio, and iron accumulation predominantly in hepatocytes, we diagnosed this patient with nonclassical ferroportin disease. Unfortunately, we could not examine mutations in SLC40A1 in his family lineage.
We initiated treatment with phlebotomy in January in 2017. Phlebotomy (400 ml at a time) was performed monthly for 3 years (Fig. 4). The serum ferritin level rapidly declined in a few months and the serum iron and transferrin saturation ratio started to decline in approximately one year after the commencement of phlebotomy, and then gradually decreased until the last phlebotomy. Serum ALT also returned to the normal range in a few months after the commencement of phlebotomy, and remained normal thereafter. The hemoglobin level did not decrease for the first 2 years after the commencement of phlebotomy and then gradually decreased until the last phlebotomy, but slightly increased after the cessation of phlebotomy (Fig. 4). MRI after 3 years of phlebotomy demonstrated a marked restoration in the signal intensity reduction in the liver in T1- and T2-weighted images compared to that before the commencement of phlebotomy (Fig. 1d-f), suggesting a marked reduction in hepatic iron accumulation by phlebotomy. Importantly, the serum hepcidin level (1.0 ng/ml) was normal when the serum ferritin level was normal and hepatic iron accumulation was remarkably reduced after 3 years of phlebotomy (Fig. 4). Serum ALT and ferritin levels have remained normal to date since cessation of phlebotomy (for 6 months).
Discussion and conclusion
A systemic meta-analysis of ferroportin disease reported that eighty of the 176 individuals with SLC40A1 mutations were classified as classical phenotype with hyperferritinemia and normal transferrin saturation, and 53 were nonclassical phenotype with hyperferritinemia and elevated transferrin saturation [10]. A patient with mutant ferroportin p.H507R who was originally reported in the United Kingdom showed hyperferritinemia, elevated transferrin saturation and iron accumulation predominantly in hepatocytes (nonclassical form) [11], which is consistent with the present case in this study. Functional analysis of mutant ferroportin p.H507R revealed that p.H507R conferred resistance to hepcidin [11]. Hepcidin binding to ferroportin causes ubiquitination, endocytosis, and degradation of the ligand-receptor complex, thereby decreasing iron supply to plasma [5, 12]. Of note, structure and function analysis of ferroportin demonstrated that nonclassical ferroportin disease was caused by ferroportin mutations that suppress hepcidin binding or hinder conformational changes required for ubiquitination and endocytosis [13]. A mutant ferroportin p.H507R has been shown to have impaired hepcidin-mediated ubiquitination activity, even though it has normal hepcidin binding activity [13]. The association of p.H507R with this phenotype is explained by mapping the residues onto computational models of the human ferroportin structure, which indicated that mutations causing ubiquitination resistance were positioned at helix–helix interfaces, likely preventing the hepcidin-induced conformational change [13]. On the other hand, a recent analysis of cryogenic electron-microscopy indicated a possibility that p.H507R disrupts hepcidin binding to ferroportin [14]. In this report two metal-binding sites within the N and C domains of ferroportin have been identified. The N domain metal site is crucial for iron efflux, whereas the C domain metal-binding site is important for hepcidin binding. The mutation of p.H507R was predicted to disrupt the precise tetrahedral coordination geometry that is required for the metal binding within the C domain [14]. Thus, it seems to be reasonable that the present patient with a mutation of p.H507R in SLC40A1 exhibited nonclassical ferroportin disease. To the best of our knowledge, based on the literature, the present case is the fifth reported patient with mutant ferroportin p.H507R and the third family lineage in the world [11, 15].
Hepcidin is expressed from the HAMP gene located on the long arm of chromosome 19 [9]. The increase in iron levels and inflammation upregulate the transcription of the HAMP gene [16,17,18,19]. HJV, HFE, TRF1, and TFR2, which are located at the surface of hepatocytes, are considered to be “iron sensors.” The HJV-hepcidin axis is the most important mechanism for the upregulation of HAMP expression during iron overload [15]. In this patient, the increased levels of both ferritin and hepcidin-25 in serum suggested that hepcidin secretion was properly regulated, which was consistent with another Japanese patient with mutant ferroportin p.H507R [15]. In addition, we have reported for the first time that serum hepcidin-25 levels can return to the normal range after reduction in hepatic iron accumulation by phlebotomy in this patient.
Phlebotomy is the standard treatment for hereditary hemochromatosis, but there are no evidence-based guidelines on the use of therapeutic phlebotomy. It has been reported that all patients with homozygous hereditary hemochromatosis and evidence of iron overload (i.e., transferrin saturation greater than 45% and serum ferritin level greater than 300 ng/ml in men and greater than 200 ng/ml in women) should be treated, regardless of symptoms [20]. Although this patient had a heterozygous A > G transition at c.1520 in exon 8 (p.H507R) in the SLC40A1 gene without any symptoms, we treated this patient with phlebotomy since iron overload (91.3% transferrin saturation ratio and 1722 ng/ml serum ferritin) was prominent. Considering that each 500 ml unit of whole blood removes 200 to 250 mg of iron and reduces serum ferritin levels by approximately 30 ng/ml [21], 400 ml of whole blood was removed monthly for 3 years in this patient. The 3-year-phlebotomy successfully alleviated hepatic iron accumulation. We discontinued phlebotomy when the patient had a hemoglobin level of 12.7 g/dl and a serum ferritin level of 16 ng/ml, because phlebotomy is advised to be withheld when the hemoglobin level is less than 12.5 g/dl [16].
In conclusion, we reported a patient with ferroportin disease with the p.H507R mutation of in SLC40A1 in whom hepatic iron accumulation was successfully alleviated by long-term phlebotomy. The present case demonstrated for the first time that there was a correlation between hepatic iron levels as measured by MRI and serum hepcidin levels through long-term phlebotomy in a patient with ferroportin disease with the p.H507R mutation of in SLC40A1.
Availability of data and materials
All data generated or analyzed for this study are included in this published article.
Abbreviations
- DM:
-
diabetes mellitus
- HAMP:
-
human antimicrobial peptide
- HJV:
-
hemojuvelin
- TFR2:
-
transferrin receptor 2
- ALT:
-
alanine aminotransferase
- MRI:
-
magnetic resonance imaging
References
Hattori A, Miyajima H, Tomosugi N, et al. Clinicopathological study of Japanese patients with genetic iron overload syndromes. Pathol Int. 2012;62:612–8.
Hino K, Harada M. Metal metabolism and liver. In: Ohira H, editor. The liver in systemic diseases. Singapore: Springer; 2016. p. 123–46.
Park CH, Valore EV, Waring AJ, et al. Hepcidin, a urinary antimicrobial peptide synthesized in the liver. J Biol Chem. 2001;276:7806–10.
Ganz T. Hepcidin, a key regulator of iron metabolism and mediator of anemia of inflammation. Blood. 2003;102:783–8.
Nemeth E, Tuttle MS, Powelson J, et al. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. 2004;306:2090–3.
Pietrangelo A. Hereditary hemochromatosis: pathogenesis, diagnosis, and treatment. Gastroenterology. 2010;139:393–408.
Sham RL, Phatak PD, West C, et al. Autosomal dominant hereditary hemochromatosis associated with a novel ferroportin mutation and unique clinical features. Blood Cells Mol Dis. 2005;34:157–61.
Lim RP, Tuvia K, Hajdu CH, et al. Quantification of hepatic iron deposition in patients with liver disease: comparison of chemical shift imaging with single-echo T2*-weighted imaging. Am J Roentgenol. 2010;194:1288–95.
Hino K, Nishina S. Liver cirrhosis with inherited liver disease: Hemochromatosis. In: Yoshiji H, Kaji K, editors. The evolving landscape of liver cirrhosis management. Singapore: Springer; 2019. p. 47–57.
Mayr R, Janecke AR, Schranz M, et al. Ferroportin disease: a systemic meta-analysis of clinical and molecular findings. J Hepatol. 2010;53:941–9.
Mayr R, Griffiths WJH, Hermann M, et al. Identification of mutations in SLC40A1 that affect ferroportin function and phenotype of human ferroportin iron overload. Gastroenterology. 2011;140:2056–63.
Qiao B, Sugianto P, Fung E, et al. Hepcidin-induced endocytosis of ferroportin is dependent on ferroportin ubiquitination. Cell Metab. 2012;15:918–24.
Aschemeyer S, Qiao B, Stefanova D, et al. Structure-function analysis of ferroportin defines the binding site and an alternative mechanism of action of hepcidin. Blood. 2018;131:899–910.
Billesbølle CB, Azumaya CM, Kretsch RC, et al. Structure of hepcidin-bound ferroportin reveals iron homeostatic mechanisms. Nature. 2020;586:807–11.
Yamakawa N, Oe K, Yukawa N, et al. A novel phenotype of a hereditary hemochromatosis type 4 with ferroportin-1 mutation, presenting with juvenile cataracts. Intern Med. 2016;55:2697–701.
Zhang AS, Gao J, Koeberl DD, et al. The role of hepatocyte hemojuvelin in the regulation of bone morphogenic protein-6 and hepcidin expression in vivo. J Biol Chem. 2010;285:16416–23.
Schmidt PJ, Toran PT, Giannetti AM, et al. The transferrin receptor modulates Hfe-dependent regulation of hepcidin expression. Cell Metab. 2008;7:205–14.
Gao J, Chen J, Kramer M, et al. Interaction of the hereditary hemochromatosis protein HFE with transferrin receptor 2 is required for transferrin-induced hepcidin expression. Cell Metab. 2009;9:217–27.
Pietrangelo A, Dierssen U, Valli L, et al. STAT3 is required for IL-6-gp130-dependent activation of hepcidin in vivo. Gastroenterology. 2007;132:294–300.
Crownover BK, Covey CJ. Hereditary hemochromatosis. Am Fam Physician. 2013;87:183–90.
Harrison SA, Bacon BR. Hereditary hemochromatosis: update for 2003. J Hepatol. 2003;38(suppl 1):S14-23.
Acknowledgements
Not applicable.
Funding
Sohji Nishina was supported by a Grant-in Aid for Scientific Research (C) (18K07923), Keisuke Hino was supported by a Grant-in Aid for Scientific Research (B) (19H03644) from the Japan Society for the Promotion of Science.
Author information
Authors and Affiliations
Contributions
SN collected the clinical data and wrote the manuscript. YT1 applied and obtained the approval for genetic examination from the Kawasaki Medical School Ethics Committee. KI and YT assessed the results of genetic examination and discussed the obtained results. YT2, AK and KK performed genetic examination. NY, KS and YH collected the clinical data. KH treated the patient and wrote the manuscript. All authors have read and approved the final manuscript. YT1 corresponding to Yasuyuki Tomiyama, and YT2 corresponding to Yasuaki Tatsumi.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Genetic examination was approved by the Kawasaki Medical School Ethics Committee and written informed consent was obtained from the patient.
Consent to publication
Written informed consent was obtained from the patients for publication of this report and any accompanying images. A copy of the written consent is available for review by the Editor of this journal.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Nishina, S., Tomiyama, Y., Ikuta, K. et al. Long-term phlebotomy successfully alleviated hepatic iron accumulation in a ferroportin disease patient with a mutation in SLC40A1: a case report. BMC Gastroenterol 21, 111 (2021). https://doi.org/10.1186/s12876-021-01674-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12876-021-01674-z