Abstract
Background
Biliary decompression can reduce symptoms and improve quality of life in patients with malignant biliary obstruction. Endoscopically placed stents have become the standard of care for biliary drainage with the aim of improving hepatic function, relieving jaundice, and reducing adverse effects of obstruction. The purpose of this study was to evaluate the performance characteristics of a newly-designed, uncovered metal biliary stent for the palliation of malignant biliary obstruction.
Methods
This post-market, prospective study included patients with biliary obstruction due to a malignant neoplasm treated with a single-type, commercially available uncovered self-expanding metal stent (SEMS). Stents were placed as clinically indicated for palliation of jaundice and to potentially facilitate neo-adjuvant chemotherapy. The main outcome measure was freedom from recurrent biliary obstruction (within the stent) requiring re-intervention within 1, 3, and 6 months of stent insertion. Secondary outcome measures included device-related adverse events and technical success of stent deployment.
Results
SEMS were placed in 113 patients (73 men; mean age, 69); a single stent was inserted in 106 patients, and 2 stents were placed in 7 patients. Forty-eight patients survived and/or completed the 6 month study protocol. Freedom from symptomatic recurrent biliary obstruction requiring re-intervention was achieved in 108 of 113 patients (95.6, 95%CI = 90.0–98.6%) at study exit for each patient. Per interval analysis yielded the absence of recurrent biliary obstruction in 99.0% of patients at 1 month (n = 99; 95%CI = 97.0–100%), 96.6% of patients at 3 months (n = 77; 95%CI = 92.7–100%), and 93.3% of patients at 6 months (n = 48; 95%CI = 86.8–99.9%). In total, only 5 patients (4.4%) were considered failures of the primary endpoint. Most of these failures (4/5) were due to stent occlusion from tumor ingrowth or overgrowth. Overall technical success rate of stent deployment was 99.2%. There were 2 cases of stent-related adverse events (1.8%). There were no cases of post-procedure stent migration, stent-related perforation, or stent-related deaths.
Conclusions
This newly designed and marketed biliary SEMS system appears to be effective at relieving biliary obstruction and preventing re-intervention within 6 months of insertion in the overwhelming majority of patients. The device has an excellent safety profile, and associated high technical success rate during deployment.
Trial registration
The study was registered on clinicaltrials.gov on 14 October 2013 and the study registration number is NCT01962168. University of Massachusetts Medical School did not participate in the study.
Similar content being viewed by others
Background
Biliary decompression can reduce symptoms and improve quality of life in patients with malignant biliary obstruction [1,2,3,4]. Resolving the obstruction may also allow for palliative chemotherapy [3]. Due to their minimal invasiveness compared to percutaneous drainage, endoscopically placed stents have become the standard of care for non-surgical biliary drainage [1, 5]. The goal of stent placement is to improve hepatic function, normalize bilirubin levels, relieve symptoms of jaundice, and reduce other adverse effects of obstruction [1, 3, 6, 7].
There are a variety of stents available and the choice of device is often based on several factors, including certainty of malignancy diagnosis, potential need for removal, expected length of survival, location of obstruction, and cost-effectiveness [2, 3, 7,8,9]. Plastic stents are less expensive than self-expanding metal stents (SEMS) and can be removed easily, if necessary [7]. However, plastic stents are known to have a high rate of occlusion and the risk of occlusion increases over time [1, 3, 5, 9,10,11,12]. While SEMS are more expensive than plastic stents, long stent patency and reduced need for re-intervention makes SEMS a more cost-effective option [1, 5, 10, 12, 13].
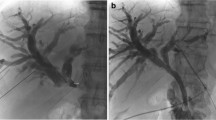
The aim of this study was to evaluate the performance of a newly-marketed, uncovered biliary stent system (Evolution® Biliary Stent System – Uncovered; Cook Ireland Ltd., Limerick, Ireland) for the palliation of malignant biliary obstruction (Fig. 1). The primary outcome measure was freedom from symptomatic recurrent biliary obstruction requiring another intervention. Secondary outcome measures included technical success of attempted stent placement, ease-of-use of the device, and device-related adverse events.
Methods
Patients
Patients who underwent biliary stent placement for the treatment of biopsy-proven, malignant biliary tree obstruction were prospectively enrolled at 8 medical centers (7 US, 1 Canada) between December 2013 and October 2014. This trial was registered with ClinicalTrials.gov under the following trial number: NCT01962168. University of Massachusetts Medical School did not participate in the study. Patients who were 18 years or older, with the ability to provide informed consent, as well as comply with the post-procedural follow-up schedule, were eligible for inclusion in the study. SEMS were placed as clinically indicated, for the purposes of palliation of jaundice and/or to facilitate expected neo-adjuvant chemotherapy.
Ethics approval
The study was approved by the institutional review boards (IRB) at each of the respective medical centers (see ‘Ethics Approval and Consent to Participate’ section below in the ‘Declaration’ for list of individual sites). All patients provided informed consent prior to participation in the study. This study was sponsored by Cook Endoscopy.
Uncovered SEMS
The uncovered SEMS (Cook Evolution® Biliary Stent – Uncovered; Cook Ireland Ltd., Limerick, Ireland) used in this study is constructed of a single, woven, nitinol wire with a radiopaque core. The stent has flanged ends to mitigate migration. The stent is available in 8 or 10 mm diameters and 4, 6, 8, and 10 cm lengths and contains 4 radiopaque markers on the delivery system to assist in deployment under fluoroscopic guidance. The stent is mounted on an inner catheter, which accepts a 0.035 in. wire guide and is restrained from expansion by an outer catheter. The stent is deployed using a controlled-release, trigger-driven delivery system. This system allows for recapturing of the SEMS prior to complete deployment if repositioning is required.
Stent placement
The placement of the SEMS was performed by experienced endoscopists during endoscopic retrograde cholangiopancreatography (ERCP) with patients under both moderate and deep sedation at the various 8 participating centers. Table 1 outlines the number of patients enrolled at each of the 8 different centers.
Follow-up assessment
Follow-up assessments were conducted at 30 days (1 month), 3 months, and 6 months post-procedure for symptoms of recurrent biliary obstruction, additional hospitalizations or procedures, and adverse events. Assessments were conducted by telephone or, if preferred by the physician and/or patient, by clinical examination.
Definition of endpoints
The primary endpoint was clinical success, defined as freedom from symptomatic recurrent biliary obstruction (within the stent) requiring re-intervention. Clinical success was assessed for each patient at the time of study exit. Study exit may be due to completion of 6 month follow-up protocol, withdrawal from the study, patient lost to follow-up, symptoms of obstruction requiring re-intervention, stent removal due to reasons other than obstruction, or patient death. Clinical failure occurred when the patient presented with symptoms of biliary obstruction that the physician considered significant enough to warrant re-intervention. If a patient refused re-intervention, the case was conservatively considered as a clinical failure. If re-intervention was performed, confirmation of complete or partial occlusion of the biliary stent was needed to be considered a failure of the primary endpoint. Cases in which the stent was found to be patent (e.g., obstruction occurred distal or proximal to the stent) were not classified as clinical failures. If a patient died without a known recurrence, they were not considered a failure of the primary endpoint.
Secondary endpoints included: 1) ease-of-use, based on physician assessment of the device after each deployment; 2) incidence of device-related adverse events, including stent migration, stent-related perforation, and stent-related death; 3) technical success, defined as successful delivery and placement of a stent at its intended location, assessed using fluoroscopy at the end of the stent placement procedure. Positional adjustments to the stent during the procedure were not considered technical failures.
Statistical analysis
A minimum sample size of 112 was calculated based on the rate of freedom from symptomatic biliary obstruction requiring re-intervention for a similar uncovered biliary stents. Data were collected and managed by a centralized data coordinating center. Statistical analyses were performed by a biomedical statistician using Statistical Analysis Software (SAS) for Windows (release 9.3 or higher). Continuous variables are reported as mean (standard deviation) unless otherwise noted and categorical variables are reported as percentages. A Clopper-Pearson exact 95% binomial confidence interval was calculated for the primary study endpoint of freedom from recurrent biliary obstruction requiring re-intervention, based on clinical success assessment for each patient at the time of study exit. Additionally, a Kaplan-Meier analysis was performed to estimate clinical success during follow-up at 30 days, 3 months, and 6 months post-procedure. The analysis accounts for patients who exited the study without a documented clinical failure and who were censored at the time of study exit.
Results
A total of 113 patients (73 [64.6%] male; mean age, 69 ± 12 years) with malignant biliary obstruction were enrolled. Thirty patients (26.5%) underwent pre-procedural or adjunctive tumor reduction therapy with systemic chemotherapy. A previous biliary sphincterotomy was noted in 28 patients (24.8%), and sphincterotomy was performed at the time of SEMS placement in 75 patients (66.4%). Prior attempts at biliary decompression of the stricture were noted in 54 patients. This included 40 patients (35.4%) with plastic biliary stents, 5 patients (4.4%) with percutaneous biliary drain placement, and 9 patients (8.0%) with balloon dilation of the stricture. The majority of patients (79, or 69.9%) had pancreatic cancer, and advanced stage disease (T3N1) at initial presentation (Table 2).
Most strictures (92, 81.4%) were completely or partially localized to the common bile duct; 96 stents (80.0%) did not extend above the biliary confluence (Table 2). The majority of stents (100, 83.3%) crossed the papilla. A single SEMS was placed in 106 patients (93.8%); 2 SEMS were placed in 7 patients (6.2%). The most common stent diameter and length used in this study were 10 mm (85.8%) and 60 mm (59.2%), respectively.
Figure 2 represents a summary of patient enrollment and retention for procedure and clinical follow-up. Over the course of the study, 13 patients (11.5%) were lost to follow-up and 1 patient (0.9%) voluntarily withdrew from the study. A total of 48 patients (42.5%) had a follow-up assessment at 6 months. Throughout the course of the study, a total of 10 patients (9 + 1, or total of 8.8%) developed symptomatic recurrent biliary obstruction. In addition to the 9 patients, one patient exited the study due to death but developed biliary obstruction prior to dying. Of the 9 patients with recurrent biliary obstruction, only 5 patients underwent re-intervention (see Clinical Success below).
Clinical success
The primary study endpoint, clinical success, defined as freedom from symptomatic recurrent biliary obstruction (within the stent) requiring re-intervention, was achieved in 108 of 113 patients (95.6, 95%CI = 90.0–98.6%) at study exit for each patient. Kaplan-Meier analysis was performed to assess clinical success during follow-up (Fig. 3), which indicated freedom from obstruction requiring re-intervention in 99.0% ± 1.0% at 1 month (95%CI = 97.0–100%; n = 99 remaining patients), 96.6% ± 1.9% at 3 months (95%CI = 92.7–100%; n = 77 remaining patients), and 93.3% ± 3.0% at 6 months (95%CI = 86.8–99.9%; n = 48 remaining patients). As noted, 10 patients presented with symptoms of recurrent biliary obstruction over the course of the study; however, only 5 cases (4.4%) were considered failures of the primary endpoint. The majority of these failures (4/5) were due to stent occlusion from tumor ingrowth and/or overgrowth. The other 5 cases not considered failures were due to obstruction either proximal (n = 4) or distal (n = 1) to the existing metal stent, but not involving the actual stent. This was due to biliary stones or sludge, food debris, or tumor in some cases.
Technical success and device performance
Technical success was achieved in 112/113 patients (99.1%, CI 95% = 95.1–100.0%) and in 119/120 stent placements (99.2%). The single failure was due to difficulty removing the delivery system from a tight stricture, resulting in distal stent migration (1 cm) during the procedure. As a result, a non-study stent was placed inside the SEMS to ensure adequate stent coverage in the duct.
Ease of stent deployment was rated as ‘easy’ (53/120) or ‘very easy’ (57/120) in the majority of cases (91.7%). The deployment of 3 stents, placed in 2 patients, was rated as ‘difficult’ (1/120, 0.8%) or ‘very difficult’ (2/120, 1.7%). One of these patients had a moderately tortuous stricture at the confluence of the left and right main intrahepatic ducts, and 2 stents were placed. In all 3 cases deployment was successful. In addition, fluoroscopic visualization of the SEMS was rated as ‘easy’ (n = 25) or ‘very easy’ (n = 87) for 112 stents (93.3%). Stent recapture was required during 6 deployments (5.0, 95%CI = 1.9–10.6%); 1 stent was recaptured twice. Recapture was rated as ‘very easy’ in all cases and the stents were successfully deployed.
Adverse events and deaths
Only two patients (1.8, 95%CI = 0.4–10.1%) had device-related adverse events, which were classified by the physician as probably or definitely related to the device deployment. In both cases, the device-related adverse event resulted in biliary obstruction requiring re-intervention before 6 months’ time. Both patients were successfully treated with the placement of a non-study stent. The remaining adverse events were not stent-related; and they were categorized as gastrointestinal (GI) in 18 patients, cardiovascular in 3 patients, and miscellaneous in 32 patients. In addition, 7 patients underwent surgical removal of the study stent via pancreatoduodenectomy. There were no cases of post-procedure stent migration or stent-related perforation. There were a total of 38 deaths over the course of the study. None of these deaths were stent-related.
Discussion
As we are faced with the rising incidence of pancreatic cancer and other malignancies of the upper gastrointestinal tract, the role of non-surgical endoscopic techniques for managing pancreatico-biliary disorders is expanding. As a result, available technologies which enable the endoscopist to intervene in this setting are also growing. The growth in industry and technology, however, does not always parallel the availability of appropriately-designed scientific studies of new devices used in critical patient care settings. In this study, we prospectively evaluated the basic performance characteristics and safety of a newly-designed uncovered biliary SEMS used in the palliation of malignant biliary obstruction. We not only evaluated device-specific endpoints, but we critically evaluated patient-specific outcomes with recurrent biliary obstruction mandating re-intervention as our primary endpoint.
It has long been known that SEMS provide more optimal patency than plastic biliary stents. A recent meta-analysis performed by Sawas and colleagues [10] on the comparison of plastic versus metal biliary stents found a 30-day occlusion rate of 3% for SEMS, and a long-term occlusion rate of 27% for SEMS. In the present study, the overall stent occlusion rate at 6 months was 4.4%. Tumor ingrowth was the cause of occlusion in nearly all of these patients. This is not unexpected when utilizing uncovered SEMS that have open interstices. Furthermore, there were no cases of post-procedure stent migration reported, likely related to the open mesh design enabling the stent to embed itself into the bile duct wall.
The technical success rate of 99.2% achieved in this study is comparable to that observed with other SEMS. Meta-analyses investigating other types of SEMS found an overall technical success rate ranging from 94.6 to 98.8%, [1, 10, 14], and 88.3% with plastic stents [1]. The higher technical success rate of SEMS is likely due to their expandability, facilitating placement in tight strictures while using endoscopes with relatively small working channels [7]. Here, of the 23 stents (19.2%) placed in strictures rated as ‘moderately’ or ‘severely tortuous’ by the treating endoscopists, only one stent was unsuccessfully deployed. This single failure was due to the removal of the delivery system from a tight stricture, resulting in distal stent migration during the procedure.
Overall, ease-of-use was rated extremely favorably by the physicians who performed the procedures in this study. We suspect that much of this positive rating is related to the lack of stent foreshortening while using this device. In general, the low foreshortening rate associated with uncovered SEMS is suggested to make deployment easier compared to covered stents [9]. Dumonceau and colleagues [7] suggest that SEMS with a low foreshortening ratio are superior in cases of long, tight strictures; but they may be associated with a jerky deployment. Deployment with the current device appeared devoid of erratic or uncontrolled release from the delivery system, likely because of its incremental trigger-release system which allows a smoother exit from the catheter.
Despite some of these favorable outcomes noted in our study, it is not without its limitations. Studying patients with advanced malignancies of the upper gastrointestinal tract is frequently challenged by the fact that patients often succumb to their disease process prior to reaching the desired endpoints of the study. This is clearly the case here, as approximately one-third of the patients died prior to reaching the 6 month post-stent follow-up analysis. Most of these patients (81.6%) died of their underlying malignancy. When coupled with a relatively high rate of patients lost to follow-up (14/113, or 12.4%), the number of patients available for analysis is limited, which may reduce the accuracy of the endpoint estimate. Furthermore, additional clinical characteristics of the enrolled patients, such as performance status, comorbidity indices, and duration of stent/drain placement prior to SEMS, were not collected. These important parameters may have an effect on procedure-related complications, likelihood of palliation of jaundice, and overall survival; which, in turn, may affect recurrent obstruction and the need for re-intervention.
Conclusions
In summary, the recently designed and marketed biliary SEMS system (Cook Evolution® Biliary Stent – Uncovered; Cook Ireland Ltd., Limerick, Ireland) appears to be effective at relieving biliary obstruction and preventing re-intervention within 6 months of insertion in the overwhelming majority of patients. The device has an excellent safety profile, and possess a high technical success rate during deployment in a variety of types of biliary strictures.
Availability of data and materials
Data underlying the results reported in this article are available upon reasonable request from the corresponding author, immediately after publication and ending 5 years after publication, subject to review and approval by the study sponsor. Interested researchers may review the “Cook Research Incorporated Policy on Access to Clinical Study Data” at https://www.cookresearchinc.com/extranet/data-access.html and submit a complete research proposal to request data access.
Abbreviations
- CI:
-
Confidence interval
- ERCP:
-
Endoscopic retrograde cholangiopancreatography
- GI:
-
Gastrointestinal
- SEMS:
-
Self-expanding metal stent
References
Liberato MJ, Canena JM. Endoscopic stenting for hilar cholangiocarcinoma: efficacy of unilateral and bilateral placement of plastic and metal stents in a retrospective review of 480 patients. BMC Gastroenterol. 2012;12:103.
Webb K, Saunders M. Endoscopic management of malignant bile duct strictures. Gastrointest Endosc Clin N Am. 2013;23(2):313–31.
Boulay BR, Parepally M. Managing malignant biliary obstruction in pancreas cancer: choosing the appropriate strategy. World J Gastroenterol. 2014;20(28):9345–53.
van den Bosch RP, van der Schelling GP, Klinkenbijl JH, Mulder PG, van Blankenstein M, Jeekel J. Guidelines for the application of surgery and endoprostheses in the palliation of obstructive jaundice in advanced cancer of the pancreas. Ann Surg. 1994;219(1):18–24.
Kaw M, Singh S, Gagneja H. Clinical outcome of simultaneous self-expandable metal stents for palliation of malignant biliary and duodenal obstruction. Surg Endosc. 2003;17(3):457–61.
Hsieh J, Thosani A, Grunwald M, Nagula S, Bucobo JC, Buscaglia JM. Serial insertion of bilateral uncovered metal stents for malignant hilar obstruction using an 8 Fr biliary system: a case series of 17 consecutive patients. Hepatobiliary Surg Nutr. 2015;4(5):348–53.
Dumonceau JM, Heresbach D, Devière J, Costamagna G, Beilenhoff U, Riphaus A. Biliary stents: models and methods for endoscopic stenting: European Society of Gastrointestinal Endoscopy (ESGE) technology review. Endoscopy. 2011;43(7):617–26.
Tsetis D, Krokidis M, Negru D, Prassopoulos P. Malignant biliary obstruction: the current role of interventional radiology. Ann Gastroenterol. 2016;29(1):33–6.
Itoi T, Sofuni A, Itokawa F, Tonozuka R, Ishii K. Current status and issues regarding biliary stenting in unresectable biliary obstruction. Dig Endosc. 2013;25(Suppl 2):63–70.
Sawas T, Al Halabi S, Parsi MA, Vargo JJ. Self-expandable metal stents versus plastic stents for malignant biliary obstruction: a meta-analysis. Gastrointest Endosc. 2015;82(2):256–67 e7.
Hong WD, Chen XW, Wu WZ, Zhu QH, Chen XR. Metal versus plastic stents for malignant biliary obstruction: an update meta-analysis. Clin Res Hepatol Gastroenterol. 2013;37(5):496–500.
Pfau PR, Pleskow DK, Banerjee S, Barth BA, Bhat YM, Desilets DJ, et al. Pancreatic and biliary stents. Gastrointest Endosc. 2013;77(3):319–27.
Yoon WJ, Ryu JK, Yang KY, Paik WH, Lee JK, Woo SM, et al. A comparison of metal and plastic stents for the relief of jaundice in unresectable malignant biliary obstruction in Korea: an emphasis on cost-effectiveness in a country with a low ERCP cost. Gastrointest Endosc. 2009;70(2):284–9.
Moss AC, Morris E, Leyden J, MacMathuna P. Malignant distal biliary obstruction: a systematic review and meta-analysis of endoscopic and surgical bypass results. Cancer Treat Rev. 2007;33(2):213–21.
Acknowledgements
The authors thank the research staff and the nursing staff for supporting this study and coordinating data collection.
Ethics approval for consent to participate
The study was approved by the institutional review boards (IRB) at each of the respective medical centers. All patients provided written informed consent. The list of participating IRB sites are as follows: Aurora Health Care Research Subject Protection Program, Baptist Medical Center Downtown IRB, CHUM Research Ethics Board, Florida Hospital IRB, Mobile Infirmary IRB, Northwest Community Hospital IRB, Schulman IRB, and Chesapeake IRB.
Funding
This study was sponsored by Cook Endoscopy. Funding was granted to each institution to support data collection and facilitate patient follow-up visits. The stents used in this study were not paid for by Cook Endoscopy. No physician researcher and/or co-author of this manuscript was paid or provided honoraria by Cook Endoscopy for participating and completing this study. Cook Research Incorporated was involved in the study design, as well as collection, analysis, and interpretation of the data. Cook Research Incorporated also contributed to manuscript preparation, and all employees who contributed in this manner are reflected as authors, or in the acknowledgements.
Author information
Authors and Affiliations
Consortia
Contributions
J.M.B., J.N., W.G.P., A.R., N.M.G., S.E.S., M.K.H., J.C.B., S.N., and C.L. performed research and made critical revisions; N.D.D. interpreted the data and drafted the article. All authors provided final approval of the version of the article to be published.
Corresponding author
Ethics declarations
Consent for publication
Not applicable.
Competing interests
Author J.M.B. is a speaker for AbbVie and Boston Scientific, author W.G.P. is a consultant for Cook Medical and Medtronic, author N.M.G. is a consultant for Boston Scientific, author M.K.H. is a consultant for Boston Scientific, J.C.B. is a consultant for Boston Scientific, and author N.D.D. is a paid employee of Cook Research Incorporated., a contract research organization and Cook Group Company. Authors J.N., A.R., S.E.S., S.N., and C.L. declare no conflicts of interests for this article.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Lawrence, C., Nieto, J., Parsons, W.G. et al. A newly designed uncovered biliary stent for palliation of malignant obstruction: results of a prospective study. BMC Gastroenterol 20, 184 (2020). https://doi.org/10.1186/s12876-020-01325-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12876-020-01325-9