Abstract
Background
Whether gastrointestinal motor and sensory function is primary cause or secondary effect of abnormal body weight is uncertain. Moreover, studies relating continuous postprandial sensations of satiation to measurable pathology are scarce. This work assessed postprandial gastrointestinal function and concurrent sensations of satiation across a wide range of body weight and after weight change.
Methods
Patients with anorexia nervosa (AN) and obesity (OB) were investigated in reference to normal weight controls (HC). AN were additionally investigated longitudinally. Gastric emptying, antral contractions and oro-cecal transit after ingestion of a solid meal were investigated by MRI and 13C-lactose-ureide breath test. The dependency of self-reported sensations of satiation on the varying degree of stomach filling during gastric emptying was compared between groups.
Results
24 AN (BMI 14.4 (11.9–16.0) kg/m2), 16 OB (34.9 (29.6–41.5) kg/m2) and 20 HC (21.9 (18.9–24.9) kg/m2) were studied. Gastric half-emptying time (t50) was slower in AN than HC (p = 0.016) and OB (p = 0.007), and a negative association between t50 and BMI was observed between BMI 12 and 25 kg/m2 (p = 0.007). Antral contractions and oro-cecal transit were not different. For any given gastric content volume, self-reported postprandial fullness was greater in AN than in HC or OB (p < 0.001). After weight rehabilitation, t50 in AN tended to become shorter (p = 0.09) and postprandial fullness was less marked (p < 0.01).
Conclusions
A relationship between body weight and gastric emptying as well as self-reported feelings of satiation is present. AN have slower gastric emptying and heightened visceral perception compared to HC and OB. Longitudinal follow-up after weight rehabilitation in AN suggests these abnormalities are not a primary feature, but secondary to other factors that determine abnormal body weight.
Trial registration
Registered July 20, 2009 at ClinicalTrials.gov (NCT00946816).
Similar content being viewed by others
Background
Clinically relevant over- and underweight states have been attributed to genetic, psychological and environmental factors that influence food intake [1–4]. The central perception of satiation and satiety regulates food intake and is modulated by biophysical and neurohormonal feedback mechanisms originating from the gastrointestinal (GI) tract [5–8]. By regulating digestive function, these signals link central perceptions with GI motility [9]. However, observational and interventional trials studying the interrelation between GI motor and postprandial sensory function with body weight have not provided definitive findings. Previous studies have focused on either under- or overweight patients. In addition, the interpretation of published data is difficult because the technologies applied to assess GI function (e.g., γ-scintigraphy, 13C-breath test) varied greatly between studies. Moreover, the relation of self-reported postprandial sensations of satiation and stomach volumes with body weight has not been demonstrated without invasive methods.
Individuals with morbid obesity have a delayed postprandial onset of satiation and less fullness [10]. Some authors suggest that this is related to relatively rapid gastric emptying compared to normal weight controls, but continuous assessments of sensations in the postprandial period are missing [11–15]. Moreover, observations varied between studies [16–21], so the role of altered gastric emptying and postprandial sensations in obesity remains controversial.
At the other extreme, many morbidly underweight patients with anorexia nervosa complain of prolonged fullness, bloating and nausea after meals [22]. These sensations have been linked to abnormal stomach function [23–26] – in particular, prolonged gastric emptying [25, 27–32]. However, the association between slow solid gastric emptying and clinically relevant postprandial symptoms is weak [33, 34] and it is not known whether this abnormality restricts meal consumption in this patient group.
This study aimed to clarify the relationship of postprandial GI motor and sensory function with body weight. The specific hypothesis tested was that the “GI response to feeding varies inversely with body weight” such that gastric emptying and oro-cecal transit increase and postprandial sensations of satiation decrease from under- to overweight. If present, then these findings would support the idea that the neurohormonal response to feeding is heightened in underweight and weakened in overweight patients and provide a physiological basis for the peripheral GI control of body weight (mediated by food intake). To this end, GI motor and sensory function in participants across a wide range of body weight including anorexic, normal weight and obese participants were investigated. In order to further test whether GI motor and sensory function are a primary cause or a secondary effect of abnormal body weight the participants with abnormal body weight were intended to be tested before and after weight rehabilitation.
Methods
Participants
Anorexia nervosa (AN) patients met DSM-IV criteria [35] and had a BMI <15.5 kg/m2 at inclusion. AN were recruited from an inpatient psychotherapy unit for patients with severe eating disorders of the Psychiatric Department of the University Hospital. After a somatic and psychiatric stabilization period at the Eating Disorders Centre of the Clinic for Psychiatry and Psychotherapy within the first 4 weeks following admission, the participants should regularly gain approximately 1 kg of weight per week. This was attained by interdisciplinary multimodal inpatients therapy, consisting of assisted meals, group and individual psychotherapy, body-perception therapy, creativity therapy, nutritional counselling and other therapies. Recruitment and visit 1 occurred within the first 2 to 4 weeks of the program (orientation phase), before patients start to gain weight. Visit 2 (group AN2) occurred after AN patients attained BMI >17.5 kg/m2. Normal weight, healthy controls (HC) with BMI 18.5–24.9 kg/m2 were recruited via public announcements. Obese participants (OB) with BMI >30 kg/m2 were recruited via the outpatient clinic of the Endocrinology Department of the University Hospital. Visit 2 was planned after patients lost >5% of body weight. Exclusion criteria were age <18 years and >60 years; history of gastrointestinal, cardiorespiratory (including hypertension), hematologic, renal or atopic disorders, diabetes, drug or alcohol abuse; abdominal surgery; regular intake of medication altering gut function (e.g. anticholinergics, laxatives, proton-pump inhibitors); presence of metallic foreign bodies interacting with magnetic resonance imaging (MRI); claustrophobia; body dimensions too large to fit into MRI scanner; pregnancy and lactation.
Study design
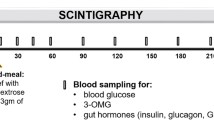
At the screening visits for the cross-sectional and longitudinal comparison, participants meeting the inclusion criteria completed the short-form Leeds Dyspepsia Questionnaire (LDQ), Gastroparesis Cardinal Symptom Index (GCSI), Beck Depression Inventory II (BDI) and the State-Trait-Anxiety Inventory (STAI). Participants fasted for minimum eight hours and refrained from smoking prior to study days. On study days, women were tested for pregnancy by urine analysis, normogylcaemia was confirmed. Participants were not allowed to eat or drink apart from the offered food and beverages. As outlined in Fig. 1, following baseline measurements the muffin test meal (430 kcal, 21% fat, 63% carbohydrate, 16% protein) and the 13C-breath test marker were eaten together with 200 ml of tap water while seated on the MRI scanner table. After ingestion (time t = 0 min), participants were placed in supine position inside the scanner and postprandial gastric volumes, antral motility, breath samples and sensation scores were acquired at regular time intervals (ref. Fig. 1). After 240 min, participants had to eat another unlabelled muffin meal. After 360 min, an ad libitum buffet was provided consisting of water, tomato soup, cheese, apples, chocolate, crackers and butter.
Magnetic resonance imaging
MRI was performed in a 1.5 T whole-body system (1.5 T Achieva; Philips Healthcare, The Netherlands). Gastric volume was derived from 30 axial MRI image planes covering the gastric region, performed during a single breath hold. Gastric content volume (GCV) was semi-automatically segmented at each time point using a custom designed image analysis software developed for MATLAB 7.4 (MathWorks Inc., Natick, MA, USA) [36]. Gastric motility scans consisted of 70 consecutive dynamic scans covering the gastric antrum and pylorus and recorded over 100 s during free-breathing [37].
13C-Lactose-ureide breath test
Oro-cecal transit time (OCTT) was assessed by 13C-lactose-ureide (13C-LU) (Euriso-Top, Saint-Aubin Cedex, France) breath test after priming of the colonic bacterial flora with five 100 milligram doses of unlabelled lactose-ureide the day prior to the study (validated by authors). On the study day 500 mg 13C-lactose-ureide blended into 2 grams of butter on 1x1 cm white bread was ingested immediately before the muffin meal [38]. Breath samples were collected into aluminized bags and analysed by non-dispersive isotope selective infrared spectroscopy (NDIRS, IRIS, Wagner Analysen Technik, Bremen, Germany). The increase of 13CO2 concentration in the exhaled air referenced to the baseline value (delta over baseline, DOB) was used for data analyses.
Sensation scores
Participants were asked to rate their sensations of hunger, fullness, nausea, bloating, abdominal pain, desire to eat and amount desired to eat on a scale from 0 (zero, no sensation) to 10 (ten, maximal possible sensation) as described previously [39, 40].
Data analyses and statistics
Group size and power calculations were based on the primary study outcome measurement: half-time of gastric emptying (t50). Published MRI data reported a mean difference of 29 min in t50 between a solid and a liquid test meal in 8 healthy participants, which was used as a clinically relevant effect size [41]. To detect the same difference with α = 0.05 and power 90%, the required group size was estimated to be 16. Data plots and statistical analyses were performed with the software R, version 2.13.1 (R Foundation for Statistical Computing, Vienna, Austria).
MRI gastric content emptying curves were fitted by a power-exponential function to derive t50 [42]. To compensate for heteroscedasticity (reduction of the number of outliers and homogenization of variances), values of t50 were log-transformed for linear model analysis. Differences in log(t50) between groups are given as ratios (with confidence intervals). Gastric motility data were analyzed by a linear mixed-effect model.
OCTT was determined from the DOB-versus-time plots by a web-based consensus application (mean from four separate raters). Results were censored at OCTT >480 min. Analysis was based on Kaplan-Meier estimates and log-rank (Mantel-Haenszel) tests.
LDQ and GCSI were analyzed as previously described [43, 44]. The LDQ asks for the frequency and severity of indigestion, heartburn, regurgitation and nausea within the preceding 2 months. Each item scores 0 to 4, and the sum (0 to 32) indicates severity of dyspeptic symptoms. The GCSI scores 0 to 5 for nausea, retching, vomiting, stomach and excessive fullness, inability to finish a meal, loss of appetite, bloating, and abdominal distention. A mean score of <3 indicates mild, a score of 3.0 to 3.9 moderate and a score of ≥4.0 severe gastroparesis symptoms [45]. BDI and STAI were analyzed according to the German manuals [46, 47]. The BDI evaluates self-reported depression symptoms using 21 questions. Each answer scores between 0 and 3, and the sum of scores indicates depressive symptoms (≤12: no depression symptoms, 13–19: mild symptoms, 20–29 moderate symptoms, ≥30 severe symptoms). The STAI uses 20 questions to evaluate “state” anxiety (temporary emotion) and 20 questions to evaluate “trait” anxiety (anxious personality). Both parts are interpreted separately, and each answer scores between 1 and 4. The higher the score, the higher the anxiety. Differences in distributions of questionnaire scores and demographic data between the groups were analyzed by Kruskal-Wallis test. Healthy controls were expected to have the lowest scores.
Differences in initial group-wise sensation scores were tested with a bootstrapped median test because of the highly skewed distribution (package pairwiseCI for program R) [48]. To analyze the dependency of sensations of satiation on gastric content volume, the cumulative link mixed model package ordinal and function clmm2 for the program R were applied. Linear fits were computed to the cumulative logits (ordinal logistic regression) of hunger and fullness scores with group as fixed effect [49]. This was followed by a logistic transformation in order to obtain the cumulative probability that a participant chooses a defined satiation score.
All tests based on linear models were corrected for 3-fold multiple testing using the Tukey method. Computed data are given as mean and 95% confidence interval (CI) unless otherwise stated.
Results
Demographics and descriptive statistics
The study was conducted between October 2010 and July 2012. Demographic data of the study population included in the cross-sectional comparison are given in Table 1. The majority of patients in all groups were female. The age in the obese participant group was wider spread (p < 0.001). The distributions of dyspepsia, anxiety and depression scores (ref. Table 1) were different between groups (all p < 0.01).
12 of 24 AN patients included in the cross-sectional study were re-investigated after 112 (69 to 161) days of weight rehabilitation. These AN2 patients achieved a mean BMI increase of 3.4 (2.7 to 4.1) kg/m2 to a mean BMI of 18.1 kg/m2. The individual changes between visits for dyspepsia, anxiety and depression are displayed in Additional file 1: Figure S1. No participant with OB lost sufficient weight to meet the criteria for re-investigation during the study period.
Gastrointestinal motor function
GI motor function was determined by MRI (assessment of gastric emptying and antral contraction frequency) and 13C-lactose-ureide breath test (oro-cecal transit). Respective t50 values were: AN 138.7 (121.3 to 158.5) minutes, HC 110 (94.9 to 127.4) minutes and OB 105.5 (89.4 to 124.5) minutes. The ratio of log-transformed t50 data showed slower gastric emptying in AN patients compared to HC and OB participants (HC:AN = 0.8 (0.7 to 1), p = 0.016; OB:AN = 0.8 (0.6 to 0.9), p = 0.007 and OB:HC = 1.0 (0.8 to 1.2), p = 0.89). A correlation between body weight and gastric emptying was observed up to a BMI of 25 kg/m2 (p = 0.0068), but not at higher values (Fig. 2). All groups had similar mean antral contraction frequencies (~3/min; p > 0.3).
Oro-cecal transit time showed a tendency to decrease with increasing body weight, but median values were statistically not different: AN = 346 (294 to NA) minutes, HC = 308 (277 to 430) minutes and OB = 280 (266 to 401) minutes (log-rank test over all groups, p = 0.564).
In the longitudinal study, after weight rehabilitation in AN, gastric emptying time slightly decreased. The average t50 was 134.3 (109.9 to 148.4) and 121.5 (99.5 to 134.3) minutes for AN1 and AN2, respectively. The ratio of log-transformed values of t50 was 0.90 (0.82 to 1.00), p = 0.087. Mean antral contraction frequency did not change. For AN1 it was 2.7 (2.5 to 3)/min and for AN2 2.8 (2.6 to 3)/min, p = 0.45.
Gastrointestinal sensory function
GI sensory function was determined by self-reported sensation scores and food intake at an ad libitum buffet. Postprandial sensations of nausea, bloating and abdominal pain were rarely reported after the test meal and were not different between groups. The dependency of self-reported sensations of fullness and hunger on the varying postprandial gastric content volume is displayed in Fig. 3. Fullness was maximal immediately after food intake (Fig. 3a and c). Postprandial fullness was greater in AN patients than in HC and OB participants at any given volume (p < 0.001). For example, all AN patients still rated fullness > zero when gastric content volume was 200 ml, whereas median fullness in HC dropped to zero between gastric content volume 300 to 250 ml. In contrast, OB participants occasionally rated fullness as zero even at maximal gastric volume after the meal.
Sensation scores for hunger and fullness in relation to gastric content volume. The plots display the probability (y-axis) that participants report fullness or hunger higher than a stated threshold (indicated above each panel) at a given gastric content volume (x-axis). The maximal postprandial content volume after the meal is plotted at the right of the x-axis. Compared to HC, the sensation of fullness reported by AN patients was significantly shifted to the left (i.e., increased visceral sensation) and for OB the relationship was shifted to the right (i.e., decreased visceral sensation). This is illustrated for fullness scores > zero (a) and > two (c). Conversely, at any given volume, the sensation of hunger reported by HC and OB was significantly greater than that reported by AN patients. This is illustrated for hunger scores > zero (b) and > two (d). The 12 AN patients included in the longitudinal analysis sensed less fullness (e) and more hunger (f) after the weight rehabilitation program. 1 = AN at visit 1, 2 = AN at visit 2
The sensation of hunger was reported by all groups when postprandial gastric content volume decreased below 300 to 250 ml (ref. Fig. 3b and d). In AN patients, hunger increased more slowly as gastric emptying proceeded than in HC and OB participants (p < 0.04).
After weight gain, sensation scores in AN2 for both fullness and hunger were shifted towards larger gastric content volumes (p < 0.001), i.e. less abnormal values (ref. Fig. 3e and f).
Satiety was measured by ad libitum food intake, with higher satiety corresponding to lower food intake. AN ate less than HC and OB; however, there was no difference between OB and HC (HC-AN: 376 (178 to 574) kcal, p < 0.001; OB-AN: 511 (292 to 731) kcal, p < 0.001; OB-HC: 135 (-90 to 361) kcal, p = 0.33). AN preferred to eat carbohydrates, while HC and OB ate comparable amounts of fat and carbohydrates (detailed data on file). After weight rehabilitation, AN2 patients increased food intake by 6.1% (p = 0.034). The composition of food ingested remained unchanged.
Discussion
Postprandial gastrointestinal (GI) motor and sensory function was investigated across a wide spectrum of body weight. A relationship was detected between body weight and gastric emptying such that anorexic patients (AN) had slower gastric emptying than healthy controls (HC) or obese participants (OB). The dependency of postprandial sensations of satiation on the changing gastric content volume during emptying was extracted and displayed using an ordinal logistic regression approach based on the concurrent and continuous MRI volume data and sensation ratings. For any postprandial gastric content volume, AN reported markedly more fullness and less hunger compared to HC and OB participants. Taken together, these findings confirmed the hypothesis that GI response to feeding varies inversely with body weight. The additional longitudinal follow-up in anorexic patients demonstrated that both gastric emptying and sensations can become less abnormal following weight rehabilitation.
Gastrointestinal motor function and body weight
A relationship between gastric emptying and body weight was present in the BMI range from 12 to 25 kg/m2, corresponding to AN patients and HCs. These findings are consistent with previous studies that often reported delayed gastric emptying for AN, but similar emptying in OB compared to HC [16–19, 25, 27–31, 50–52]. Interestingly, no difference in antral contraction frequency was present between groups. In this regard, it can be inferred that relatively slow gastric emptying in AN patients is not due to impaired breakdown (grinding) of the solid test meal by antral contraction waves, but due to other factors such as enhanced nutrient feedback from the small intestine. The oro-cecal transit time (OCTT) documented by 13C-lactose-ureide breath test followed the same pattern as gastric emptying, i.e., it decreased with increasing BMI from AN to HC and OB. However, no statistical difference between groups was detected, due to high variability of OCTT. The latter might result from an undiagnosed small intestinal bacterial overgrowth (not tested during screening) that would result in a premature rise of 13CO2 in the exhaled air. Previous studies have also reported normal or prolonged OCTT in AN [53, 54] with no consistent abnormality in OB patients [16, 55, 56]. The rate of gastric emptying and OCTT is thought to be modulated by secretion of GI peptide hormones (e.g. CCK, GLP-1, PYY) in response to nutrient sensing in the small bowel (termed “the ileal brake”) [16, 29, 57–59]. The relevance of the major GI peptide hormones for gastric emptying and satiation in the diseased state, i.e., AN and OB compared to HC, will be evaluated by state-of-the-art multivariate modelling. Since this adds sizeable complexity to the data analyses, it was considered beyond the focus of this work. Besides any direct effects of GI peptide hormones, the differences in GI motor function for AN may also be explained by different dietary habits at study entry. As AN patients notoriously avoid fatty food (also observed during ad libitum buffet), it can be assumed that the muffin represents a high-fat meal for this group [60, 61]. Acute fat consumption following a fat-restricted diet has been shown to prolong gastric emptying in lean and obese humans [62–64]. Conversely, with chronic high-fat diet the gastric emptying rate increases over time [65, 66]. These effects are thought to be due to up- and down-regulation of intraluminal fat digestion and mucosal fat sensing, respectively, impacting on the neurohormonal GI response (“ileal brake”) [66–68]. However, here the relationship of GI motility and nutritional habits remains speculative, as this study did not control for long-term food intake. The influence of female sex steroid hormones on gastric emptying might add to the observed differences in gastric emptying between the groups. While some studies could not find differences between the follicular and luteal phase of the menstrual cycle in women, other studies showed slower gastric emptying in premenopausal women investigated in the follicular phase and postmenopausal women taking hormone replacement [69–71]. This was accompanied by reduced contractility in the gastric antrum [71]. Here, the study days were not timed with the menstrual cycle of the female participants. However, a difference in antral contractions was not observed between groups. Furthermore, AN participants were amenorrheal and the majority took oral contraceptives. If this has the same effect on gastric emptying as hormone replacement in postmenopausal women has not yet been investigated.
In the longitudinal investigation of anorexic patients, a trend to faster gastric emptying after successful weight rehabilitation was observed. It should be noted that AN2 patients were still underweight (average BMI of 18.1 kg/m2) and that this effect might have been more distinct with more pronounced increase in BMI. This finding is consistent with previous studies in AN with comparable follow-up time and BMI at follow-up [25, 27, 29, 50, 72]. These investigations found accelerated gastric emptying particularly in the restrictive type of anorexia nervosa, while gastric emptying in the purging type remained unchanged (here 9 of 12 patients that gained weight were classified as restrictive type AN at study entry). While AN patients at visit 1 had a restrictive eating behaviour, all AN2 patients were close to discharge and ate regularly a balanced, ca. 2400 kcal diet. Thus, at re-investigation the test meal was more similar to the habitual diet than at study entry, thereby contributing to a more normal gastric emptying. Psychological comorbidities, like anxiety or major depression, can additionally influence gastrointestinal motility [73]. This was addressed in a covariable analysis with data from 8 AN patients with complete data sets for t50, BDI and STAI for both study visits (Additional file 1: Figure S1). None of the tests with this small data set showed evidence for an effect of STAI and BDI on the estimated differences in t50. Recruitment of constitutionally thin and overweight individuals with comparable dietary habits and without psychiatric comorbidities could possibly overcome such confounders [74].
Whereas 50% of the AN patients attained the pre-defined weight change required for re-investigation, this was not achieved by any of the OB patients. This could have several reasons. First, the out-patient consultations of OB did not include behavioural and psychotherapy. As OB are less persistent and self-directive than AN patients [75, 76], this could have contributed to the failure of the weight rehabilitation program. Second, during the study period bariatric surgery became a health insurance paid treatment for BMI ≥35 kg/m2, which might have reduced the motivation in some OB participants to lose weight conventionally [77].
Taken together, the results of the longitudinal study suggest that abnormal gastric emptying is not a primary feature of underweight, but more likely secondary to other factors (e.g. eating habits, diet, psychiatric comorbidities). However, the lack of control for these factors does not allow for discrimination between these possibilities.
Gastrointestinal sensory function and body weight
Important differences in self-reported postprandial sensations of satiation were present between the three study groups. By combining continuous MRI volume data with postprandial sensation ratings and using an ordinal logistic regression approach, fullness and hunger ratings could directly be related to the degree of gastric filling after intake of the ~400 ml muffin test meal (Fig. 3a-d). Previous MRI studies described a linear relationship between fullness and gastric distention in healthy participants and patients with functional dyspepsia [78, 79]. The presented approach allows for a more detailed observation of this relationship without using invasive techniques such as barostat. This allowed the following conclusions: (i) Most HC sensed an “empty” stomach (i.e., fullness ratings ~ zero) while the stomach still contained 200 ml and started to report hunger (i.e., hunger ratings > zero) at a gastric content volume of <100 ml. (ii). Interestingly, a number of OB patients did not feel any fullness even at maximal stomach filling; however, overall, the sensation of fullness and hunger was comparable to HCs. (iii) In contrast, AN patients report heightened sensitivity to gastric filling compared to HC and OB patients with approximately 1 in 3 AN patients still reporting fullness and no hunger at all when the stomach was completely empty. Consistent with this experimental data, questionnaires also demonstrated more dyspeptic and gastroparesis symptoms in AN patients (Table 1). Additionally, satiety, as assessed by the ad libitum buffet, was also more marked in AN patients than in HC and, in particular, OB patients. These findings complement a previous study in obese individuals that reported reduced fullness at one single postprandial time point compared to normal weight controls [10]. In AN, a previous epidemiological study found severe postprandial dyspeptic symptoms, i.e., fullness and early satiety [22].
The relationship between sensations of satiation and gastric distention is mediated by gastric mechanosensitive receptors with the former being modulated by the macronutrient composition (especially fat content) of the ingested meal [79–81]. Chemo-sensitive nutrient sensors in the small intestine trigger the release of GI peptides (e.g. CCK, GLP-1, PYY). These act as neuro-hormones that modulate vagal afferent activity and central perception [5, 82, 83]. For example, CCK enhances the satiating effect of gastric filling (i.e., reduces hunger) and high postprandial CCK levels may explain the altered sensation of fullness and hunger in AN patients [83–85]. However, a meta-analysis of studies in AN patients revealed only slightly increased CCK levels at baseline compared to HC, but equivalent levels after meals [86]. Thus, analogous to the effects on gastric emptying, altered perception of fullness in AN and OB might not be related to direct effects of GI peptide hormones on gastric function, but rather to the habitual diet at study entry. It has been shown that chronic, high-fat diet leads to a decrease in postprandial fullness and satiety respectively [80, 87]. Therefore, it may be the composition of the test meal relative to the habitual diet of participants that explains the observed differences. Psychiatric comorbidity provides several other, possible explanations for differences in fullness and hunger sensations between the study groups. First, anxiety among AN patients (Table 1) could reduce gastric accommodation which would be expected to increase postprandial fullness [88]. Second, reduced reporting of hunger and higher satiety ratings may represent a “secondary gain” in AN patients undergoing weight rehabilitation [27]. Third, interoceptive awareness and visceral sensitivity are decreased in AN patients, resulting in diminished ability to discriminate hunger and satiety sensations [89, 90]. In summary, abnormal reports of sensations in this group could be exaggerated by abnormal GI physiology, attempts by patients to excuse eating less, or the psychiatric disease and related comorbidities.
Longitudinal investigation of AN1 vs. AN2 revealed that fullness and hunger ratings shifted towards more normal levels (Fig. 3e and f). There was also less satiety, as indicated by increased intake at the ad libitum buffet. As discussed above, such improvements could be the result of physiological adaptation to increased oral intake or behavioural change. Previous studies found an improvement of interoceptive awareness in AN patients with weight gain [91]. However, it is not possible to assess from study results whether this is a consequence of weight rehabilitation, adaption to regular food intake or improved psychological state. Of note, food intake increased by only 6%, thus, the trend to more normal postprandial sensations is more likely related to regular food intake and weight gain, than to psychological rehabilitation. The latter is also mirrored by unchanged anxiety, i.e., values of the STAI (state). Indeed, if the latter occurred, then one would expect AN patients to choose chocolate or cheese rather than apples and soup at the ad libitum buffet, which was never observed.
Conclusions
By investigating participants across a wide range of BMI, this study provides detailed insight into the relationship of GI motor and sensory function with body weight. Results indicate that gastric emptying rate is decreased and postprandial sensations of satiation are increased in AN patients compared to HC and OB patients. In principle, these findings support the hypothesis that the physiological response to feeding is heightened in lower body weight. The improvement in gastric emptying and postprandial sensations of satiation in AN after weight rehabilitation suggest that the differences in GI motor and sensory function are unlikely to be a primary cause of abnormal body weight. Secondary causes, such as dietary habits and psychiatric comorbidities, are more likely to determine GI motor and sensory function in the investigated groups.
Abbreviations
- AN:
-
Patients with anorexia nervosa
- AN2:
-
AN at follow-up (after weight rehabilitation)
- BDI:
-
Beck Depression Inventory II
- BMI:
-
Body mass index
- GCSI:
-
Gastroparesis Cardinal Symptom Index
- GI:
-
Gastrointestinal
- HC:
-
Healthy controls
- LDQ:
-
Leeds Dyspepsia Questionnaire
- MRI:
-
Magnetic resonance imaging
- OB:
-
Patients with obesity
- OCTT:
-
Oro-cecal transit time
- STAI:
-
State-Trait-Anxiety Inventory
- t50 :
-
Gastric content half-emptying time
References
Dubois L, Ohm Kyvik K, Girard M, Tatone-Tokuda F, Perusse D, Hjelmborg J, et al. Genetic and environmental contributions to weight, height, and BMI from birth to 19 years of age: an international study of over 12,000 twin pairs. PLoS One. 2012;7(2):e30153.
Collins JCB, Jon E. Behavioral and psychological factors in obesity. J Lancaster General Hospital. 2009;4(4):4.
Schmidhauser SE, Klaus; Brügger, Urs..Environmental determinants of overweight and obesity: Extended international literature review. Final report. Study commissioned by the Federal Office of Public Health Switzerland. 2009:170.
Fairburn CG, Cooper Z, Doll HA, Welch SL. Risk factors for anorexia nervosa: three integrated case-control comparisons. Arch Gen Psychiatry. 1999;56(5):468–76.
Janssen P, Vanden Berghe P, Verschueren S, Lehmann A, Depoortere I, Tack J. Review article: the role of gastric motility in the control of food intake. Aliment Pharmacol Ther. 2011;33(8):880–94.
Woods SC. The control of food intake: behavioral versus molecular perspectives. Cell Metab. 2009;9(6):489–98.
Cummings DE, Overduin J. Gastrointestinal regulation of food intake. J Clin Invest. 2007;117(1):13–23.
Rui L. Brain regulation of energy balance and body weight. Rev Endocr Metab Disord. 2013;14(4):387–407.
Camilleri M. Integrated upper gastrointestinal response to food intake. Gastroenterology. 2006;131(2):640–58.
Delgado-Aros S, Cremonini F, Castillo JE, Chial HJ, Burton DD, Ferber I, et al. Independent influences of body mass and gastric volumes on satiation in humans. Gastroenterology. 2004;126(2):432–40.
Delgado-Aros S, Camilleri M, Castillo EJ, Cremonini F, Stephens D, Ferber I, et al. Effect of gastric volume or emptying on meal-related symptoms after liquid nutrients in obesity: a pharmacologic study. Clin Gastroenterol Hepatol. 2005;3(10):997–1006.
Cardoso-Junior A, Coelho LG, Savassi-Rocha PR, Vignolo MC, Abrantes MM, de Almeida AM, et al. Gastric emptying of solids and semi-solids in morbidly obese and non-obese subjects: an assessment using the 13C-octanoic acid and 13C-acetic acid breath tests. Obes Surg. 2007;17(2):236–41.
Wright RA, Krinsky S, Fleeman C, Trujillo J, Teague E. Gastric emptying and obesity. Gastroenterology. 1983;84(4):747–51.
Tosetti C, Corinaldesi R, Stanghellini V, Pasquali R, Corbelli C, Zoccoli G, et al. Gastric emptying of solids in morbid obesity. Int J Obes Relat Metab Disord. 1996;20(3):200–5.
Gryback P, Naslund E, Hellstrom PM, Jacobsson H, Backman L. Gastric emptying of solids in humans: improved evaluation by Kaplan-Meier plots, with special reference to obesity and gender. Eur J Nucl Med. 1996;23(12):1562–7.
Seimon RV, Brennan IM, Russo A, Little TJ, Jones KL, Standfield S, et al. Gastric emptying, mouth-to-cecum transit, and glycemic, insulin, incretin, and energy intake responses to a mixed-nutrient liquid in lean, overweight, and obese males. Am J Physiol Endocrinol Metab. 2013;304(3):E294–300.
Buchholz V, Berkenstadt H, Goitein D, Dickman R, Bernstine H, Rubin M. Gastric emptying is not prolonged in obese patients. Surg Obes Relat Dis. 2013;9(5):714–7.
Chiloiro M, Caroli M, Guerra V, Lodadea Piepoli A, Riezzo G. Gastric emptying in normal weight and obese children--an ultrasound study. Int J Obes Relat Metab Disord. 1999;23(12):1303–6.
Verdich C, Madsen JL, Toubro S, Buemann B, Holst JJ, Astrup A. Effect of obesity and major weight reduction on gastric emptying. Int J Obes Relat Metab Disord. 2000;24(7):899–905.
Jackson SJ, Leahy FE, McGowan AA, Bluck LJ, Coward WA, Jebb SA. Delayed gastric emptying in the obese: an assessment using the non-invasive (13)C-octanoic acid breath test. Diabetes Obes Metab. 2004;6(4):264–70.
Vazquez Roque MI, Camilleri M, Stephens DA, Jensen MD, Burton DD, Baxter KL, et al. Gastric sensorimotor functions and hormone profile in normal weight, overweight, and obese people. Gastroenterology. 2006;131(6):1717–24.
Santonicola A, Siniscalchi M, Capone P, Gallotta S, Ciacci C, Iovino P. Prevalence of functional dyspepsia and its subgroups in patients with eating disorders. World J Gastroenterol. 2012;18(32):4379–85.
Robinson PH. Gastric function in eating disorders. Ann N Y Acad Sci. 1989;575:456–64. discussion 64-5.
Robinson PH. Perceptivity and paraceptivity during measurement of gastric emptying in anorexia and bulimia nervosa. Br J Psychiatry. 1989;154:400–5.
Rigaud D, Bedig G, Merrouche M, Vulpillat M, Bonfils S, Apfelbaum M. Delayed gastric emptying in anorexia nervosa is improved by completion of a renutrition program. Dig Dis Sci. 1988;33(8):919–25.
Waldholtz BD, Andersen AE. Gastrointestinal symptoms in anorexia nervosa. A prospective study. Gastroenterology. 1990;98(6):1415–9.
Benini L, Todesco T, Dalle Grave R, Deiorio F, Salandini L, Vantini I. Gastric emptying in patients with restricting and binge/purging subtypes of anorexia nervosa. Am J Gastroenterol. 2004;99(8):1448–54.
Hutson WR, Wald A. Gastric emptying in patients with bulimia nervosa and anorexia nervosa. Am J Gastroenterol. 1990;85(1):41–6.
Abell TL, Malagelada JR, Lucas AR, Brown ML, Camilleri M, Go VL, et al. Gastric electromechanical and neurohormonal function in anorexia nervosa. Gastroenterology. 1987;93(5):958–65.
McCallum RW, Grill BB, Lange R, Planky M, Glass EE, Greenfeld DG. Definition of a gastric emptying abnormality in patients with anorexia nervosa. Dig Dis Sci. 1985;30(8):713–22.
Holt S, Ford MJ, Grant S, Heading RC. Abnormal gastric emptying in primary anorexia nervosa. Br J Psychiatry. 1981;139:550–2.
Buchman AL, Ament ME, Weiner M, Kodner A, Mayer EA. Reversal of megaduodenum and duodenal dysmotility associated with improvement in nutritional status in primary anorexia nervosa. Dig Dis Sci. 1994;39(2):433–40.
Pasricha PJ, Colvin R, Yates K, Hasler WL, Abell TL, Unalp-Arida A, et al. Characteristics of patients with chronic unexplained nausea and vomiting and normal gastric emptying. Clin Gastroenterol Hepatol. 2011;9(7):567–76. e1-4.
Norris ML, Harrison ME, Isserlin L, Robinson A, Feder S, Sampson M. Gastrointestinal complications associated with anorexia nervosa: A systematic review. Int J Eat Disord. 2016;49(3):216–37.
Eating Disorders. Core Interventions in the Treatment and Management of Anorexia Nervosa. Leicester (UK): Bulimia Nervosa and Related Eating Disorders; 2004.
Roy S, Fox MR, Curcic J, Schwizer W, Pal A. The gastro-esophageal reflux barrier: biophysical analysis on 3D models of anatomy from magnetic resonance imaging. Neurogastroenterol Motil. 2012;24(7):616–25. e269.
Kwiatek MA, Fox MR, Steingoetter A, Menne D, Pal A, Fruehauf H, et al. Effects of clonidine and sumatriptan on postprandial gastric volume response, antral contraction waves and emptying: an MRI study. Neurogastroenterol Motil. 2009;21(9):928–e71.
Priebe MG, Wachters-Hagedoorn RE, Landman K, Heimweg J, Elzinga H, Vonk RJ. Influence of a subsequent meal on the oro-cecal transit time of a solid test meal. Eur J Clin Investig. 2006;36(2):123–6.
Marciani L, Cox EF, Hoad CL, Pritchard S, Totman JJ, Foley S, et al. Postprandial changes in small bowel water content in healthy subjects and patients with irritable bowel syndrome. Gastroenterology. 2010;138(2):469–77. 77 e1.
Marciani L, Hall N, Pritchard SE, Cox EF, Totman JJ, Lad M, et al. Preventing gastric sieving by blending a solid/water meal enhances satiation in healthy humans. J Nutr. 2012;142(7):1253–8.
Kunz P, Feinle C, Schwizer W, Fried M, Boesiger P. Assessment of gastric motor function during the emptying of solid and liquid meals in humans by MRI. J Magn Reson Imaging. 1999;9(1):75–80.
Elashoff JD, Reedy TJ, Meyer JH. Analysis of gastric emptying data. Gastroenterology. 1982;83(6):1306–12.
Fraser A, Delaney BC, Ford AC, Qume M, Moayyedi P. The Short-Form Leeds Dyspepsia Questionnaire validation study. Aliment Pharmacol Ther. 2007;25(4):477–86.
Revicki DA, Rentz AM, Dubois D, Kahrilas P, Stanghellini V, Talley NJ, et al. Development and validation of a patient-assessed gastroparesis symptom severity measure: the Gastroparesis Cardinal Symptom Index. Aliment Pharmacol Ther. 2003;18(1):141–50.
Parkman HP, Yates K, Hasler WL, Nguyen L, Pasricha PJ, Snape WJ, et al. Clinical features of idiopathic gastroparesis vary with sex, body mass, symptom onset, delay in gastric emptying, and gastroparesis severity. Gastroenterology. 2011;140(1):101–15.
Hautzinger M, Bailer M, Worall H, Keller F. BDI Beck-Depressions-Inventar Testhandbuch. 2nd ed. Bern: Verlag Hans Huber; 1995.
Laux L, Glanzmann P, Schaffner P, Spielberger CD. Das State-Trait-Angstinventar. Theoretische Grundlagen und Handanweisung. Weinheim: Beltz Test GmbH; 1981.
Follmann DA, Proschan MA, Geller NL. Monitoring pairwise comparisons in multi-armed clinical trials. Biometrics. 1994;50(2):325–36.
Christensen RBP. Analysis of sensory ratings data with cumulative link models. Journal de la Société Française de Statistique. 2013;154(3):22.
Perez ME, Coley B, Crandall W, Di Lorenzo C, Bravender T. Effect of nutritional rehabilitation on gastric motility and somatization in adolescents with anorexia. J Pediatr. 2013;163(3):867–72. e1.
Glasbrenner B, Pieramico O, Brecht-Krauss D, Baur M, Malfertheiner P. Gastric emptying of solids and liquids in obesity. Clin Investig. 1993;71(7):542–6.
Domstad PA, Shih WJ, Humphries L, DeLand FH, Digenis GA. Radionuclide gastric emptying studies in patients with anorexia nervosa. J Nucl Med. 1987;28(5):816–9.
Kamal N, Chami T, Andersen A, Rosell FA, Schuster MM, Whitehead WE. Delayed gastrointestinal transit times in anorexia nervosa and bulimia nervosa. Gastroenterology. 1991;101(5):1320–4.
Hirakawa M, Okada T, Iida M, Tamai H, Kobayashi N, Nakagawa T, et al. Small bowel transit time measured by hydrogen breath test in patients with anorexia nervosa. Dig Dis Sci. 1990;35(6):733–6.
Rerksuppaphol S, Rerksuppaphol L. Rapid orocecal transit time in obese children measured by hydrogen breath test. J Med Assoc Thail. 2012;95 Suppl 12:S26–31.
Basilisco G, Camboni G, Bozzani A, Vita P, Doldi S, Bianchi PA. Orocecal transit delay in obese patients. Dig Dis Sci. 1989;34(4):509–12.
Shin HS, Ingram JR, McGill AT, Poppitt SD. Lipids, CHOs, proteins: can all macronutrients put a ‘brake’ on eating? Physiol Behav. 2013;120:114–23.
Van Citters GW, Lin HC. Ileal brake: neuropeptidergic control of intestinal transit. Curr Gastroenterol Rep. 2006;8(5):367–73.
Spiller RC, Trotman IF, Higgins BE, Ghatei MA, Grimble GK, Lee YC, et al. The ileal brake--inhibition of jejunal motility after ileal fat perfusion in man. Gut. 1984;25(4):365–74.
Fernstrom MH, Weltzin TE, Neuberger S, Srinivasagam N, Kaye WH. Twenty-four-hour food intake in patients with anorexia nervosa and in healthy control subjects. Biol Psychiatry. 1994;36(10):696–702.
Jauregui Lobera I, Bolanos RP. Choice of diet in patients with anorexia nervosa. Nutr Hosp. 2009;24(6):682–7.
Cecil JE, Francis J, Read NW. Comparison of the effects of a high-fat and high-carbohydrate soup delivered orally and intragastrically on gastric emptying, appetite, and eating behaviour. Physiol Behav. 1999;67(2):299–306.
Boyd KA, O’Donovan DG, Doran S, Wishart J, Chapman IM, Horowitz M, et al. High-fat diet effects on gut motility, hormone, and appetite responses to duodenal lipid in healthy men. Am J Physiol Gastrointest Liver Physiol. 2003;284(2):G188–96.
Seimon RV, Taylor P, Little TJ, Noakes M, Standfield S, Clifton PM, et al. Effects of acute and longer-term dietary restriction on upper gut motility, hormone, appetite, and energy-intake responses to duodenal lipid in lean and obese men. Am J Clin Nutr. 2014;99(1):24–34.
Cunningham KM, Daly J, Horowitz M, Read NW. Gastrointestinal adaptation to diets of differing fat composition in human volunteers. Gut. 1991;32(5):483–6.
Castiglione KE, Read NW, French SJ. Adaptation to high-fat diet accelerates emptying of fat but not carbohydrate test meals in humans. Am J Physiol Regul Integr Comp Physiol. 2002;282(2):R366–71.
Little TJ, Horowitz M, Feinle-Bisset C. Modulation by high-fat diets of gastrointestinal function and hormones associated with the regulation of energy intake: implications for the pathophysiology of obesity. Am J Clin Nutr. 2007;86(3):531–41.
Meyer-Gerspach AC, Wolnerhanssen B, Beglinger B, Nessenius F, Napitupulu M, Schulte FH, et al. Gastric and intestinal satiation in obese and normal weight healthy people. Physiol Behav. 2014;129:265–71.
Mones J, Carrio I, Calabuig R, Estorch M, Sainz S, Berna L, et al. Influence of the menstrual cycle and of menopause on the gastric emptying rate of solids in female volunteers. Eur J Nucl Med. 1993;20(7):600–2.
Hutson WR, Roehrkasse RL, Wald A. Influence of gender and menopause on gastric emptying and motility. Gastroenterology. 1989;96(1):11–7.
Knight LC, Parkman HP, Brown KL, Miller MA, Trate DM, Maurer AH, et al. Delayed gastric emptying and decreased antral contractility in normal premenopausal women compared with men. Am J Gastroenterol. 1997;92(6):968–75.
Szmukler GI, Young GP, Lichtenstein M, Andrews JT. A serial study of gastric emptying in anorexia nervosa and bulimia. Aust NZ J Med. 1990;20(3):220–5.
Bennett EJ, Evans P, Scott AM, Badcock CA, Shuter B, Hoschl R, et al. Psychological and sex features of delayed gut transit in functional gastrointestinal disorders. Gut. 2000;46(1):83–7.
Germain N, Galusca B, Le Roux CW, Bossu C, Ghatei MA, Lang F, et al. Constitutional thinness and lean anorexia nervosa display opposite concentrations of peptide YY, glucagon-like peptide 1, ghrelin, and leptin. Am J Clin Nutr. 2007;85(4):967–71.
Atiye M, Miettunen J, Raevuori-Helkamaa A. A meta-analysis of temperament in eating disorders. Eur Eat Disord Rev. 2015;23(2):89–99.
Sarisoy G, Atmaca A, Ecemis G, Gumus K, Pazvantoglu O. Personality characteristics and body image in obese individuals. Asia-Pacific Psychiatry. 2014;6(2):191–9.
Schiesser M. Adipositaschirurgie im Wandel: Aktuelle Indikationen, Operationstechniken und Resultate. Swiss Med Forum. 2015;15(10):5.
Fruehauf H, Steingoetter A, Fox MR, Kwiatek MA, Boesiger P, Schwizer W, et al. Characterization of gastric volume responses and liquid emptying in functional dyspepsia and health by MRI or barostat and simultaneous C-acetate breath test. Neurogastroenterol Motil. 2009;21(7):697–e37.
Marciani L, Cox EF, Pritchard SE, Major G, Hoad CL, Mellows M, et al. Additive effects of gastric volumes and macronutrient composition on the sensation of postprandial fullness in humans. Eur J Clin Nutr. 2015;69(3):380–4.
Brennan IM, Luscombe-Marsh ND, Seimon RV, Otto B, Horowitz M, Wishart JM, et al. Effects of fat, protein, and carbohydrate and protein load on appetite, plasma cholecystokinin, peptide YY, and ghrelin, and energy intake in lean and obese men. Am J Physiol Gastrointest Liver Physiol. 2012;303(1):G129–40.
Kwiatek MA, Menne D, Steingoetter A, Goetze O, Forras-Kaufman Z, Kaufman E, et al. Effect of meal volume and calorie load on postprandial gastric function and emptying: studies under physiological conditions by combined fiber-optic pressure measurement and MRI. Am J Physiol Gastrointest Liver Physiol. 2009;297(5):G894–901.
Geeraerts B, Van Oudenhove L, Dupont P, Vanderghinste D, Bormans G, Van Laere K, et al. Different regional brain activity during physiological gastric distension compared to balloon distension: a H2 15O-PET study. Neurogastroenterol Motil. 2011;23(6):533–e203.
Kanoski SE, Walls EK, Davidson TL. Interoceptive “satiety” signals produced by leptin and CCK. Peptides. 2007;28(5):988–1002.
Kissileff HR, Carretta JC, Geliebter A, Pi-Sunyer FX. Cholecystokinin and stomach distension combine to reduce food intake in humans. Am J Physiol Regul Integr Comp Physiol. 2003;285(5):R992–8.
Feinle C, D’Amato M, Read NW. Cholecystokinin-A receptors modulate gastric sensory and motor responses to gastric distension and duodenal lipid. Gastroenterology. 1996;110(5):1379–85.
Prince AC, Brooks SJ, Stahl D, Treasure J. Systematic review and meta-analysis of the baseline concentrations and physiologic responses of gut hormones to food in eating disorders. Am J Clin Nutr. 2009;89(3):755–65.
French SJ, Murray B, Rumsey RD, Fadzlin R, Read NW. Adaptation to high-fat diets: effects on eating behaviour and plasma cholecystokinin. Br J Nutr. 1995;73(2):179–89.
Geeraerts B, Vandenberghe J, Van Oudenhove L, Gregory LJ, Aziz Q, Dupont P, et al. Influence of experimentally induced anxiety on gastric sensorimotor function in humans. Gastroenterology. 2005;129(5):1437–44.
Pollatos O, Kurz AL, Albrecht J, Schreder T, Kleemann AM, Schopf V, et al. Reduced perception of bodily signals in anorexia nervosa. Eat Behav. 2008;9(4):381–8.
Fassino S, Piero A, Gramaglia C, Abbate-Daga G. Clinical, psychopathological and personality correlates of interoceptive awareness in anorexia nervosa, bulimia nervosa and obesity. Psychopathology. 2004;37(4):168–74.
Matsumoto R, Kitabayashi Y, Narumoto J, Wada Y, Okamoto A, Ushijima Y, et al. Regional cerebral blood flow changes associated with interoceptive awareness in the recovery process of anorexia nervosa. Prog Neuro-Psychopharmacol Biol Psychiatry. 2006;30(7):1265–70.
Acknowledgements
The authors gratefully acknowledge Judit Valentini, Tobias Hahn and Jelena Curcic for MRI technical assistance and Simon Bütikofer for recruitment and investigation of study participants.
Funding
The study was funded by the Swiss National Science Foundation (grant 320030/125333). SB was supported by a personal grant from the Swiss National Science Foundation (P2SKP3_158649).
Availability of data and materials
The database supporting the conclusions of this article can not be shared at present, as analyses testing the predictive value of GI peptide hormones for gastric emptying are not yet completed.
Authors’ contributions
SB performed the recruitment of participants, data acquisition, data management and drafted the manuscript. DM performed statistical analyses and data plots. GM, MFx, OG and AS wrote the study protocol. GM, OG, MF and WS supervised the study. MFx and AS critically revised the manuscript. All authors approved the final version of the manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
The study was carried out according to Good Clinical Practice and the Declaration of Helsinki. The protocol was approved by the University of Zurich Ethics Commission (KEK-ZH-No 2009-0115/1) and registered at ClinicalTrials.gov (NCT00946816). All participants provided written informed consent.
Author information
Authors and Affiliations
Corresponding author
Additional file
Additional file 1: Figure S1.
Longitudinal comparison of AN patients. Displayed are the data of the 12 participants that were investigated at visit 1 and visit 2. Paired data: n = 11 for LDQ and GCSI; n = 9 for BDI and STAI. (TIF 10832 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Bluemel, S., Menne, D., Milos, G. et al. Relationship of body weight with gastrointestinal motor and sensory function: studies in anorexia nervosa and obesity. BMC Gastroenterol 17, 4 (2017). https://doi.org/10.1186/s12876-016-0560-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12876-016-0560-y