Abstract
Background
The plasticity region of Helicobacter pylori (H. pylori) is a large chromosomal segment containing strain-specific genes. The prevalence of the plasticity region genes of the H. pylori strains in China remains unknown. The aim of this study was to examine the status of these genes and to assess the relationship between the genes and the diseases caused by H. pylori infection.
Methods
A total of 141 strains were isolated from patients with chronic active gastritis (CAG), peptic ulcer disease (PUD) and gastric carcinoma (GC). The prevalence of jhp0940, jhp0945, jhp0947, jhp0949 and jhp0951 was determined using PCR, and the results were analyzed using the chi-squared test.
Results
The prevalence rates of jhp0940, jhp0945, jhp0947, jhp0949 and jhp0951 in the H. pylori strains were 42.55, 51.06, 20.57, 56.03 and 63.12 %, respectively. The prevalence rates of jhp0940 were similar in the isolates from the CAG, PUD and GC patients, and there was no association between the jhp0940 status and any of the diseases. In contrast, the prevalence rates of jhp0945, jhp0947, jhp0949 and jhp0951 were significantly higher in the PUD and GC isolates than in the CAG isolates (p < 0.01). A univariate analysis showed that jhp0945, jhp0947, jhp0949 and jhp0951 increased the risk of PUD, while only jhp0951 was significantly associated with PUD in the multivariate analysis (p = 0.0149). The jhp0945-positive isolates were significantly associated with an increased risk for GC (p = 0.0097).
Conclusion
The plasticity region genes are widely distributed in Chinese patients, and a high prevalence of these genes occurs in more serious diseases. Therefore, jhp0951 status is an independent factor associated with the development of PUD, and jhp0945 may predict the future development of GC in patients with CAG and is considered to be the best candidate disease marker for H. pylori-related diseases.
Similar content being viewed by others
Background
H. pylori is the major cause of chronic gastritis, peptic ulcer disease, gastric carcinoma and mucosa-associated lymphoid tissue lymphoma [1, 2]. The majority of infected individuals remain asymptomatic throughout their lifetime, and only approximately 15 % develop gastroduodenal diseases. Variations in the clinical outcomes of these diseases have been attributed to differences in environmental factors, bacterial strains and host genetics [3, 4]. A number of bacterial virulence factors that are associated with these diseases have been described for H. pylori, such as the cag pathogenicity island (cag PAI) [5, 6].
A comparison of the genomes of two H. pylori strains revealed that in addition to the cag PAI, a large region of approximately 45 kb in strain J99 and 68 kb in strain 26695 is present in both strains and has been termed the “plasticity region” [7, 8] . Up to 50 % of the strain-specific genes transferred from other species are located in the plasticity region [9]. Whether these strain-specific genes influence the severity of different diseases or the biological functions of the ORFs in the plasticity region still remains unknown. Recent studies have revealed that the jhp0940, jhp0945, jhp0947, jhp0949 and jhp0951 genes from H. pylori are associated with an increased risk for gastroduodenal diseases [3, 9–11]. In a Brazilian study, the jhp0947 gene was found to be involved in the development of duodenal ulcers (DUs) and GC [11]. In addition, Romo-González et al. found that jhp0951 is also associated with DUs [9]. In China, the number of H. pylori infections has exceeded 60 %, and the high-risk incidence of GC poses a serious health and economic burden. Moreover, there are no reports discussing the prevalence of these genes or the relationship between the genes and the severity of the clinical outcomes of the abovementioned diseases in China. The aim of this study was to assess the prevalence of jhp0940, jhp0945, jhp0947, jhp0949 and jhp0951 and to determine their association with H. pylori-related diseases.
Methods
Strains
A total of 141 H. pylori strains were selected from the H. pylori strain bank of China at the National Institute for Communicable Disease Control and Prevention, Chinese Center for Disease Control and Prevention. A total of 40 strains were isolated from patients in Heilongjiang (HLJ) Province (located in northern China), and another 101 strains were isolated from patients from Jiangxi (JX) Province (located in southeast China). These strains were related with CAG only (n = 58), PUD (n = 45) or GC (n = 38). Two strains with fully sequenced genomes, including strain 26695, which was isolated from a gastritis patient, and strain J99, which was isolated from a patient with a duodenal ulcer, were used as controls. This study was approved by the Ethics Committee of National Institute for Communicable Disease Control and Prevention, Chinese Center for Disease Control and Prevention. The written informed consent was obtained from all patients.
Culture and extraction of genomic DNA
The strains stored in brain heart infusion in −80 °C were recovered on Columbia agar plates (Oxoid) supplemented with 5 % fresh defibrinated sheep blood and kept under microaerophilic conditions (5 % O2, 10 % CO2 and 85 % N2) at 37 °C for 3 days. Colonies displaying typical H. pylori morphology were selected and identified by Gram staining and urease, oxidase, and catalase activity testing. The bacterial cells on chocolate agar plate were washed twice with phosphate buffer saline (PBS, pH7.5) and centrifuged at 5000 rpm for 10 min. The chromosomal DNA was extracted using the QIAamp DNA Mini Kit (Qiagen, Germany) according to the manufacturer’s instructions.
Determination of the jhp0940, jhp0945, jhp0947, jhp0949 and jhp0951 status via PCR
The status of the genes was determined via PCR using the primer pairs shown in Table 1. The amplification of the genes was performed in a volume of 25 μl containing 25 pmol of both forward and reverse primers. The PCR conditions were 95 °C for 5 min, followed by 35 cycles of 95 °C for 45 s, 52 °C for 45 s, and 72 °C for 45 s, and finally 72 °C for 5 min. Two primer sets were used for the jhp0940 gene, and when the PCR using one pair was positive, the jhp0940 status was determined to be positive. The status of the genes was positive in strain J99 and negative in strain 26695.
Statistical analysis
The prevalence rates of all five genes were evaluated. The association between each genotype and the clinical outcomes were quantified using the chi-squared test. A probability (p) value equal to or less than 0.05 was considered to be statistically significant, and a value of 0.001 or less was considered to be highly significant. Univariate analysis and a multivariate logistic regression model were used to calculate the odds ratios (ORs) of the clinical outcomes using SAS 9.3.
Results
Distribution of the jhp0940, jhp0945, jhp0947, jhp0949 and jhp0951 genes
The prevalence rates of jhp0940, jhp0945, jhp0947, jhp0949 and jhp0951 in the tested H. pylori were 42.55 % (60/141), 51.06 % (72/141), 20.57 % (29/141), 56.03 % (79/141) and 63.12 % (89/141), respectively (Table 2).
Geographic variation of plasticity region genes
The results were divided into two groups based on their geographic variation. By comparing the results of the strains from HLJ Province and JX Province, we found no significant differences in the prevalence of jhp0940 (40 %, 16/40 and 43.56 %, 44/101, respectively, p = 0.699), jhp0945 (42.5 %, 17/40 and 54.46 %, 55/101, p = 0.201), jhp0947 (10 %, 4/40 and 24.75 %, 25/101, p = 0.051), jhp0949 (52.5 %, 21/40 and 57.43 %, 58/101, p = 0.595), or jhp0951 (57.5 %, 23/40 and 65.35 %, 66/101, p = 0.384).
Plasticity region genes and diseases
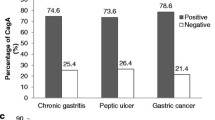
The prevalence rates of jhp0940 in the strains from the CAG, PUD and GC patients were 34.48 % (20/58), 48.89 % (22/45) and 47.37 % (18/38), respectively, and there were no differences between the three diseases. The prevalence rates of jhp0945 in the isolates from the patients with PUD (62.22 %, 28/45) and GC (73.68 %, 28/38) were significantly higher than from the CAG patients (27.59 %, 16/58; p = 0.0004 and p < 0.0001, respectively). The jhp0947 gene occurred in only 4 (6.89 %) of the strains isolated from the CAG patients, and the occurrence was much lower than in the PUD and GC patients (33.33 %, 15/45 and 26.32 %, 10/38; p = 0.0006 and p = 0.0084, respectively). The prevalence of jhp0949 was similar (68.89 %, 31/45 and 65.79 %, 25/38) in the PUD and GC isolates and was much higher than that in the CAG patients (39.66 %, 23/58; p = 0.0032 and p = 0.0123, respectively). For jhp0951, the prevalence rate in the PUD patients was higher than that in the CAG patients (77.78 %, 35/45 and 53.45 %, 31/58, respectively; p = 0.0107); however, there was no difference between GC and CAG.
The relationship between jhp0940, jhp0945, jhp0947, jhp0949 and jhp0951
The status of all of the genes showed that they are significantly associated with each other (Table 3). The status of jhp0945 was associated with jhp0949 (p = 0.009) and jhp0951 (p = 0.022). All of the jhp0947-positive isolates possessed jhp0949, and both were significantly associated with jhp0951 (p = 0.0008 and p < 0.0001, respectively).
When combining the five genes together, the majority of the genotypes were all negative or all positive (the −/−/−/−/- genotype or the +/+/+/+/+ genotype, 12.06 %, 17/141). The rates of the all-positive genotypes were 15.79 % (6/38), 20 % (9/45) and 3.45 % (2/58) in the isolates from the patients with GC, PUD and CAG only, respectively. In contrast, the rates of the all-negative genotypes were 5.26 % (2/38), 2.22 % (1/45) and 24.14 % (14/58) for GC, PUD, and CAG, respectively.
The relationship between the gene status and the clinical outcomes
A univariate analysis showed that there was no significant association between the jhp0940 status and the selected diseases (Table 4), but the status of jhp0945, jhp0947, jhp0949 and jhp0951 was significantly associated with a lower risk for CAG (odds ratio (OR), [95 % confidence interval (CI)], 0.176 [0.081 to 0.381]; 0.116 [0.035 to 0.389]; 0.300 [0.145 to 0.623] and 0.445 [0.214 to 0.928], respectively). On the converse, they were also related to an increased risk for PUD (OR [95 % CI], 2.306 [1.08 to 4.927]; 2.835 [1.191 to 6.751], 2.452 [1.126 to 5.336] and 2.825 [1.226 to 6.51], respectively). Moreover, only the jhp0945-positive isolates were associated with an increased risk for GC (OR, 5.9259; 95 % CI, 1.267 to 27.714).
A multivariate analysis, including age and jhp0940, jhp0945, jhp0947, jhp0949 and jhp0951 status, was performed to determine the factors that were related to the clinical outcomes of the selected diseases (Table 5). The jhp0945 status for GC and the jhp0951 status for PUD significantly increased the risk of a clinical outcome. In contrast, the jhp0945 or jhp0949 status significantly decreased the risk for CAG (OR [95 % CI]: 0.214 [0.099 to 0.466]; 0.373 [0.172 to 0.807], respectively). Age significantly decreased the risk of CAG and PUD (OR [95 % CI]: 0.964 [0.937 to 0.992]; 0.963 [0.937 to 0.991], respectively), while it increased the risk for GC (1.098 [1.059 to 1.139]).
Discussion
The plasticity region is a recently identified locus found in the chromosome of H. pylori strains J99 and 26695 that displays similar characteristics to pathogenicity islands [7, 8]. The majority of the H. pylori strain-specific genes that are transferred from other species are located in the plasticity region [9, 12, 13]. The genes present in the plasticity region have been highlighted as potential pathogenic markers and may account for the differences in the H. pylori strain virulence, resulting in various clinical outcomes.
The jhp0940, jhp0945, jhp0947, jhp0949 and jhp0951 genes, which are specific for strain J99, have recently been reported to be associated with H. pylori-related diseases and are potential markers for the risk of gastrointestinal diseases [10]. Because of their geographic variation, the relationship between these genes and the severity of certain diseases has been discussed, but the results are often not in agreement. The prevalence of these genes and their relationship with certain diseases in China is currently unknown; therefore, we examined the distribution of these virulence markers and their relationship to the clinical outcomes of patients infected with H. pylori.
Previous study found that there were no significant associations between the gastroduodenal diseases and the status of jhp0940, jhp0945 and jhp0949 in East Asia strains [10]. Yakoob et al. demonstrated that jhp0940 and jhp0947 in Pakistan strains were associated with GC and PUD [14]. In our study, there was no association between jhp0940 and diseases. The positive rates of jhp0945, jhp0947, jhp0949 and jhp0951 were much higher in PUD, and there was a significant association between the genes. However, the multivariate analysis showed that only jhp0951 was independently associated with the development of PUD in our study. Jhp0951, which encodes an integrase from the XerCD family, is involved in the response to acidic environments [15]. In DUs, acid secretion increases, causing the mucosa to be continuously exposed to a low pH, which is consistent with our results [9]. As the virulence genes were associated with each other in our study, the strong linkage of jhp0945, jhp0947 and jhp0949 with PUD may be due to the significant association between jhp0951 and PUD. We also speculate that all of these factors act synergistically in causing damage to the host.
It is well known that the development of GC is marked by a slow progression that begins with H. pylori-induced chronic superficial gastritis, which then progresses to atrophic gastritis, intestinal metaplasia, dysplasia and eventually GC [16, 17]. In our study, the multivariate analysis showed that the only independent virulence gene that increased the risk of GC was jhp0945, indicating a significant association between the two. However, the jhp0945 status showed a negative association with CAG (Table 5), which conflicts with the process of GC development. In our study, we speculate that the majority of the individuals with CAG do not carry the jhp0945 gene, while the few jhp0945-positive individuals potentially develop GC due to a combination of the bacteria, the host and other environmental factors, which is consistent with the fact that of the H. pylori-infected individuals, 80–90 % have clinically asymptomatic gastritis, while only 1–2 % develop GC [18]. Jhp0945-positive isolates may be more likely to develop severe diseases, and jhp0945 status may be a risk indicator for GC development. Additional prospective studies are still necessary to further confirm our speculations.
Conclusions
In conclusion, this was the first study in China to evaluate the relationship between plasticity region genes and clinical disease outcomes. We found that jhp0951 status is an independent factor for discriminating PUD and may influence the association of other virulence factors with certain diseases. Jhp0945 may predict the future development of GC in patients with CAG and is considered to be the best candidate disease marker for H. pylori-related diseases.
Abbreviations
- H. pylori :
-
Helicobacter pylori
- CAG:
-
Chronic active gastritis
- PUD:
-
Peptic ulcer disease
- GC:
-
Gastric carcinoma
- HLJ:
-
Heilongjiang
- JX:
-
Jiangxi
- OR:
-
Odds ratio
- CI:
-
Confidence interval
References
Parsonnet J, Friedman GD, Vandersteen DP, Chang Y, Volgeman JH, Orentreich N, et al. Helicobacter pylori infection and the risk of gastric carcinoma. N Engl J Med. 1991;325:1127–31.
Uemura N, Okamoto S, Yamamoto S, Matsumura N, Yamaguchi S, Yamakido M, et al. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med. 2001;345:784–9.
Occhialini A, Marais A, Richard A, Garcia F, Sierra R, Mégraud F. Distribution of Open Reading Frames of Plasticity Region of Strain J99 in Helicobacter pylori Strains Isolated from Gastric Carcinoma and Gastritis Patients in Costa Rica. Infect Immun. 2000;68:6240–9.
Santos A, Queiroz DM, Ménard A, Marais A, Rocha GA, Oliveira CA, et al. New pathogenicity marker found in the plasticity region of the Helicobacter pylori genome. J Clin Microbiol. 2003;41:1651–5.
Backert S, Schwarz T, Miehlke S, Kirsch C, Sommer C, Kwok T, et al. Functional analysis of the cag pathogenicity island in Helicobacter pylori isolates from patients with gastritis, peptic ulcer, and gastric cancer. Infect Immun. 2004;72:1043–56.
Yamaoka Y. Mechanisms of disease: Helicobacter pylori virulence factors. Nat Rev Gastroenterol Hepatol. 2000;7:629–41.
Alm RA, Ling LS, Moir DT, King BL, Brown ED, Doig PC, et al. Genomic-sequence comparison of two unrelated isolates of the human gastric pathogen Helicobacter pylori. Nature. 1999;397:176–80.
Doig P, de Jonge BL, Alm RA, Brown ED, Uria-Nickelsen M, Noonan B, et al. Helicobacter pylori physiology predicted from genomic comparison of two strains. Microbiol Mol Biol Rev. 1999;63:675–707.
Romo-González C, Salama NR, Burgeño-Ferreira J, Ponce-Castañeda V, Lazcano-Ponce E, Camorlinga-Ponce M, et al. Differences in genome content among Helicobacter pylori isolates from patients with gastritis, duodenal ulcer, or gastric cancer reveal novel disease-associated genes. Infect Immun. 2009;77:2201–11.
Sugimoto M, Watada M, Jung SW, Graham DY, Yamaoka Y. Role of Helicobacter pylori Plasticity Region Genes in development of Gastroduodenal Diseases. J Clin Microbiol. 2012;50:441–8.
Proença Módena JL, Lopes Sales AI, Olszanski Acrani G, Russo R, Vilela Ribeiro MA, Fukuhara Y, et al. Association between Helicobacter pylori genotypes and gastric disorders in relation to the cag pathogenicity island. Diagn Microbiol Infect Dis. 2007;59:7–16.
Rizwan M, Alvi A, Ahmed N. Novel protein antigen (JHP940) from the genomic plasticity region of Helicobacter pylori induces tumor necrosis factor alpha and interleukin-8 secretion by human macrophages. J Bacteriol. 2008;190:1146–51.
de Jonge R, Kuipers EJ, Langeveld SC, Loffeld RJ, Stoof J, van Vliet AH, et al. The Helicobacter pylori plasticity region locus jhp0947-jhp0949 is associated with duodenal ulcer disease and interleukin-12 production in monocyte cells. FEMS Immunol Med Microbiol. 2004;41:161–7.
Yakoob J, Abbas Z, Nas S, Islam M, Abid S, Jafri W. Associations between the plasticity region genes of Helicobacter pylori and gastroduodenal diseases in a high-prevalence area. Gut and Liver. 2010;4:345–50.
Gancz H, Censini S, Merrell DS. Iron and pH homeostasis intersect at the level of Fur regulation in the gastric pathogen Helicobacter pylori. Infect Immun. 2006;74:602–14.
Conteduca V, Sansonno D, Lauletta G, Russi S, Ingravallo G, Dammacco F. H. pylori infection and gastric cancer: State of the art. Int J Oncol. 2013;42:5–18.
Matysiak-Budnik T, Mégraud F. Helicobacter pylori infection and gastric cancer. Eur J Cancer. 2006;42:708–16.
Wu MS, Chow LP, Lin JT, Chiou SH. Proteomic identification of biomarkers related to Helicobacter pylori-associated gastroduodenal disease: Challenges and opportunities. J Gastroenterol Hepatol. 2008;23:1657–61.
Watada M, Shiota S, Matsunari O, Suzuki R, Murakami K, Fujioka T, et al. Association between Helicobacter pylori cagA-related genes and clinical outcomes in Colombia and Japan. BMC Gastroenterol. 2011;11:141–8.
Acknowledgments
This study was supported by the Grant from National Technology R & D Program in the 12th Five-Year Plan of China (No. 2012BAI06B02), the Major Technology Project as part of “Prevention and Control of Major Infectious Diseases including AIDS and Viral Hepatitis” (No. 2013ZX10004216-002) and the grant from National Key Scientific Instrument and Equipment Development Project (No. 2012YQ180117).
We would like to thank all of the participants from the Department of Diagnosis for Communicable Diseases, the National Institute for Communicable Disease Control and Prevention, and the Chinese Center for Disease Control and Prevention.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
ZJZ designed the research. GYN performed the study and wrote the paper. PXH and LH analyzed the results. HLH and YYH participated in strains recovery. All authors read and approved the final manuscript.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Gong, Y., Peng, X., He, L. et al. The distribution of jhp0940, jhp0945, jhp0947, jhp0949 and jhp0951 genes of Helicobacter pylori in China. BMC Gastroenterol 15, 115 (2015). https://doi.org/10.1186/s12876-015-0341-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12876-015-0341-z